Estimating the Impact of Air Pollution on Healthcare-Seeking Behaviour by Applying a Difference-in-Differences Method to Syndromic Surveillance Data
Abstract
:1. Introduction
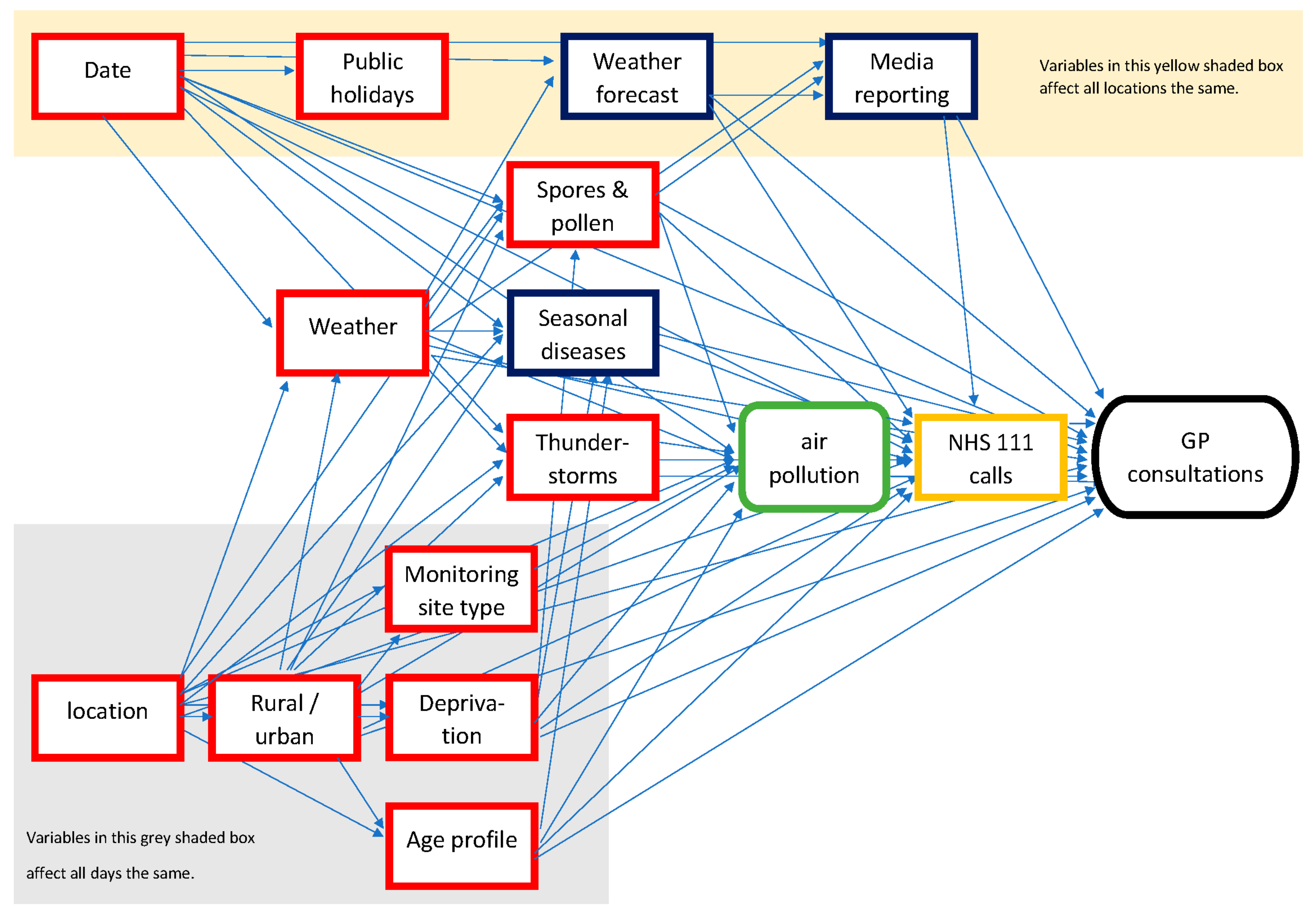
2. Method
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Exposure Period | Number of Upper Tier Local Authorities | ||||
|---|---|---|---|---|---|
| At Least 1 Day with Moderate or Higher DAQI Level | Low DAQI Levels Only | ||||
| Start Date | End Date | At Least 1 Day with Data | No Missing Data (Cases) | At Least 1 Day with Data | No Missing Data (Controls) |
| 4 May 2012 | 6 May 2012 | 1 | 0 | 52 | 12 |
| 21 May 2012 | 30 May 2012 | 30 | 3 | 22 | 3 |
| 24 July 2012 | 27 July 2012 | 10 | 0 | 44 | 3 |
| 19 August 2012 | 19 August 2012 | 1 | 0 | 51 | 9 |
| 11 October 2012 | 11 October 2012 | 1 | 0 | 53 | 15 |
| 22 October 2012 | 24 October 2012 | 22 | 9 | 31 | 6 |
| 04 November 2012 | 06 November 2012 | 14 | 5 | 39 | 10 |
| 15 November 2012 | 16 November 2012 | 13 | 6 | 40 | 8 |
| 29 November 2012 | 02 December 2012 | 10 | 3 | 44 | 9 |
| 11 December 2012 | 13 December 2012 | 14 | 3 | 40 | 8 |
| 11 January 2013 | 25 January 2013 | 20 | 3 | 34 | 2 |
| 11 February 2013 | 8 March 2013 | 42 | 4 | 12 | 0 |
| 20 March 2013 | 12 April 2013 | 39 | 10 | 16 | 0 |
| 7 May 2013 | 7 May 2013 | 1 | 0 | 52 | 9 |
| 19 May 2013 | 19 May 2013 | 2 | 1 | 50 | 9 |
| 19 June 2013 | 20 June 2013 | 9 | 2 | 45 | 5 |
| 6 July 2013 | 22 July 2013 | 6 | 0 | 49 | 2 |
| 23 August 2013 | 24 August 2013 | 14 | 4 | 40 | 6 |
| 04 September 2013 | 05 September 2013 | 7 | 1 | 47 | 10 |
| 24 September 2013 | 01 October 2013 | 30 | 6 | 25 | 4 |
| 18 October 2013 | 18 October 2013 | 1 | 1 | 55 | 12 |
| 5 November 2013 | 5 November 2013 | 1 | 0 | 54 | 15 |
| 16 November 2013 | 28 November 2013 | 2 | 1 | 54 | 11 |
| 10 December 2013 | 13 December 2013 | 21 | 10 | 35 | 3 |
| 20 January 2014 | 21 January 2014 | 9 | 4 | 46 | 6 |
| 30 January 2014 | 30 January 2014 | 2 | 0 | 53 | 8 |
| 28 February 2014 | 28 February 2014 | 1 | 0 | 53 | 8 |
| 08 March 2014 | 14 March 2014 | 37 | 7 | 18 | 1 |
| 28 March 2014 | 04 April 2014 | 40 | 11 | 16 | 1 |
| 20 April 2014 | 30 April 2014 | 22 | 6 | 34 | 2 |
| 18 May 2014 | 20 May 2014 | 8 | 1 | 47 | 9 |
| 11 June 2014 | 12 June 2014 | 1 | 0 | 54 | 6 |
| 11 July 2014 | 21 July 2014 | 5 | 0 | 50 | 5 |
| 30 July 2014 | 7 August 2014 | 1 | 0 | 52 | 3 |
| 5 September 2014 | 29 September 2014 | 34 | 3 | 21 | 0 |
| 30 October 2014 | 6 November 2014 | 18 | 4 | 38 | 6 |
| 19 November 2014 | 21 November 2014 | 20 | 6 | 34 | 6 |
| 29 November 2014 | 04 December 2014 | 8 | 2 | 46 | 13 |
