A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects
Abstract
:1. Introduction
2. Seaweed Uses: A Commercial Perspective
3. Seaweed Aquaculture and Sustainable Development Goals
4. The Emergence of Seaweed Diseases and Prevalent Threats to Seaweed Farming
4.1. Bacteria-Induced Diseases
4.2. Epiphytic Attachment
4.3. Herbivory Grazing
4.4. Virus Infection
4.5. Abiotic Factors
5. Current Production and Farming Practices of Commercial Seaweeds
Single Crop Farming and Harvesting Systems
6. Existing and Future Mitigation Strategies
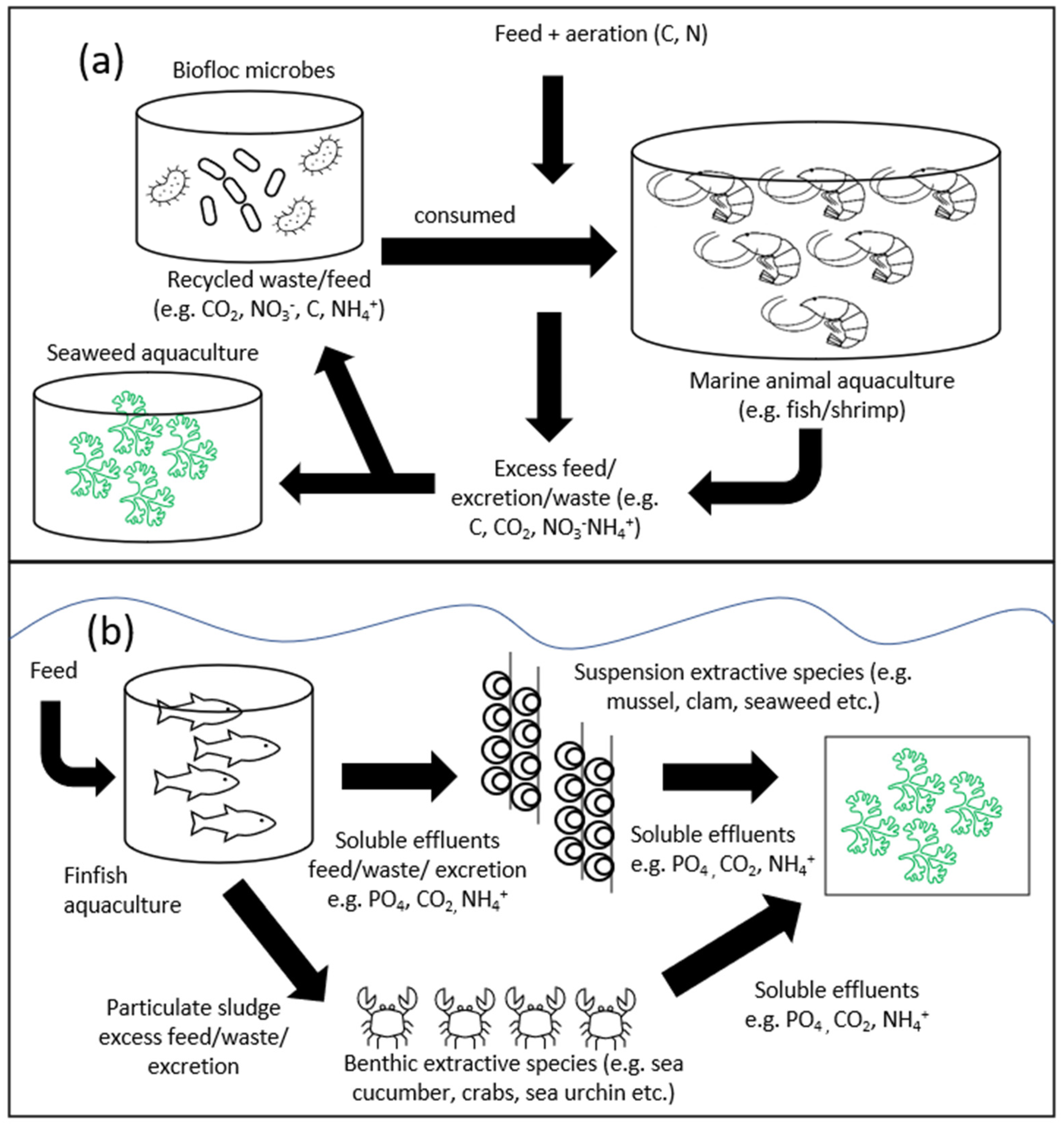
6.1. Integrated Multi-Trophic Aquaculture (IMTA) Systems and Bioremediation Strategies
6.2. Disease Management Practices in Seaweed Farms
6.3. Genetic Manipulation and Strain Improvement
6.3.1. Micropropagation and Hybridization
6.3.2. Molecular Identification for Strain Selection
6.3.3. Induction of Stress Signaling to Increase Resistance
6.4. Enhancement through Biostimulant and Biocontrol Strategies
6.4.1. Seaweed-Derived Biostimulant
6.4.2. Biocontrol in Seaweed-Bacteria Interaction
7. Prospects and Recommendations
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; 224p. [Google Scholar] [CrossRef]
- Chopin, T.; Tacon, A.G.J. Importance of seaweeds and extractive species in global aquaculture production. Rev. Fish. Sci. Aquacult. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- FAO. The Global Status of Seaweed Production, Trade and Utilization. Globefish Research Program 124; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; 120p. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; 200p. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, A.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Ma, Y.; Huan, L.; Wang, Y.; Xia, B.; Wang, G. Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Res. 2020, 46, 101817. [Google Scholar] [CrossRef]
- Hwang, E.K.; Park, C.S. Seaweed cultivation and utilization of Korea. Algae 2020, 35, 107–121. [Google Scholar] [CrossRef]
- Tanaka, K.; Ohno, M.; Largo, D.B. An update on the seaweed resources of Japan. Bot. Mar. 2020, 63, 105–117. [Google Scholar] [CrossRef]
- Alemañ, A.E.; Robledo, D.; Hayashi, L. Development of seaweed cultivation in Latin America: Current trends and future prospects. Phycologia 2019, 58, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Lowe, B.; Anil, S.; Manivasagan, P.; Kheraif, A.A.A.; Kang, K.H.; Kim, S.K. Seaweed polysaccharides and their potential biomedical applications. Starch-Stärke 2015, 67, 381–390. [Google Scholar] [CrossRef]
- Valero, M.; Guillemin, M.; Destombe, C.; Jacquemin, B.; Gachon, C.; Badis, Y.; Buschmann, A.; Camus, C.; Faugeron, S. Perspectives on domestication research for sustainable seaweed aquaculture. Perspect. Phycol. 2017, 4, 33–36. [Google Scholar] [CrossRef]
- World Meteorological Organization. The Global Climate in 2015–2019. Centre for Research on the Epidemiology of Disasters National Institute for Space Research; World Meteorological Organization: Geneva, Switzerland, 2019; 32p. [Google Scholar]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Fernández, P.A.; Leal, P.P.; Henríquez, L.A. Co-culture in marine farms: Macroalgae can act as chemical refuge for shell-forming molluscs under an ocean acidification scenario. Phycologia 2019, 58, 542–551. [Google Scholar] [CrossRef]
- Smith, T.E.; Smith, J. Sustainable bioethanol production from a marine alga, Enteromorpha flexuosa (Wulfen) J Agardh (Chlorophyta). Int. J. Algae 2016, 20, 175–180. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Praveen, M.A.; Karthika Parvathy, K.R.; Jayabalan, R.; Balasubramanian, P. Dietary fiber from Indian edible seaweeds and its in-vitro prebiotic effect on the gut microbiota. Food Hydrocoll. 2019, 96, 343–353. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J. Appl. Phycol. 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- Kraan, S. Seaweed and alcohol: Biofuel or booze? In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press Cambridge: Massachusetts, MA, USA, 2016; pp. 169–184. [Google Scholar] [CrossRef]
- Uchida, M.; Kurushima, H.; Ishihara, K.; Murata, Y.; Touhata, K.; Ishida, N.; Niwa, K.; Araki, T. Characterization of fermented seaweed sauce prepared from nori (Pyropia yezoensis). J. Biosci. Bioeng. 2016, 123, 327–332. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Seaweed application in cosmetics. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press Cambridge: Massachusetts, MA, USA, 2016; pp. 423–441. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [Green Version]