| 29 December 2014 | 31 December 2014 | 9 | 4 | 47 | 13 |
| 22 January 2015 | 23 January 2015 | 16 | 5 | 38 | 3 |
| 09 February 2015 | 15 February 2015 | 18 | 4 | 37 | 2 |
| 24 February 2015 | 24 February 2015 | 1 | 0 | 53 | 10 |
| 12 March 2015 | 20 March 2015 | 43 | 12 | 12 | 0 |
| 8 April 2015 | 10 April 2015 | 41 | 12 | 13 | 0 |
| 23 April 2015 | 24 April 2015 | 2 | 2 | 53 | 12 |
| 21 May 2015 | 22 May 2015 | 1 | 0 | 56 | 11 |
| 12 June 2015 | 02 July 2015 | 30 | 4 | 27 | 5 |
| 23 August 2015 | 23 August 2015 | 5 | 2 | 52 | 8 |
| 25 September 2015 | 25 September 2015 | 1 | 0 | 56 | 11 |
| 3 October 2015 | 6 October 2015 | 26 | 6 | 31 | 3 |
| 22 October 2015 | 5 November 2015 | 18 | 1 | 38 | 4 |
| 17 December 2015 | 17 December 2015 | 1 | 1 | 56 | 8 |
| 27 December 2015 | 02 January 2016 | 18 | 5 | 39 | 3 |
| 19 January 2016 | 21 January 2016 | 23 | 5 | 32 | 3 |
| 11 February 2016 | 11 February 2016 | 1 | 1 | 55 | 6 |
| 24 February 2016 | 25 February 2016 | 2 | 0 | 53 | 10 |
| 10 March 2016 | 23 March 2016 | 40 | 10 | 18 | 0 |
| 05 May 2016 | 12 May 2016 | 23 | 4 | 35 | 11 |
| 07 June 2016 | 07 June 2016 | 1 | 0 | 56 | 12 |
| 19 July 2016 | 20 July 2016 | 3 | 0 | 53 | 7 |
| 08 August 2016 | 08 August 2016 | 1 | 0 | 56 | 6 |
| 14 September 2016 | 15 September 2016 | 7 | 0 | 50 | 9 |
| 25 October 2016 | 10 November 2016 | 21 | 7 | 36 | 4 |
| 25 November 2016 | 06 December 2016 | 32 | 7 | 25 | 3 |
| 16 December 2016 | 19 December 2016 | 15 | 5 | 42 | 9 |
| 27 December 2016 | 06 January 2017 | 10 | 5 | 46 | 6 |
| 18 January 2017 | 27 January 2017 | 43 | 9 | 15 | 0 |
| 09 February 2017 | 15 February 2017 | 40 | 8 | 19 | 1 |
| 27 March 2017 | 28 March 2017 | 28 | 10 | 32 | 8 |
| 08 April 2017 | 09 April 2017 | 6 | 4 | 54 | 13 |
| 30 April 2017 | 30 April 2017 | 1 | 0 | 59 | 14 |
| 11 May 2017 | 11 May 2017 | 4 | 1 | 55 | 15 |
| 18 June 2017 | 21 June 2017 | 6 | 0 | 49 | 11 |
| 25 September 2017 | 27 September 2017 | 11 | 5 | 45 | 6 |
| 16 October 2017 | 18 October 2017 | 1 | 0 | 57 | 12 |
| 02 November 2017 | 06 November 2017 | 18 | 6 | 42 | 12 |
| 19 December 2017 | 19 December 2017 | 4 | 2 | 57 | 17 |
| 11 December 2017 | 11 December 2017 | 1 | 0 | 59 | 20 |
| System | Syndrome | Daily Mean Counts in UTLAs | Minimum | Maximum |
|---|---|---|---|---|
| GP out of hours consultations | Total with a clinical code | 99.1 | 1 | 1766 |
| Stroke | 0.2 | 0 | 10 | |
| Acute bronchitis | 0.2 | 0 | 38 | |
| Eye irritation | 0.9 | 0 | 34 | |
| Chest pain | 1.0 | 0 | 29 | |
| Cardiac | 1.7 | 0 | 38 | |
| Asthma/Wheeze/DB | 2.3 | 0 | 68 | |
| Gastroenteritis | 4.6 | 0 | 130 | |
| GP in-hours consultations | Registered patients in GP practices (thousands) | 188.0 | 0.4 | 1284 |
| Acute presenting asthma | 2.6 | 0 | 113 | |
| Allergic rhinitis | 5.8 | 0 | 463 | |
| Conjunctivitis | 8.6 | 0 | 160 | |
| Pharyngitis or scarlet fever | 15.1 | 0 | 289 | |
| Gastroenteritis | 19.4 | 0 | 287 | |
| NHS 111 telephone calls | Total calls | 198.4 | 1 | 3364 |
| Eye problems | 2.7 | 0 | 64 | |
| Diarrhoea | 4.1 | 0 | 81 | |
| Sore throat | 4.5 | 0 | 149 | |
| Difficulty breathing | 7.0 | 0 | 233 | |
| Cough | 7.0 | 0 | 467 |
| System | System: Syndrome | Lag (Days) | Rate Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| GP out of hours consultations | Asthma/Wheeze/DB | 0 | 1.06 | 0.91 | 1.24 |
| 1 | 1.09 | 0.93 | 1.27 | ||
| 2 | 1.17 | 1.00 | 1.35 | ||
| 3 | 1.05 | 0.91 | 1.22 | ||
| 4 | 1.01 | 0.87 | 1.17 | ||
| Acute bronchitis | 0 | 1.98 | 1.02 | 3.86 | |
| 1 | 1.00 | 0.54 | 1.87 | ||
| 2 | 1.53 | 0.82 | 2.84 | ||
| 3 | 1.11 | 0.58 | 2.12 | ||
| 4 | 1.62 | 0.87 | 3.01 | ||
| Cardiac | 0 | 1.26 | 1.06 | 1.49 | |
| 1 | 1.11 | 0.93 | 1.31 | ||
| 2 | 1.15 | 0.97 | 1.36 | ||
| 3 | 1.17 | 0.99 | 1.38 | ||
| 4 | 1.09 | 0.92 | 1.30 | ||
| Eye irritation | 0 | 0.98 | 0.74 | 1.32 | |
| 1 | 0.72 | 0.53 | 0.96 | ||
| 2 | 0.83 | 0.62 | 1.10 | ||
| 3 | 1.10 | 0.82 | 1.48 | ||
| 4 | 1.35 | 1.01 | 1.80 | ||
| Gastroenteritis | 0 | 1.07 | 0.96 | 1.18 | |
| 1 | 1.13 | 1.02 | 1.25 | ||
| 2 | 1.08 | 0.98 | 1.19 | ||
| 3 | 1.08 | 0.98 | 1.18 | ||
| 4 | 1.07 | 0.97 | 1.18 | ||
| Chest pain | 0 | 1.23 | 0.97 | 1.54 | |
| 1 | 1.09 | 0.87 | 1.37 | ||
| 2 | 1.19 | 0.95 | 1.49 | ||
| 3 | 1.27 | 1.01 | 1.58 | ||
| 4 | 1.12 | 0.89 | 1.41 | ||
| Stroke | 0 | 0.52 | 0.12 | 2.26 | |
| 1 | 1.45 | 0.40 | 5.34 | ||
| 2 | 0.69 | 0.20 | 2.39 | ||
| 3 | 0.94 | 0.27 | 3.30 | ||
| 4 | 0.74 | 0.20 | 2.71 | ||
| GP in-hours consultations | Allergic rhinitis | 0 | 0.99 | 0.89 | 1.10 |
| 1 | 1.04 | 0.93 | 1.16 | ||
| 2 | 1.00 | 0.90 | 1.12 | ||
| 3 | 1.07 | 0.96 | 1.20 | ||
| 4 | 0.97 | 0.86 | 1.09 | ||
| Conjunctivitis | 0 | 1.00 | 0.95 | 1.06 | |
| 1 | 1.00 | 0.94 | 1.06 | ||
| 2 | 0.97 | 0.92 | 1.04 | ||
| 3 | 0.98 | 0.93 | 1.05 | ||
| 4 | 1.00 | 0.93 | 1.06 | ||
| Gastroenteritis | 0 | 0.98 | 0.94 | 1.02 | |
| 1 | 0.98 | 0.94 | 1.03 | ||
| 2 | 1.00 | 0.95 | 1.04 | ||
| 3 | 0.97 | 0.93 | 1.01 | ||
| 4 | 1.02 | 0.98 | 1.07 | ||
| Pharyngitis or scarlet fever | 0 | 0.98 | 0.93 | 1.04 | |
| 1 | 0.98 | 0.92 | 1.03 | ||
| 2 | 1.04 | 0.98 | 1.10 | ||
| 3 | 1.04 | 0.98 | 1.10 | ||
| 4 | 1.02 | 0.96 | 1.08 | ||
| Acute presenting asthma | 0 | 1.02 | 0.93 | 1.11 | |
| 1 | 1.03 | 0.94 | 1.14 | ||
| 2 | 1.06 | 0.95 | 1.17 | ||
| 3 | 0.97 | 0.87 | 1.07 | ||
| 4 | 0.96 | 0.87 | 1.07 | ||
| NHS 111 telephone calls | Cough | 0 | 1.00 | 0.92 | 1.09 |