- Hitoe, S.; Shimoda, S. Seaweed fucoxanthin supplementation improves obesity parameters in mildly obese Japanese subjects. Funct. Foods Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Dörschmann, P.; Klettner, A. Fucoidans as Potential Therapeutics for Age-Related Macular Degeneration-Current Evidence from In Vitro Research. Int. J. Mol. Sci. 2020, 21, 9272. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Vijn, S.; Compart, D.P.; Dutta, N.; Foukis, A.; Hess, M.; Hristov, A.N.; Kalscheur, K.F.; Kebreab, E.; Nuzhdin, S.V.; Price, N.N.; et al. Key considerations for the use of seaweed to reduce enteric methane emissions from cattle. Front. Vet. Sci. 2020, 7, 597430. [Google Scholar] [CrossRef] [PubMed]
- Ragaza, J.A.; Koshio, S.; Mamauag, R.E.; Ishikawa, M.; Yokoyama, S.; Villamor, S.S. Dietary supplemental effects of red seaweed Eucheuma denticulatum on growth performance, carcass composition and blood chemistry of juvenile Japanese flounder, Paralichthys Olivaceus. Aquac. Res. 2015, 46, 647–657. [Google Scholar] [CrossRef]
- Zhou, M.; Huenerberg, M.; Chen, Y.; Reuter, T.; McAllister, T.A. Effects of the seaweed Ascophyllum nodosum on the rumen microbiome and fecal pathogenic Escherichia coli serotypes in sheep. J. Anim. Sci. 2017, 95, 299–300. [Google Scholar] [CrossRef]
- Shimazu, T.; Borjigin, L.; Katoh, K.; Roh, S.; Kitazawa, H.; Abe, K.; Suda, Y.; Saito, H.; Kunii, H.; Nihei, K.; et al. Addition of Wakame seaweed (Undaria pinnatifida) stalk to animal feed enhances immune response and improves intestinal microflora in pigs. Anim. Sci. J. 2019, 90, 1248–1260. [Google Scholar] [CrossRef]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Mantri, V.A.; Eswaran, K.; Shanmugam, M.; Ganesan, M.; Veeragurunathan, V.; Thiruppathi, S.; Reddy, C.R.K.; Seth, A. An appraisal on commercial farming of Kappaphycus alvarezii in India: Success in diversification of livelihood and prospects. J. Appl. Phycol. 2017, 29, 335–357. [Google Scholar] [CrossRef]
- Shah, M.T.; Zodape, S.T.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Seaweed sap as an alternative liquid fertilizer for yield and quality improvement of wheat. J. Plant Nutr. 2013, 36, 192–200. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.K.; Pal, S.K.; Trivedi, K.; Yesuraj, D.; Singh, C.S.; Vijay Anand, K.G.; Chandramohan, M.; Patidar, R.; Kubavat, D.; et al. Sustainable enhancement in yield and quality of rain-fed maize through Gracilaria edulis and Kappaphycus alvarezii seaweed sap. J. Appl. Phycol. 2016, 28, 2099–2112. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of short-term application of seaweed fertilizer on bacterial diversity and community structure, soil nitrogen contents, and plant growth in maize rhizosphere soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Salim, M.R.; Salam, Z.; Sivakumar, P.; Chong, C.T.; Elumalai, S.; Suresh, V.; Ani, F.N. Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina tetrastromatica. Energy Convers. 2017, 135, 351–361. [Google Scholar] [CrossRef]
- Hessami, M.J.; Phang, S.M.; Salleh, A.; Rabiei, R. Evaluation of tropical seaweeds as feedstock for bioethanol production. Int. J. Environ. Sci. Technol. 2017, 15, 977–992. [Google Scholar] [CrossRef]
- Ra, C.H.; Sunwoo, I.Y.; Nguyen, T.H.; Sukwang, P.; Sirisuk, P.; Jeong, G.T.; Kim, S.K. Butanol and butyric acid production from Saccharina japonica by Clostridium acetobutylicum and Clostridium tyrobutyricum with adaptive evolution. Bioprocess Biosyst. Eng. 2019, 42, 1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleymani, M.; Rosentrater, A. Techno-economic analysis of biofuel production from macroalgae (seaweed). Bioengineering 2017, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renew. Sust. Energ. Rev. 2017, 68, 136–146. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocol. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocol. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Kok, J.M.L.; Wong, C.L. Physicochemical properties of edible alginate film from Malaysian Sargassum polycystum C. Agardh. Sustain. Chem. Pharm. 2018, 9, 87–94. [Google Scholar] [CrossRef]
- Notpla Technology. Available online: https://www.notpla.com/ (accessed on 25 April 2022).
- Evoware. Available online: https://rethink-plastic.com/home/ (accessed on 25 April 2022).
- Algeon Materials. Available online: https://www.algeonmaterials.com/ (accessed on 25 April 2022).
- Sway. Available online: https://swaythefuture.com/ (accessed on 25 April 2022).
- FlexSea. Available online: https://flex-sea.com/ (accessed on 25 April 2022).
- Helmes, R.J.K.; López-Contreras, A.M.; Benoit, M.; Abreu, H.; Maguire, J.; Moejes, F.; Burg, S.W.K. Environmental impacts of experimental production of lactic acid for bioplastics from Ulva spp. Sustainability 2018, 10, 2462. [Google Scholar] [CrossRef] [Green Version]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development 21 October 2015, A/RES/70/1. Available online: https://www.refworld.org/docid/57b6e3e44.html (accessed on 31 January 2021).