| 1 | 1.04 | 0.95 | 1.13 | ||
| 2 | 1.00 | 0.92 | 1.09 | ||
| 3 | 1.02 | 0.94 | 1.11 | ||
| 4 | 1.01 | 0.93 | 1.10 | ||
| Diarrhoea | 0 | 1.06 | 0.96 | 1.17 | |
| 1 | 1.05 | 0.95 | 1.16 | ||
| 2 | 0.98 | 0.89 | 1.08 | ||
| 3 | 1.03 | 0.94 | 1.14 | ||
| 4 | 1.06 | 0.96 | 1.16 | ||
| Difficulty breathing | 0 | 1.03 | 0.95 | 1.12 | |
| 1 | 1.01 | 0.92 | 1.09 | ||
| 2 | 1.01 | 0.93 | 1.10 | ||
| 3 | 1.00 | 0.92 | 1.08 | ||
| 4 | 1.01 | 0.93 | 1.10 | ||
| Eye problems | 0 | 1.16 | 1.03 | 1.31 | |
| 1 | 1.16 | 1.03 | 1.30 | ||
| 2 | 1.09 | 0.97 | 1.23 | ||
| 3 | 1.08 | 0.96 | 1.21 | ||
| 4 | 1.09 | 0.97 | 1.22 | ||
| Sore throat | 0 | 1.02 | 0.92 | 1.14 | |
| 1 | 1.07 | 0.97 | 1.19 | ||
| 2 | 1.05 | 0.94 | 1.16 | ||
| 3 | 1.02 | 0.92 | 1.13 | ||
| 4 | 1.03 | 0.93 | 1.14 | ||
References
- Hu, L.-W.; Lawrence, W.R.; Liu, Y.; Yang, B.-Y.; Zeng, X.-W.; Chen, W.; Dong, G.-H. Ambient Air Pollution and Morbidity in Chinese. Adv. Exp. Med. Biol. 2017, 1017, 123–151. [Google Scholar] [PubMed]
- Samek, L. Overall human mortality and morbidity due to exposure to air pollution. Int. J. Occup. Med. Environ. Health 2016, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Brabin, B.; Smith, M.; Milligan, P.; Benjamin, C.; Dunne, E.; Pearson, M. Respiratory morbidity in Merseyside schoolchildren exposed to coal dust and air pollution. Arch. Dis. Child. 1994, 70, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansel, N.N.; Romero, K.M.; Pollard, S.L.; Bose, S.; Psoter, K.J.; Underhill, L.J.; Johnson, C.; Williams, D.; Curriero, F.C.; Breysse, P.; et al. Ambient Air Pollution and Variation in Multiple Domains of Asthma Morbidity among Peruvian Children. Ann. Am. Thorac. Soc. 2019, 16, 348–355. [Google Scholar] [PubMed]
- Burbank, A.J.; Peden, D. Assessing the impact of air pollution on childhood asthma morbidity: How, when, and what to do. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Carpenter, D.O.; Zeb, J.; Aburizaiza, A.J.; Pantea, C.; Hussain, M.M.; Khwaja, H.A. Association of fine particulate air pollution with cardiopulmonary morbidity in Western Coast of Saudi Arabia. Saudi Med. J. 2017, 38, 905–912. [Google Scholar] [CrossRef]
- Xie, W.; Li, G.; Zhao, D.; Xie, X.; Wei, Z.; Wang, W.; Wang, M.; Li, G.; Liu, W.; Sun, J.; et al. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart 2015, 101, 257–263. [Google Scholar] [CrossRef]
- Fuller, C.H.; Jones, J.; Roblin, D.W. Evaluating changes in ambient ozone and respiratory-related healthcare utilization in the Washington, DC metropolitan area. Environ. Res. 2020, 186, 109603. [Google Scholar] [CrossRef]