- Felaco, L.; Olvera-Novoa, M.A.; Robledo, D. Multitrophic integration of the tropical red seaweed Solieria filiformis with sea cucumbers and fish. Aquaculture 2020, 527, 735475. [Google Scholar] [CrossRef]
- Padam, B.S.; Chye, F.Y. Chapter 2: Seaweed components, properties, and applications. In Sustainable Seaweed Technologies: Cultivation, Biorefinery, and Applications; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–87. [Google Scholar] [CrossRef]
- Hasselström, L.; Visch, W.; Gröndahl, F.; Nylund, G.M.; Pavia, H. The impact of seaweed cultivation on ecosystem services—A case study from the west coast of Sweden. Mar. Pol. Bul. 2018, 133, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.Q.; Neish, I.C.; Critchley, A.T. Phyconomy: The extensive cultivation of seaweeds, their sustainability and economic value, with particular reference to important lessons to be learned and transferred from the practice of eucheumatoid farming. Phycologia 2019, 58, 472–483. [Google Scholar] [CrossRef]
- Mariño, M.; Breckwoldt, A.; Teichberg, M.; Kase, A.; Reuter, H. Livelihood aspects of seaweed farming in Rote Island, Indonesia. Mar. Pol. 2019, 107, 103600. [Google Scholar] [CrossRef]
- Larson, S.; Stoeckl, N.; Fachry, M.E.; Dalvi Mustafa, M.; Lapong, I.; Purnomo, A.H.; Rimmer, M.A.; Paul, N.A. Women’s well-being and household benefits from seaweed farming in Indonesia. Aquaculture 2021, 530, 735711. [Google Scholar] [CrossRef]
- Hill, N.A.O.; Rowcliffe, J.M.; Koldewey, H.J.; Milner-Gulland, E.J. The interaction between seaweed farming as an alternative occupation and fisher numbers in the central Philippines. Conserv. Biol. 2012, 26, 324–334. [Google Scholar] [CrossRef]
- Monagail, M.M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Hedberg, N.; von Schreeb, K.; Charisiadou, S.; Jiddawi, N.S.; Tedengren, M.; Nordlund, L.M. Habitat preference for seaweed farming—A case study from Zanzibar, Tanzania. Ocean Coast. Manag. 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Eggertsen, M.; Halling, C. Knowledge gaps and management recommendations for future paths of sustainable seaweed farming in the Western Indian Ocean. Ambio 2021, 50, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Hussin, H.; Khoso, A. Seaweed cultivation and coastal communities in Malaysia: An overview. Asian Fish. Sci. 2017, 30, 87–100. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1585p. [Google Scholar]
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Moreira, D.; Pires, J.C.M. Atmospheric CO2 capture by algae: Negative carbon dioxide emission path. Bioresour. Technol. 2016, 215, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. Eur. J. Phycol. 2017, 52, 506–522. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Lavery, P.; Serrano, O.; Marbà, N.; Masque, P.; Duarte, C.M. Sequestration of macroalgal carbon: The elephant in the Blue Carbon room. Biol. Lett. 2018, 14, 20180236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper. No. 530; FAO: Rome, Italy, 2009; 212p. [Google Scholar]
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can seaweed farming play a role in climate change mitigation and adaptation? Front. Mar. Sci. 2017, 4, 100. [Google Scholar] [CrossRef]
- Kim, G.H.; Moon, K.H.; Kim, J.Y.; Shim, J.; Klochkova, T.A. A revaluation of algal diseases in Korean Pyropia (Porphyra) sea farms and their economic impact. Algae 2014, 29, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Ward, G.M.; Faisan, J.P.; Cottier-Cook, E.J.; Gachon, C.; Hurtado, A.Q.; Lim, P.E.; Matoju, I.; Msuya, F.E.; Bass, D.; Brodie, J. A review of reported seaweed diseases and pests in aquaculture in Asia. J. World Aqua. Soc. 2020, 51, 815–828. [Google Scholar] [CrossRef]
- Wang, X.; Broch, O.J.; Forbord, S.; Handå, A.; Skjermo, J.; Reitan, K.I.; Vadstein, O.; Olsen, Y. Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: Implications for integrated multi-trophic aquaculture. J. Appl. Phycol. 2014, 26, 1869–1878. [Google Scholar] [CrossRef]
- Cottier-Cook, E.J.; Nagabhatla, N.; Badis, Y.; Campbell, M.L.; Chopin, T.; Dai, W.; Fang, J.; He, P.; Hewitt, C.L.; Kim, G.H. Safeguarding the Future of the Global Seaweed Aquaculture Industry; United Nations University (INWEH): Hamilton, ON, Canada; Scottish Association for Marine Science (SAMS) Policy Brief: Oban, UK, 2016; 12p, ISBN 978-92-808-6080-1. [Google Scholar]
- Kopprio, G.A.; Cuong, L.H.; Luyen, N.D.; Duc, T.M.; Ha, T.H.; Huong, L.M.; Gärdes, A. Carrageenophyte-attached and planktonic bacterial communities in two distinct bays of Vietnam: Eutrophication indicators and insights on ice-ice disease. Ecol. Indic. 2021, 121, 107067. [Google Scholar] [CrossRef]
- Tsiresy, G.; Preux, J.; Lavitra, T.; Dubois, P.; Lepoint, G.; Eeckhaut, I. Phenology of farmed seaweed Kappaphycus alvarezii infestation by the parasitic epiphyte Polysiphonia sp. in Madagascar. J. Appl. Phycol. 2016, 28, 2903–2914. [Google Scholar] [CrossRef]
- Largo, D.B.; Fukami, K.; Nishijima, T. Occasional pathogenic bacteria promoting ice-ice disease in the carrageenan-producing red algae Kappaphycus alvarezii and Eucheuma denticulatum (Solieriaceae, Gigartinales, Rhodophyta). J. Appl. Phycol. 1995, 7, 545–554. [Google Scholar] [CrossRef]
- Dobretsov, S.V.; Qian, P.Y. Effect of bacteria associated with the green alga Ulva reticulata on marine micro- and macrofouling. Biofouling 2002, 18, 217–228. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Chung, C.S.; Hurtado, A.Q.; Soya, F.E.; Lhonneur, G.B.; Critchley, A. Distribution and symptoms of epiphyte infection in major carrageenophyte-producing farms. J. Appl. Phycol. 2008, 20, 477–483. [Google Scholar] [CrossRef]
- Syafitri, E.; Prayitno, S.B.; Ma’Ruf, W.F.; Radjasa, O.K. Genetic diversity of the causative agent of ice-ice disease of the seaweed Kappaphycus alvarezii from Karimunjawa island, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 012044. [Google Scholar] [CrossRef] [Green Version]
- Arasamuthu, A.; Edward, J.K.P. Occurrence of Ice-ice disease in seaweed Kappaphycus alvarezii at Gulf of Mannar and Palk Bay, Southeastern India. Indian J. Mar. Sci. 2018, 47, 1208–1216. [Google Scholar]
- Vairappan, C.S.; Suzuki, M.; Motomura, T.; Ichimura, T. Pathogenic bacteria associated with lesions and thallus bleaching symptoms in the Japanese kelp Laminaria religiosa Miyabe (Laminariales, Phaeophyceae). Hydrobiologia 2001, 445, 183–191. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Sawabe, T.; Alexeeva, Y.V.; Lysenko, A.M.; Gorshkova, N.M.; Hayashi, K.; Zukova, N.V.; Christen, R.; Mikhailov, V.V. Pseudoalteromonas issachenkonii sp. nov., a bacterium that degrades the thallus of the brown alga Fucus evanescens. Int. J. Syst. Evol. Microbiol. 2002, 52, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Korber, D.R.; Lawrence, J.R.; Caldwell, D.E. Effect of motility on surface colonization and reproductive success of Pseudomonas fluorescens in dual-dilution continuous culture and batch culture systems. Appl. Environ. Microbiol. 1994, 60, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Klochkova, T.A.; Shim, J.B.; Hwang, M.S.; Kim, G.H. Host-parasite interactions and host species susceptibility of the marine oomycete parasite, Olpidiopsis sp., from Korea that infects red algae. J. Appl. Phycol. 2012, 24, 135–144. [Google Scholar] [CrossRef]
- Qiu, L.; Mao, Y.; Tang, L.; Tang, X.; Mo, Z. Characterization of Pythium chondricola associated with red rot disease of Pyropia yezoensis (Ueda) (Bangiales, Rhodophyta) from Lianyungang, China. J. Oceanol. Limnol. 2019, 37, 1102–1112. [Google Scholar] [CrossRef]
- Mo, Z.; Li, S.; Kong, F.; Tang, X.; Mao, Y. Characterization of a novel fungal disease that infects the gametophyte of Pyropia yezoensis (Bangiales, Rhodophyta). J. Appl. Phycol. 2016, 28, 395–404. [Google Scholar] [CrossRef]
- Martinez, J.N.; Padilla, P.I.P. Isolation and characterization of agar-digesting Vibrio species from the rotten thallus of Gracilariopsis heteroclada Zhang et Xia. Mar. Environ. Res. 2016, 119, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Ask, E.I.; Azanza, R.V. Advances in cultivation technology of commercial eucheumatoid species: A review with suggestions for future research. Aquaculture 2002, 206, 257–277. [Google Scholar] [CrossRef]
- Leonardi, P.I.; Miravalles, A.B.; Faugeron, S.; Flores, V.; Beltrán, J.; Correa, J.A. Diversity, phenomenology and epidemiology of epiphytism in farmed Gracilaria chilensis (Rhodophyta) in northern Chile. Eur. J. Phycol. 2006, 41, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Pang, T.; Liu, J.; Liu, Q.; Li, H.; Li, J. Observations on pests and diseases affecting a Eucheumatoid farm in China. J. Appl. Phycol. 2015, 27, 1975–1984. [Google Scholar] [CrossRef]
- Vairappan, C.S. Seasonal occurrences of epiphytic algae on the commercially cultivated red alga Kappaphycus alvarezii (Solieriaceae, Gigartinales, Rhodophyta). J. Appl. Phycol. 2006, 18, 611–617. [Google Scholar] [CrossRef]
- Borlongan, I.A.G.; Tibubos, K.R.; Yunque, D.A.T.; Hurtado, A.Q.; Critchley, A.T. Impact of AMPEP on the growth and occurrence of epiphytic Neosiphonia infestation on two varieties of commercially cultivated Kappaphycus alvarezii grown at different depths in the Philippines. J. Appl. Phycol. 2011, 23, 615–621. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yoshikawa, S.; Ohki, K.; Kamiya, M. Unique distribution of epiphytic Neosiphonia harveyi (Rhodomelaceae, Rhodophyta) along sargassacean hosts. Phycol. Res. 2012, 60, 70–75. [Google Scholar] [CrossRef]
- Araújo, P.G.; Schmidt, É.C.; Kreusch, M.G.; Kano, C.H.; Guimarães, S.M.P.B.; Bouzon, Z.L.; Fujiji, M.T.; Yokoya, N.S. Ultrastructural, morphological, and molecular characterization of Colaconema infestans (Colaconematales, Rhodophyta) and its host Kappaphycus alvarezii (Gigartinales, Rhodophyta) cultivated in the Brazilian tropical region. J. Appl. Phycol. 2014, 26, 1953–1961. [Google Scholar] [CrossRef]
- Largo, D.B.; Msuya, F.E.; Menezes, A. Understanding Diseases and Control in Seaweed Farming in Zanzibar; FAO Fisheries and Aquaculture Technical Paper No. 662; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Anangdan, S.P.; Tan, K.L.; Matsunaga, S. Role of secondary metabolites as defense chemicals against ice-ice disease bacteria in biofouler at carrageenophyte farms. J. Appl. Phycol. 2010, 22, 305–311. [Google Scholar] [CrossRef]
- Russell, D.J. Ecology of the imported red seaweed Eucheuma striatum Schmitz on Coconut Island, Oahu, Hawaii. Pac. Sci. 1983, 37, 87–107. [Google Scholar]
- Eggertsen, M.; Tano, S.A.; Chacin, D.H.; Eklöf, J.S.; Larsson, J.; Berkström, C.; Buriyo, A.S.; Halling, C. Different environmental variables predict distribution and cover of the introduced red seaweed Eucheuma denticulatum in two geographical locations. Biol. Invasions 2020, 23, 1049–1067. [Google Scholar] [CrossRef]
- Endo, H.; Sato, Y.; Kaneko, K.; Takahashi, D.; Nagasawa, K.; Okumura, Y.; Agatsuma, Y. Ocean warming combined with nutrient enrichment increases the risk of herbivory during cultivation of the marine macroalga Undaria Pinnatifida. ICES J. Mar. Sci. 2021, 78, 402–409. [Google Scholar] [CrossRef]
- Sano, M.; Omori, M.; Taniguchi, K. Predator-prey systems of drifting seaweed communities off the Tohoku coast, northern Japan, as determined by feeding habit analysis of phytal animals. Fish. Sci. 2003, 69, 260–268. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Graves, M.V.; Müller, D.G.; Boland, W.; Delaroque, N. Phycodnaviridae—Large DNA algal viruses. Arch. Virol. 2002, 147, 1479–1516. [Google Scholar] [CrossRef] [PubMed]
- McKeown, D.A.; Stevens, K.; Peters, A.F.; Bond, P.; Harper, G.M.; Brownlee, C.; Brown, M.T.; Schroeder, D.C. Phaeoviruses discovered in kelp (Laminariales). ISME J. 2017, 11, 2869–2873. [Google Scholar] [CrossRef] [Green Version]
- West, J.A.; Pueschel, C.M.; Klochkova, T.A.; Kim, G.H.; de Goër, S.; Zuccarello, G.C. Gall structure and specificity in Bostrychia culture isolates (Rhodomelaceae, Rhodophyta). Algae 2013, 21, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Klochkova, T.A.; Lee, D.J.; Im, S.H. Chloroplast virus causes green-spot disease in cultivated Pyropia of Korea. Algal Res. 2016, 17, 293–299. [Google Scholar] [CrossRef]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Coy, S.R.; Gann, E.R.; Pound, H.L.; Short, S.M.; Wilhelm, S.W. Viruses of eukaryotic algae: Diversity, methods for detection, and future directions. Viruses 2018, 10, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunairi, M.; Tsuchiya, H.; Tsuchiya, T.; Omura, Y.; Koyanagi, Y.; Ozawa, M.; Iwabuchi, N.; Murooka, H.; Nakajima, M. Isolation of a bacterium that causes anaaki disease of the red algae Porphyra yezoensis. J. Appl. Bacteriol. 1995, 79, 225–229. [Google Scholar] [CrossRef]
- Jakobsson-Thor, S.; Toth, G.B.; Brakel, J.; Bockelmann, A.C.; Pavia, H. Seagrass wasting disease varies with salinity and depth in natural Zostera marina populations. Mar. Ecol. Prog. Ser. 2018, 587, 105–115. [Google Scholar] [CrossRef]
- Brakel, J.; Jakobsson-Thor, S.; Bockelmann, A.C.; Reusch, T.B.H. Modulation of the eelgrass—Labyrinthula zosterae interaction under predicted ocean warming, salinity change and light limitation. Front. Mar. Sci. 2019, 6, 268. [Google Scholar] [CrossRef]
- Graham, O.J.; Aoki, L.R.; Stephens, T.; Stokes, J.; Dayal, S.; Rappazzo, B.; Gomes, C.P.; Harvell, C.D. Effects of Seagrass Wasting Disease on Eelgrass Growth and Belowground Sugar in Natural Meadows. Front. Mar. Sci. 2021, 8, 768668. [Google Scholar] [CrossRef]
- O’Connor, M.I. Warming strengthens an herbivore–plant interaction. Ecology 2019, 90, 388–398. [Google Scholar] [CrossRef]
- Poore, A.G.B.; Graba-Landry, A.; Favret, M.; Sheppard Brennand, H.; Byrne, M.; Dworjanyn, S.A. Direct and indirect effects of ocean acidification and warming on a marine plant–herbivore interaction. Oecologia 2013, 173, 1113–1124. [Google Scholar] [CrossRef]
- Tietjen, M. “You are what you eat” How diet can influence the gut microbiota of marine invertebrates. Plymouth Stud. Sci. 2014, 7, 203–211. Available online: http://hdl.handle.net/10026.1/14076 (accessed on 17 November 2020).