- Mizen, A.; Lyons, J.; Milojevic, A.; Doherty, R.; Wilkinson, P.; Carruthers, D.; Akbari, A.; Lake, I.; Davies, G.A.; Al Sallakh, M.; et al. Impact of air pollution on educational attainment for respiratory health treated students: A cross sectional data linkage study. Health Place 2020, 63, 102355. [Google Scholar] [CrossRef]
- Sarnat, J.; Sarnat, S.E.; Flanders, W.D.; Chang, H.H.; Mulholland, J.; Baxter, L.; Isakov, V.; Özkaynak, H. Spatiotemporally resolved air exchange rate as a modifier of acute air pollution-related morbidity in Atlanta. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 606–615. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.J.; Fuller, G.W.; Walton, H.; Fussell, J.C. Monitoring air pollution: Use of early warning systems for public health. Respirology 2011, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Masselot, P.; Chebana, F.; Lavigne, É.; Campagna, C.; Gosselin, P.; Ouarda, T.B.M.J. Toward an Improved Air Pollution Warning System in Quebec. Int. J. Environ. Res. Public Health 2019, 16, 2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, T.A., Jr. Regional air pollution warning system. J. Air Pollut. Control Assoc. 1966, 16, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Triple, S. Assessment of syndromic surveillance in Europe. Lancet 2011, 378, 1833–1834. [Google Scholar] [CrossRef]
- Hughes, H.E.; Morbey, R.; Fouillet, A.; Caserio-Schönemann, C.; Dobney, A.; Hughes, T.; Smith, G.E.; Elliot, A.J. Retrospective observational study of emergency department syndromic surveillance data during air pollution episodes across London and Paris in 2014. BMJ Open 2018, 8, e018732. [Google Scholar] [CrossRef]
- Smith, G.E.; Bawa, Z.; Macklin, Y.; Morbey, R.; Dobney, A.; Vardoulakis, S.; Elliot, A.J. Using real-time syndromic surveillance systems to help explore the acute impact of the air pollution incident of March/April 2014 in England. Environ. Res. 2015, 136, 500–504. [Google Scholar] [CrossRef]
- Todkill, D.; Gonzalez, F.D.J.C.; Morbey, R.; Charlett, A.; Hajat, S.; Kovats, S.; Osborne, N.J.; McInnes, R.; Vardoulakis, S.; Exley, K.; et al. Environmental factors associated with general practitioner consultations for allergic rhinitis in London, England: A retrospective time series analysis. BMJ Open 2020, 10, e036724. [Google Scholar] [CrossRef]
- Department for Environment, Food & Rural Affairs. UK AIR-Air Information Resource. 2021. Available online: https://uk-air.defra.gov.uk (accessed on 1 November 2021).
- Department for Environment, Food & Rural Affairs. What Is the Daily Air Quality Index? 2021. Available online: https://uk-air.defra.gov.uk/air-pollution/daqi?view=more-info (accessed on 5 November 2019).