- Aires, T.; Serebryakova, A.; Viard, F.; Serrão, E.A.; Engelen, A.H. Acidification increases abundances of Vibrionales and Planctomycetia associated to a seaweed-grazer system: Potential consequences for disease and prey digestion efficiency. PeerJ 2018, 6, e4377. [Google Scholar] [CrossRef] [Green Version]
- Hemmi, A.; Jormalainen, V. Nutrient enhancement increases performance of a marine herbivore via quality of its food alga. Ecology 2002, 83, 1052–1064. [Google Scholar] [CrossRef]
- Andriesse, E.; Lee, Z. Viable insertion in agribusiness value chains? Seaweed farming after Typhoon Yolanda (Haiyan) in Iloilo Province, the Philippines. Singap. J. Trop. Geogr. 2017, 38, 25–40. [Google Scholar] [CrossRef]
- Nor, A.M.; Gray, T.S.; Caldwell, G.S.; Stead, S.M. A value chain analysis of Malaysia’s seaweed industry. J. Appl. Phycol. 2020, 32, 2161–2171. [Google Scholar] [CrossRef] [Green Version]
- Bak, U.G.; Mols-Mortensen, A.; Gregersen, O. Production method and cost of commercial-scale offshore cultivation of kelp in the Faroe Islands using multiple partial harvesting. Algal Res. 2018, 33, 36–47. [Google Scholar] [CrossRef]
- Goecke, F.; Klemetsdal, G.; Ergon, Å. Cultivar development of kelps for commercial cultivation—Past lessons and future prospects. Front. Mar. Sci. 2020, 8, 110. [Google Scholar] [CrossRef]
- Van den Burg, S.W.K.; Van Duijn, A.P.; Bartelings, H.; van Krimpen, M.M.; Poelman, M. The economic feasibility of seaweed production in the North Sea. Aquac. Econ. Manag. 2016, 20, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Meletiou, A.; Grace, M.; Darbi, M.; Pham-Truffert, M.; Locher-Krause, K.; Rueff, H. EU Renewable Energy Policies, Global Biodiversity, and the UN SDGs; Centre of Ecology & Hydrology: Wellington, UK, 2019. [Google Scholar]
- Zuniga-Jara, S.; Marín-Riffo, M.C.; Bulboa-Contador, C. Bioeconomic analysis of giant kelp Macrocystis pyrifera cultivation (Laminariales; Phaeophyceae) in northern Chile. J. Appl. Phycol. 2016, 28, 405–416. [Google Scholar] [CrossRef]
- Camus, C.; Infante, J.; Buschmann, A.H. Revisiting the economic profitability of giant kelp Macrocystis pyrifera (Ochrophyta) cultivation in Chile. Aquaculture 2019, 502, 80–86. [Google Scholar] [CrossRef]
- Hwang, E.K.; Yotsukura, N.; Pang, S.J.; Su, L.; Shan, T.F. Seaweed breeding programs and progress in eastern Asian countries. Phycologia 2019, 58, 484–495. [Google Scholar] [CrossRef]
- Praeger, C.; Vucko, M.J.; de Nys, R.; Cole, A. Maximising the productivity of the attached cultivation of Ulva tepida in land-based systems. Algal Res. 2019, 40, 101507. [Google Scholar] [CrossRef]
- Kim, J.K.; Yarish, C.; Hwang, E.K.; Park, M.; Kim, Y. Seaweed aquaculture: Cultivation technologies, challenges and its ecosystem services. Algae 2017, 32, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zuldin, W.H.; Yassir, S.; Shapawi, R. Growth and biochemical composition of Kappaphycus (Rhodophyta) in customized tank culture system. J. Appl. Phycol. 2016, 28, 2453–2458. [Google Scholar] [CrossRef]
- Kim, J.K.; Stekoll, M.; Yarish, C. Opportunities, challenges and future directions of open-water seaweed aquaculture in the United States. Phycologia 2019, 58, 446–461. [Google Scholar] [CrossRef]
- Kassim, M.; Mustafa, A. Comparison growth of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) cultivation in floating cage and longline in Indonesia. Aquac. Rep. 2017, 6, 49–55. [Google Scholar] [CrossRef]
- Trono, G.; Largo, D. The seaweed resources of the Philippines. Bot. Mar. 2019, 62, 483–498. [Google Scholar] [CrossRef]
- Veeragurunathan, V.; Prasad, K.; Malar Vizhi, J.; Singh, N.; Meena, R.; Mantri, V.A. Gracilaria debilis cultivation, agar characterization and economics: Bringing new species in the ambit of commercial farming in India. J. Appl. Phycol. 2019, 31, 2609–2621. [Google Scholar] [CrossRef]
- Zubia, M.; Draisma, S.G.A.; Morrissey, K.L. Concise review of the genus Caulerpa J.V. Lamouroux. J. Appl. Phycol. 2020, 32, 23–39. [Google Scholar] [CrossRef]
- Carl, C.; de Nys, R.; Paul, N.A. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS ONE 2014, 9, e98700. [Google Scholar] [CrossRef]
- Chemodanov, A.; Robin, A.; Jinjikhashvily, G.; Yitzhak, D.; Liberzon, A.; Israel, A.; Golberg, A. Feasibility study of Ulva sp. (Chlorophyta) intensive cultivation in a coastal area of the Eastern Mediterranean Sea. Biofuels Bioprod. Biorefin. 2019, 13, 864–877. [Google Scholar] [CrossRef]
- Sebök, S.; Herppich, W.B.; Hanelt, D. Outdoor cultivation of Ulva lactuca in a recently developed ring-shaped photobioreactor: Effects of elevated CO2 concentration on growth and photosynthetic performance. Bot. Mar. 2018, 62, 170–190. [Google Scholar] [CrossRef]
- Zhao, X.B.; Pang, S.J.; Liu, F.; Shan, T.F.; Li, J.; Gao, S.Q.; Kim, H.G. Intraspecific crossing of Saccharina japonica using distantly related unialgal gametophytes benefits kelp farming by improving blade quality and productivity at Sanggou Bay, China. J. Appl. Phycol. 2015, 28, 449–455. [Google Scholar] [CrossRef]
- Hwang, E.K.; Yoo, H.C.; Baek, J.M.; Park, C.S. Effect of pH and salinity on the removal of phytal animals during summer cultivation of Sargassum fusiforme and Sargassum fulvellumin Korea. J. Appl. Phycol. 2015, 27, 1985–1989. [Google Scholar] [CrossRef]
- Niwa, K.; Kobiyama, A.; Fuseya, R.; Sakamoto, T. Morphological and genetic differentiation of cultivated Undaria pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2017, 29, 1473–1482. [Google Scholar] [CrossRef]
- Vea, J.; Ask, E. Creating a sustainable commercial harvest of Laminaria hyperborea, in Norway. J. Appl. Phycol. 2011, 23, 489–494. [Google Scholar] [CrossRef]
- Stévant, P.; Rebours, C.; Chapman, A. Seaweed aquaculture in Norway: Recent industrial developments and future perspectives. Aquac. Int. 2017, 25, 1373–1390. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D.; Morrison, L. The sustainable harvesting of Ascophyllum nodosum (Fucaceae, Phaeophyceae) in Ireland, with notes on the collection and use of some other brown algae. J. Appl. Phycol. 2013, 25, 1823–1830. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chin. J. Oceanol. Limnol. 2015, 33, 410–418. [Google Scholar] [CrossRef]
- De Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value and farming in the Indo-Pacific region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Korzen, L.; Pulidindi, I.N.; Israel, A.; Abelson, A.; Gedanken, A. Single step production of bioethanol from the seaweed Ulva rigida using sonication. RSC Adv. 2015, 5, 223–229. [Google Scholar] [CrossRef]
- Broch, O.J.; Alver, M.O.; Bekkby, T.; Gundersen, H.; Forbord, S.; Handå, A.; Skjermo, J.; Hancke, K. The kelp cultivation potential in coastal and offshore regions of Norway. Front. Mar. Sci. 2019, 5, 529. [Google Scholar] [CrossRef]
- Monogail, M.M.; Morrison, L. The seaweed resources of Ireland: A twenty-first century perspective. J. Appl. Phycol. 2020, 32, 1287–1300. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Mawi, S.; Krishnan, S.; Din, M.F.M.; Arumugam, N.; Chelliapan, S. Bioremediation potential of macroalgae Gracilaria edulis and Gracilaria changii co-cultured with shrimp wastewater in an outdoor water recirculation system. Environ. Technol. Innov. 2020, 17, 100571. [Google Scholar] [CrossRef]