- Harcourt, S.E.; Smith, G.E.; Elliot, A.J.; Pebody, R.; Charlett, A.; Ibbotson, S.; Regan, M.; Hippisley-Cox, J. Use of a large general practice syndromic surveillance system to monitor the progress of the influenza A(H1N1) pandemic 2009 in the UK. Epidemiol. Infect. 2011, 140, 100–105. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Huang, G.; Brown, P.E.; Fu, S.H.; Shin, H.H. Daily mortality/morbidity and air quality: Using multivariate time series with seasonally varying covariances. J. R. Stat. Soc. Ser. C 2021, 71, 148–174. [Google Scholar] [CrossRef]
- Misailidou, M.; Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Stefanadis, C. Short-term effects of atmospheric temperature and humidity on morbidity from acute coronary syndromes in free of air pollution rural Greece. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 846–848. [Google Scholar] [CrossRef]
- Welty, L.J.; Zeger, S.L. Are the acute effects of particulate matter on mortality in the National Morbidity, Mortality, and Air Pollution Study the result of inadequate control for weather and season? A sensitivity analysis using flexible distributed lag models. Am. J. Epidemiol. 2005, 162, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bind, M.-A. Causal Modeling in Environmental Health. Annu. Rev. Public Health 2019, 40, 23–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VanderWeele, T.J.; Staudt, N. Causal diagrams for empirical legal research: A methodology for identifying causation, avoiding bias and interpreting results. Law Probab. Risk 2011, 10, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kwon, H.-J. Current State of Research on the Risk of Morbidity and Mortality Associated with Air Pollution in Korea. Yonsei Med. J. 2019, 60, 243–256. [Google Scholar] [CrossRef] [PubMed]
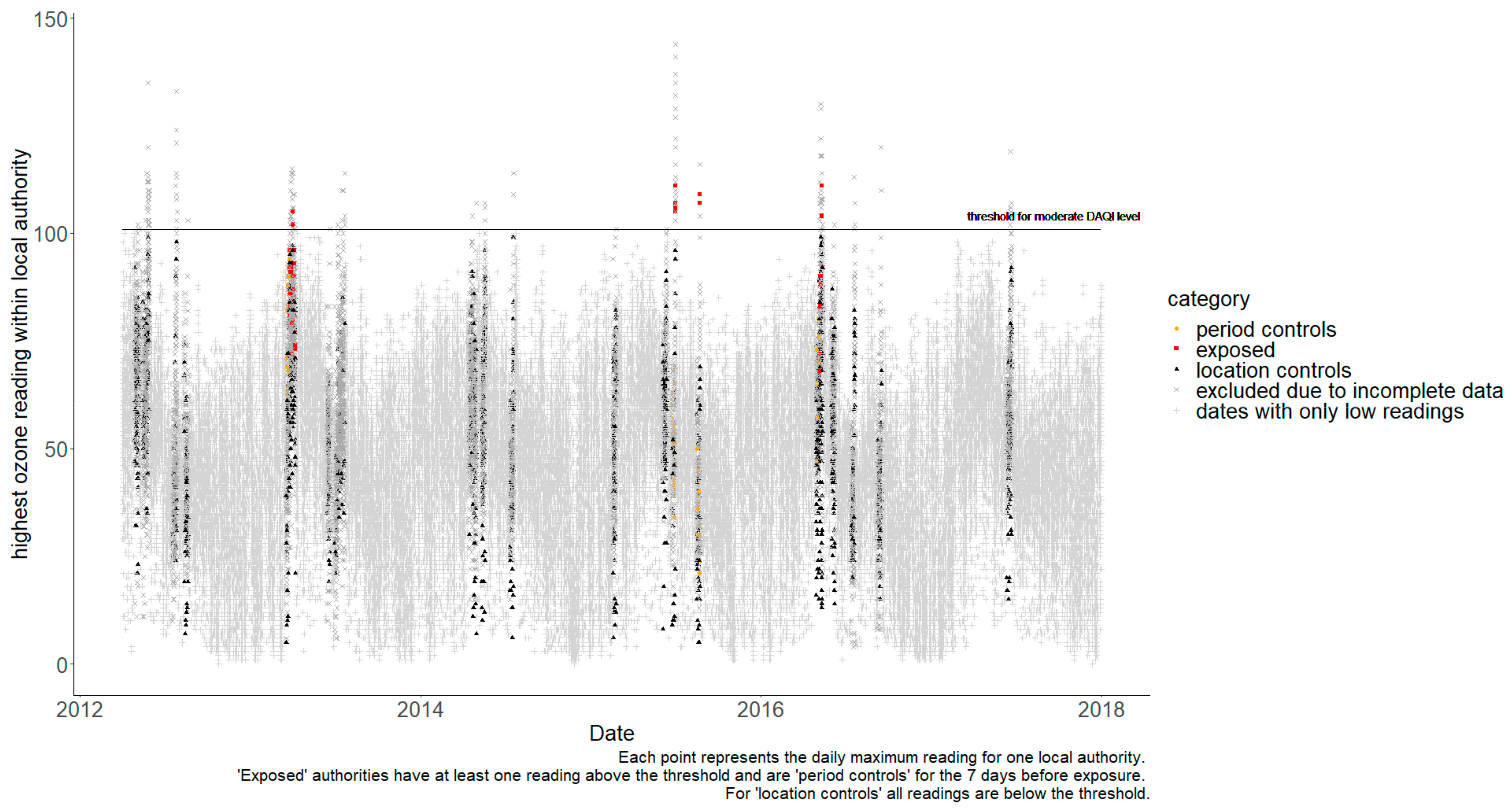

| Pollutant | Daily Air Quality Index (DAQI) | Max Value Recorded | |||
|---|---|---|---|---|---|
| Moderate | High | Very High | |||
| Lower threshold for DAQI level (μg/m3) | NO2 | 201 | 401 | 601 | 201 |
| O3 | 101 | 161 | 241 | 144 | |
| PM10 | 51 | 76 | 101 | 210 | |
| PM2.5 | 36 | 54 | 71 | 102 | |
| SO2 | 267 | 533 | 1065 | 73 | |
| Moderate | High | Very High | All levels | ||
| Number of site readings within DAQI band | NO2 | 1 | 0 | 0 | 193,098 |
| O3 | 196 | 0 | 0 | 114,952 | |
| PM10 | 1109 | 98 | 7 | 83,888 | |
| PM2.5 | 2006 | 345 | 57 | 99,725 | |
| SO2 | 0 | 0 | 0 | 36,657 | |
| Number of unique days with a reading within DAQI band | NO2 | 1 | 0 | 0 | 2101 |
| O3 | 67 | 0 | 0 | 2101 | |
| PM10 | 215 | 32 | 6 | 2101 | |
| PM2.5 | 212 | 51 | 17 | 2101 | |
| SO2 | 0 | 0 | 0 | 2101 | |
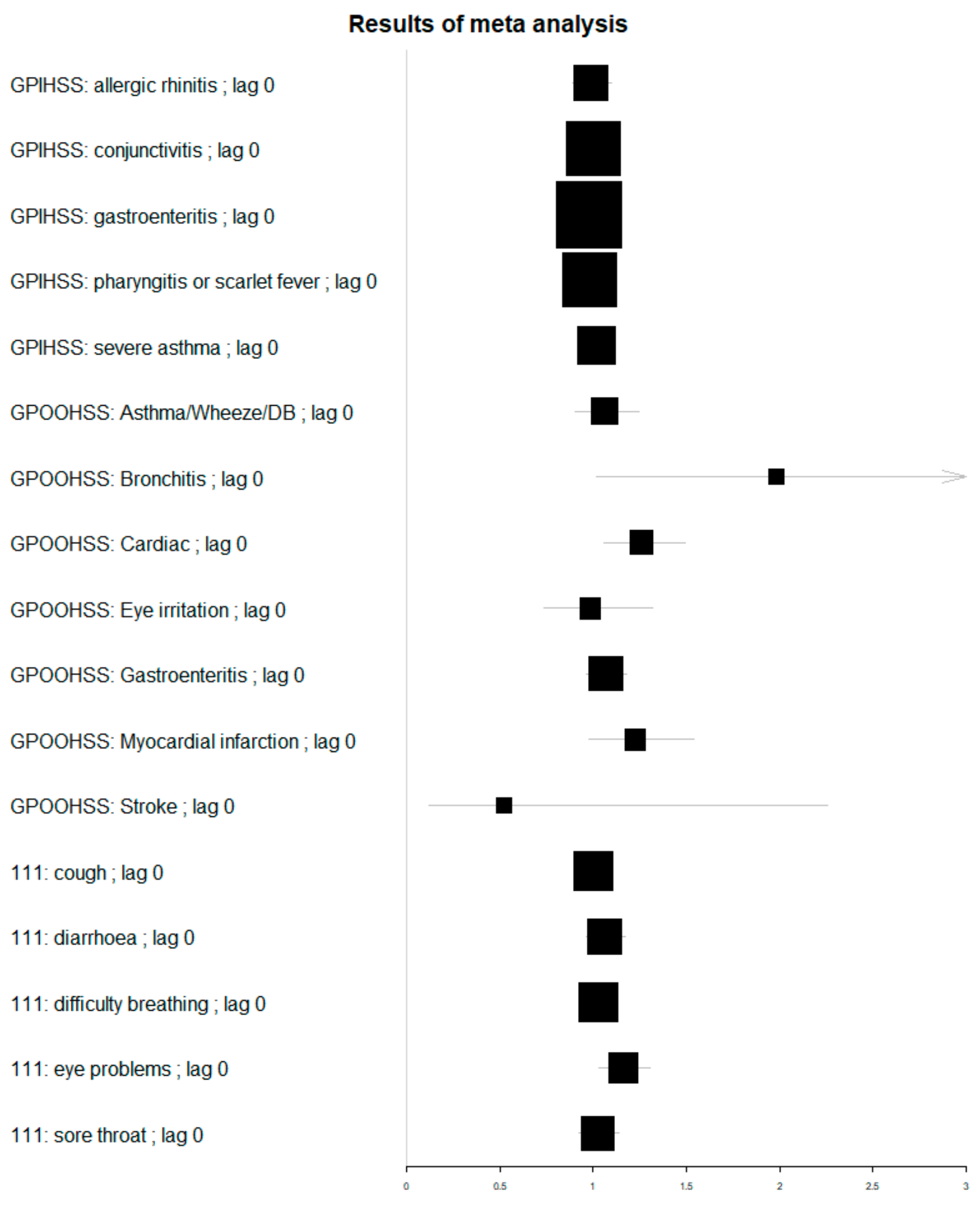
| System: Syndrome | Lag (Days) | Rate Ratio | 95% Confidence Interval | |
|---|---|---|---|---|
| GPOOH: acute bronchitis | 0 | 1.98 | 1.02 | 3.86 |
| GPOOH: Stroke | 1 | 1.45 | 0.40 | 5.34 |
| GPOOH: Eye irritation | 4 | 1.35 | 1.01 | 1.80 |
| GPOOH: chest pain | 3 | 1.27 | 1.01 | 1.58 |
| GPOOH: Cardiac | 0 | 1.26 | 1.06 | 1.49 |
| GPOOH: Asthma/Wheeze/DB | 2 | 1.17 | 1.00 | 1.35 |
| NHS 111: eye problems | 0 | 1.16 | 1.03 | 1.30 |
| GPOOH: Gastroenteritis | 1 | 1.13 | 1.02 | 1.25 |
| GPIHSS: allergic rhinitis | 3 | 1.07 | 0.96 | 1.20 |
| NHS 111: sore throat | 1 | 1.07 | 0.97 | 1.19 |
| GPIHSS: acute presenting asthma | 2 | 1.06 | 0.95 | 1.17 |
| NHS 111: diarrhoea | 4 | 1.06 | 0.96 | 1.16 |
| GPIHSS: pharyngitis or scarlet fever | 3 | 1.04 | 0.98 | 1.10 |
| NHS 111: cough | 1 | 1.04 | 0.95 | 1.13 |
| NHS 111: difficulty breathing | 0 | 1.03 | 0.95 | 1.12 |