- Roy, L.A.; Davis, D.A.; Saoud, I.P.; Boyd, C.A.; Pine, H.J.; Boyd, C.E. Shrimp culture in inland low salinity waters. Rev. Aquac. 2010, 2, 191–208. [Google Scholar] [CrossRef]
- Cardoso-Mohedano, J.G.; Paez-Osuna, F.; Amezcua-Martínez, F.; Ruiz-Fernández, A.C.; Reséndiz-Ramírez, G.; Sanchez-Cabeza, J.A. Combined environmental stress from shrimp farm and dredging releases in a subtropical coastal lagoon (SE Gulf of California). Mar. Pollut. Bull. 2016, 10, 83–91. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Figueroa, F.L.; Korbee, N.; Álvarez-Gómez, F.; Abreu, M.H. Production of mycosporine-like amino acids from Gracilaria vermiculophylla (Rhodophyta) cultured through one year in an integrated multi-trophic aquaculture (IMTA) System. Mar. Biotechnol. 2017, 1, 246–254. [Google Scholar] [CrossRef]
- La Macchia Pedra, A.G.; Ramlov, F.; Maraschin, M.; Hayashi, L. Cultivation of the red seaweed Kappaphycus alvarezii with effluents from shrimp cultivation and brown seaweed extract: Effects on growth and secondary metabolism. Aquaculture 2017, 479, 297–303. [Google Scholar] [CrossRef]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Roohani, G.K.; Roohani, G.M. Nitrogen and phosphorous budgets for integrated culture of white leg shrimp Litopenaeus vannamei with red seaweed Gracilaria corticata in zero water exchange system. Iran. J. Fish. Sci. 2018, 17, 471–486. [Google Scholar] [CrossRef]
- Chebil Ajjabi, L.; Abaab, M.; Segni, R. The red macroalga Gracilaria verrucosa in co-culture with the Mediterranean mussels Mytilus galloprovincialis: Productivity and nutrient removal performance. Aquac. Int. 2017, 26, 253–266. [Google Scholar] [CrossRef]
- Kambey, C.S.B.; Sondak, C.F.A.; Chung, I.K. Potential growth and nutrient removal of Kappaphycus alvarezii in a fish floating-net cage system in Sekotong Bay, Lombok, Indonesia. J. World Aquac. Soc. 2020, 51, 944–959. [Google Scholar] [CrossRef]
- Wei, Z.; You, J.; Wu, H.; Yang, F.; Long, L.; Liu, Q.; Huo, Y.; He, P. Bioremediation using Gracilaria lemaneiformis to manage the nitrogen and phosphorous balance in an integrated multi-trophic aquaculture system in Yantian Bay, China. Mar. Pollut. Bull. 2017, 121, 313–319. [Google Scholar] [CrossRef]
- Fossberg, J.; Forbord, S.; Broch, O.J.; Malzahn, A.M.; Jansen, H.; Handå, A.; Førde, H.; Bergvik, M.; Fleddum, A.L.; Skjermo, J.; et al. The potential for upscaling kelp (Saccharina latissima) cultivation in salmon-driven integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 418. [Google Scholar] [CrossRef]
- Kim, Y.D.; Park, M.S.; Min, B.H.; Kim, H.C.; Lee, W.C.; Lee, C.; Kim, G.S.; Do, Y.H.; Yoo, H.I. The growth of Mugil cephalus, Patinopecten yessoensis and Saccharina japonica in the IMTA System. J. Environ. Sci. Int. 2016, 25, 1445–1457. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Vásquez, J.A.J.; Zuñiga, S.; Tala, F.; Piaget, N.; Rodríguez, D.C.; Alonso Vega, J.M. Economic valuation of kelp forests in northern Chile: Values of goods and services of the ecosystem. J. Appl. Phycol. 2014, 26, 1081–1088. [Google Scholar] [CrossRef]
- Bennett, S.; Wernberg, T.; Connell, S.S.D.; Hobday, A.J.; Johnson, C.R.; Poloczanska, E.S. The “Great Southern Reef”: Social, ecological and economic value of Australia’s neglected kelp forests. Mar. Freshw. Res. 2015, 67, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Andresen, K.; Handå, A.; Jensen, B.; Reitan, K.I.; Olsen, Y. Chemical composition and release rate of waste discharge from an Atlantic salmon farm with an evaluation of IMTA feasibility. Aquac. Environ. Interact. 2013, 4, 147–162. [Google Scholar] [CrossRef] [Green Version]
- Bartley, D.M. Aquaculture Diversification as an Adaptation Approach to Climate Change and Other External Forcing Factors; FAO Aquaculture Newsletter: Rome, Italy, 2016; Volume 5, p. 17. [Google Scholar]
- Ding, H.; Ma, J. Simultaneous infection by red rot and chytrid diseases in Porphyra yezoensis Ueda. J. Appl. Phycol. 2005, 17, 51–56. [Google Scholar] [CrossRef]
- Vågsholm, I.; Djupvik, H.O.; Willumsen, F.V.; Tveit, A.M.; Tangen, K. Infectious salmon anaemia (ISA) epidemiology in Norway. Prev. Vet. Med. 1994, 19, 277–290. [Google Scholar] [CrossRef]
- Chou, H.Y.; Huang, C.Y.; Wang, C.H.; Chiang, H.C.; Lo, C.F. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis. Aquat. Org. 1995, 23, 165–173. [Google Scholar] [CrossRef]
- Zhan, W.B.; Wang, Y.H.; Fryer, J.L.; Yu, K.K.; Fukuda, H.; Meng, Q.X. White spot syndrome virus infection of cultured shrimp in China. J. Aquat. Anim. Health 1998, 10, 405–410. [Google Scholar] [CrossRef]
- Oakey, J.; Smith, C.; Underwood, D.; Afsharnasab, M.; Alday-Sanz, V.; Dhar, A.; Sivakumar, S.; Sahul Hameed, A.S.; Beattie, K.; Crook, A. Global distribution of white spot syndrome virus genotypes determined using a novel genotyping assay. Arch. Virol. 2019, 164, 2061–2082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheney, D.; Rudolph, B.; Wang, L.Z.; Metz, B.; Watson, K.; Roberts, K.; Levine, I. Genetic manipulation and strain improvement in commercially valuable red seaweeds. In New Developments in Marine Biotechnology; Le Gal, Y., Halvorson, H.O., Eds.; Springer: Boston, MA, USA, 1998; pp. 101–104. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Critchley, A.T.; Smith, J.; Sieber, V.; Lhonneur, G.B.; West, J.A. Systematics and genetic variation in commercial shape Kappaphycus and shape Eucheuma (Solieriaceae, Rhodophyta). J. Appl. Phycol. 2006, 18, 643–651. [Google Scholar] [CrossRef]
- Hayashi, L.; Yokoya, N.S.; Kikuchi, D.M.; Oliveira, E.C. Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J. Appl. Phycol. 2008, 20, 653–659. [Google Scholar] [CrossRef]
- Bindu, M.S.; Levine, I.A. The commercial red seaweed Kappaphycus alvarezii-an overview on farming and environment. J. Appl. Phycol. 2011, 23, 789–796. [Google Scholar] [CrossRef]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N.; Neish, I.C.; Hurtado, A.Q.; Msuya, F.E.; Krishnan, M.; Narayanakumar, R.; Kronen, M.; et al. The economics of Kappaphycus seaweed cultivation in developing countries: A comparative analysis of farming systems. Aquac. Econ. Manag. 2015, 19, 251–277. [Google Scholar] [CrossRef] [Green Version]
- Charrier, B.; Rolland, E.; Gupta, V.; Reddy, C.R.K. Production of genetically and developmentally modified seaweeds: Exploiting the potential of artificial selection techniques. Front. Plant Sci. 2015, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.E.; Goldberg, S.J.; Wegley Kelly, L.; Haas, A.F.; Smith, J.E.; Rohwer, F.; Carlson, C.A. Coral and macroalgal exudates vary in neutral sugar composition and differentially enrich reef bacterioplankton lineages. ISME J. 2013, 7, 962–979. [Google Scholar] [CrossRef]
- Kelly, L.W.; Nelson, C.E.; Haas, A.F.; Naliboff, D.S.; Calhoun, S.; Carlson, C.A.; Edwards, R.A.; Fox, M.D.; Hatay, M.; Johnson, M.D.; et al. Diel population and functional synchrony of microbial communities on coral reefs. Nat. Commun. 2019, 10, 1691. [Google Scholar] [CrossRef] [Green Version]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Zhou, J.; Yang, M.; Liu, Y.; Xu, X.; Xing, J. Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli. Bioresour. Technol. 2015, 185, 56–61. [Google Scholar] [CrossRef]