| GPIHSS: gastroenteritis | 4 | 1.02 | 0.98 | 1.07 |
| GPIHSS: conjunctivitis | 0 | 1.00 | 0.95 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morbey, R.; Smith, G.; Exley, K.; Charlett, A.; de Angelis, D.; Harcourt, S.; Gonzalez, F.; Lake, I.; Dobney, A.; Elliot, A. Estimating the Impact of Air Pollution on Healthcare-Seeking Behaviour by Applying a Difference-in-Differences Method to Syndromic Surveillance Data. Int. J. Environ. Res. Public Health 2022, 19, 7097. https://doi.org/10.3390/ijerph19127097
Morbey R, Smith G, Exley K, Charlett A, de Angelis D, Harcourt S, Gonzalez F, Lake I, Dobney A, Elliot A. Estimating the Impact of Air Pollution on Healthcare-Seeking Behaviour by Applying a Difference-in-Differences Method to Syndromic Surveillance Data. International Journal of Environmental Research and Public Health. 2022; 19(12):7097. https://doi.org/10.3390/ijerph19127097
Chicago/Turabian StyleMorbey, Roger, Gillian Smith, Karen Exley, André Charlett, Daniela de Angelis, Sally Harcourt, Felipe Gonzalez, Iain Lake, Alec Dobney, and Alex Elliot. 2022. "Estimating the Impact of Air Pollution on Healthcare-Seeking Behaviour by Applying a Difference-in-Differences Method to Syndromic Surveillance Data" International Journal of Environmental Research and Public Health 19, no. 12: 7097. https://doi.org/10.3390/ijerph19127097
APA StyleMorbey, R., Smith, G., Exley, K., Charlett, A., de Angelis, D., Harcourt, S., Gonzalez, F., Lake, I., Dobney, A., & Elliot, A. (2022). Estimating the Impact of Air Pollution on Healthcare-Seeking Behaviour by Applying a Difference-in-Differences Method to Syndromic Surveillance Data. International Journal of Environmental Research and Public Health, 19(12), 7097. https://doi.org/10.3390/ijerph19127097