- Camus, C.; Ballerino, P.; Delgado, R.; Olivera-Nappa, Á.; Leyton, C.; Buschmann, A.H. Scaling up bioethanol production from the farmed brown macroalga Macrocystis pyrifera in Chile. Biofuels Bioprod. Biorefin. 2016, 10, 673–685. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Lane, M.; Nelson, R.S. Sustainable seaweed biotechnology solutions for carbon capture, composition, and deconstruction. Trends Biotechnol. 2020, 38, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.K.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. J. Appl. Phycol. 2008, 20, 609–617. [Google Scholar] [CrossRef]
- Baweja, P.; Sahoo, D.; García-Jiménez, P.; Robaina, R.R. Review: Seaweed tissue culture as applied to biotechnology: Problems, achievements and prospects. Phycol. Res. 2009, 57, 45–58. [Google Scholar] [CrossRef]
- Jong, L.W.; Thien, V.Y.; Yong, Y.S.; Rodrigues, K.F.; Yong, W.T.L. Micropropagation and protein profile analysis by SDS-PAGE of Gracilaria changii (Rhodophyta, Solieriaceae). Aquac. Rep. 2015, 1, 10–14. [Google Scholar] [CrossRef]
- Muhamad, S.N.S.; Ling, A.P.K.; Wong, C.L. Effect of plant growth regulators on direct regeneration and callus induction from Sargassum polycystum C. Agardh. J. Appl. Phycol. 2018, 30, 3299–3310. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Ting, S.H.; Yong, Y.S.; Thien, V.Y.; Wong, S.H.; Chin, W.L.; Rodrigues, K.F.; Anton, A. Optimization of culture conditions for the direct regeneration of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J. Appl. Phycol. 2014, 26, 1597–1606. [Google Scholar] [CrossRef]
- Niwa, K.; Iida, S.; Kato, A.; Kawai, H.; Kikuchi, N.; Kobiyama, A.; Arugo, Y. Genetic diversity and introgression in two cultivated species (Porphyra yezoensis and Porphyra tenera) and closely related wild species of Porphyra (Bangiales, Rhodophyta). J. Phycol. 2009, 45, 493–502. [Google Scholar] [CrossRef]
- Robinson, N.; Winberg, P.; Kirkendale, L. Genetic improvement of macroalgae: Status to date and needs for the future. J. Appl. Phycol. 2013, 25, 703–716. [Google Scholar] [CrossRef]
- Li, X.; Cong, Y.; Yang, G.; Shi, Y.; Qu, S.; Li, Z.; Wang, G.; Zhang, Z.; Luo, S.; Dai, H.; et al. Trait evaluation and trial cultivation of Dongfang No. 2, the hybrid of a male gametophyte clone of Laminaria longissima (Laminariales, Phaeophyta) and a female one of L. japonica. J. Appl. Phycol. 2007, 19, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.E.; Yang, L.E.; Tan, J.; Maggs, C.A.; Brodie, J. Advancing the taxonomy of economically important red seaweeds (Rhodophyta). Eur. J. Phycol. 2017, 52, 438–451. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.E.; Phang, S.M.; Hurtado, A.Q. Biodiversity, biogeography and molecular genetics of the commercially important genera kappaphycus and eucheuma. In Tropical Seaweed Farming Trends, Problems and Opportunities; Hurtado, A., Critchley, A., Neish, I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 9, pp. 29–43. [Google Scholar] [CrossRef]
- Risjani, Y.; Abidin, G. Genetic diversity and similarity between green and brown morphotypes of Kappaphycus alvarezii using RAPD. J. Appl. Phycol. 2020, 32, 2253–2260. [Google Scholar] [CrossRef]
- Thien, V.Y.; Yong, W.T.L.; Anton, A.; Chin, G.J.W.L. A multiplex PCR method for rapid identification of commercially important seaweeds Kappaphycus alvarezii, Kappaphycus striatus and Eucheuma denticulatum (Rhodophyta, Solieriaceae). Reg. Stud. Mar. Sci. 2020, 40, 101499. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.E.; Phang, S.M.; Hong, D.D.; Sunarpi, H.; Hurtado, A.Q. Assessment of four molecular markers as potential DNA barcodes for red algae Kappaphycus doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta). PLoS ONE 2012, 7, e52905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Liu, T.; Guo, B.; Jin, D.; Weng, M.; Feng, Y.; Xu, P.; Duan, D.; Wang, B. Development of SSR primers from EST sequences and their application in germplasm identification of Porphyra lines (Rhodophyta). Eur. J. Phycol. 2006, 41, 329–336. [Google Scholar] [CrossRef]
- Ho, C.L.; Phang, S.M.; Pang, T. Application of polymerase chain reaction (PCR) using random amplified polymorphic dna (RAPD) primers in the molecular identification of selected Sargassum species (phaeophyta, fucales). Eur. J. Phycol. 1995, 30, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Li, Y.; Liu, Y.; Li, X.; Luo, S.; Zhang, Z.; Wu, R.; Liang, G.; Sun, J.; Peng, J.; et al. Determination of genetic diversity among Saccharina germplasm using ISSR and RAPD markers. CR Biol. 2017, 340, 76–86. [Google Scholar] [CrossRef]
- Chin, G.J.; Mohamad, M.Z.; Maili, S.; Yong, W.T.; Rodrigues, K. ISSR-PCR fingerprinting of Kappaphycus and Eucheuma (rhodophyta, gigartinales) seaweed varieties from Sabah, Malaysia. Trans. Sci. Technol. 2017, 4, 420–425. [Google Scholar]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Zambounis, A.; Strittmatter, M.; Gachon, C.M.M. Chronic stress and disease resistance in the genome model marine seaweed Ectocarpus siliculosus. Aquat. Bot. 2013, 104, 147–152. [Google Scholar] [CrossRef]
- Küpper, F.C. Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades. J. Exp. Bot. 2006, 57, 1991–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritter, A.; Goulitquer, S.; Salaün, J.P.; Tonon, T.; Correa, J.A.; Potin, P. Copper stress induces biosynthesis of octadecanoid and eicosanoid oxygenated derivatives in the brown algal kelp Laminaria digitata. New Phytol. 2008, 180, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Goulitquer, S.; Ritter, A.; Thomas, F.; Ferec, C.; Salaün, J.P.; Potin, P. Release of volatile aldehydes by the brown algal kelp Laminaria digitata in response to both biotic and abiotic stress. ChemBioChem 2009, 10, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Mella, D.; Moenne, A.; Correa, J.A. Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat. Toxicol. 2009, 94, 94–102. [Google Scholar] [CrossRef]
- Küpper, F.C.; Gaquerel, E.; Cosse, A.; Adas, F.; Peters, A.F.; Müller, D.G.; Kloareg, B.; Salaün, J.P.; Potin, P. Free fatty acids and methyl jasmonate trigger defense reactions in Laminaria digitata. Plant Cell Physiol. 2009, 50, 789–800. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- GÁlis, I.; Gaquerel, E.; Pandey, S.P.; Baldwin, I.T. Molecular mechanisms underlying plant memory in JA-mediated defense responses. Plant Cell Environ. 2009, 32, 617–627. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B.M. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Critchley, A.T. Time for applications of biostimulants in phyconomy: Seaweed Extracts for Enhanced Cultivation of Seaweeds (SEECS). In Sustainable Seaweed Technologies, 1st ed.; Torres, M., Kraan, S., Dominguez, H., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 103–127. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Zodape, S.T.; Mukhopadhyay, S.; Eswaran, K.; Reddy, M.P.; Chikara, J. Enhanced yield and nutritional quality in green gram (Phaseolus radiata L.) treated with seaweed (Kappaphycus alvarezii) extract. J. Sci. Ind. Res. 2010, 69, 468–471. [Google Scholar]
- Mirparsa, T.; Ganjali, H.R.; Dahmardeh, T. The effect of bio fertilizers on yield and yield components of sunflower oil seed and nut. Int. J. Agric. Biosci. 2016, 1, 46–49. [Google Scholar]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Kingman, A.R.; Moore, J. Isolation, purification and quantitation of several growth regulating substances in Ascophyllum nodosum (Phaeophyta). Bot. Mar. 1982, 25, 149–153. [Google Scholar] [CrossRef]
- Crouch, I.J.; van Staden, J. Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul. 1993, 13, 21–29. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Ugarte, R.A.; Sharp, G.; Moore, B. Changes in the brown seaweed Ascophyllum nodosum (L.) Le Jol. plant morphology and biomass produced by cutter rake harvests in southern New Brunswick, Canada. J. Appl. Phycol. 2006, 18, 351–359. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Neish, I.C.; Critchley, A.T. Developments in production technology of Kappaphycus in the Philippines: More than four decades of farming. J. Appl. Phycol. 2015, 27, 1945–1961. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Yunque, D.A.; Tibubos, K.; Critchley, A.T. Use of Acadian marine plant extract powder from Ascophyllum nodosum in tissue culture of Kappaphycus varieties. J. Appl. Phycol. 2009, 21, 633–639. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Reis, R.P.; Critchley, A.T. In vitro cultivation of three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) variants (green, red and brown) exposed to a commercial extract of the brown alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 2010, 22, 101–104. [Google Scholar] [CrossRef]
- Hurtado, A.Q.; Joe, M.; Sanares, R.C.; Fan, D.; Prithiviraj, B.; Critchley, A.T. Investigation of the application of Acadian Marine Plant Extract Powder (AMPEP) to enhance the growth, phenolic content, free radical scavenging, and iron chelating activities of Kappaphycus Doty (Solieriaceae, Gigartinales, Rhodophyta). J. Appl. Phycol. 2012, 24, 601–611. [Google Scholar] [CrossRef]
- Prasad, K.; Das, A.K.; Oza, M.D.; Brahmbhatt, H.; Siddhanta, A.K.; Meena, R.; Eswaran, K.; Rajyaguru, M.R.; Ghosh, P.K. Detection and quantification of some plant growth regulators in a seaweed-based foliar spray employing a mass spectrometric technique sans chromatographic separation. J. Agric. Food Chem. 2010, 58, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, B.; Brahmachari, K.; Ghosh, A. Effect of seaweed saps on growth and yield improvement of green gram. Afr. J. Agric. Res. 2013, 8, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Pramanick, B.; Brahmachari, K.; Kar, S.; Mahapatra, B.S. Can foliar application of seaweed sap improve the quality of rice grown under rice–potato–green gram crop sequence with better efficiency of the system? J. Appl. Phycol. 2020, 32, 3377–3386. [Google Scholar] [CrossRef]
- Bowman, J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef]
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Review chemical interactions between marine macroalgae and bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–300. [Google Scholar] [CrossRef]
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of vibrionaceae in the environment, from the genus to the population level. Front. Microbial. 2014, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Mtolera, M.S.P.; Collén, J.; Pedersén, M.; Ekdahl, A.; Abrahamsson, K.; Semesi, A.K. Stress-induced production of volatile halogenated organic compounds in Eucheuma denticulatum (Rhodophyta) caused by elevated pH and high light intensities. Eur. J. Phycol. 1996, 31, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Chye, F.Y.; Padam, B.S.; Ng, S.Y. Innovation and sustainable utilization of seaweeds as health foods. In Sustainability Challenges in the Agrofood Sector; Bhat, R., Ed.; Wiley: New Jersey, NJ, USA, 2017; pp. 390–434. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The evolution road of seaweed aquaculture: Cultivation technologies and the industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Al Sharie, A.H.; El-Elimat, T.; Al Zu’bi, Y.O.; Aleshawi, A.J.; Medina-Franco, J.L. Chemical Space and Diversity of Seaweed Metabolite Database (SWMD): A Cheminformatics Study. J. Mol. Graph. Model. 2020, 100, 107702. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.M.; Critchley, A.T.; Hurtado, A.Q. Micropropagation and sea-based nursery growth of selected commercial Kappaphycus species in Penang, Malaysia. J. Appl. Phycol. 2020, 32, 1301–1309. [Google Scholar] [CrossRef]
- Umanzor, S.; Jang, S.; Antosca, R.; Critchley, A.T.; Yarish, C.; Kim, J.K. Optimizing the application of selected biostimulants to enhance the growth of Eucheumatopsis isiformis, a carrageenophyte with commercial value, as grown in land-based nursery systems. J. Appl. Phycol. 2020, 32, 1917–1922. [Google Scholar] [CrossRef]
- Ali, M.M.; Sani, M.Z.B.; Hi, K.K.; Yasir, S.M.; Critchley, A.T.; Hurtado, A.Q. The comparative efficiency of a brown algal-derived biostimulant extract (AMPEP), with and without supplemented PGRs: The induction of direct, axis shoots as applied to the propagation of vegetative seedlings for the successful mass cultivation of three commercial strains of Kappaphycus in Sabah, Malaysia. J. Appl. Phycol. 2018, 30, 1913–1919. [Google Scholar] [CrossRef]
- Hayashi, L.; Cantarino, S.D.J.; Critchley, A.T. Challenges to the future domestication of seaweeds as cultivated species: Understanding their physiological processes for large-scale production. In Advances in Botanical Research-Seaweeds around the World: State of Art and Perspectives, 1st ed.; Bourgougnon, N., Ed.; Elsevier: London, UK, 2020; Volume 95, pp. 57–83. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.T.; Tuohy, M.G.; Kloareg, B.; Stengel, D.B. Evolution and diversity of plant cell walls: From algae to flowering plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef] [Green Version]
- Suvega, T.; Arunkumar, K. Probiotic bacteria promote the growth of associating host (red seaweed, Gracilaria edulis) also synthesize antibacterial protein. Biocatal. Agric. Biotechnol. 2019, 19, 101136. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Singh, R.P.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Effect of quorum sensing signals produced by seaweed-associated bacteria on carpospore liberation from Gracilaria dura. Front. Plant Sci. 2015, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.; Oku, N.; Kasai, H. Wenyingzhuangia gracilariae sp. nov., a novel marine bacterium of the phylum Bacteroidetes isolated from the red alga Gracilaria vermiculophylla. Antonie Van Leeuwenhoek 2015, 107, 1607–1613. [Google Scholar] [CrossRef]
- Singh, R.P.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Role of bacterial isolates in enhancing the bud induction in the industrially important red alga Gracilaria dura. FEMS Microbiol. Ecol. 2011, 76, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J.F. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, X.Q.; Mun, W.; Teo, S.S. Comparison study of anti-microbial activity between crude extract of Kappaphycus alvarezii and Andrographis paniculata. Asian Pac. J. Trop. Biomed. 2017, 7, 729–731. [Google Scholar] [CrossRef]


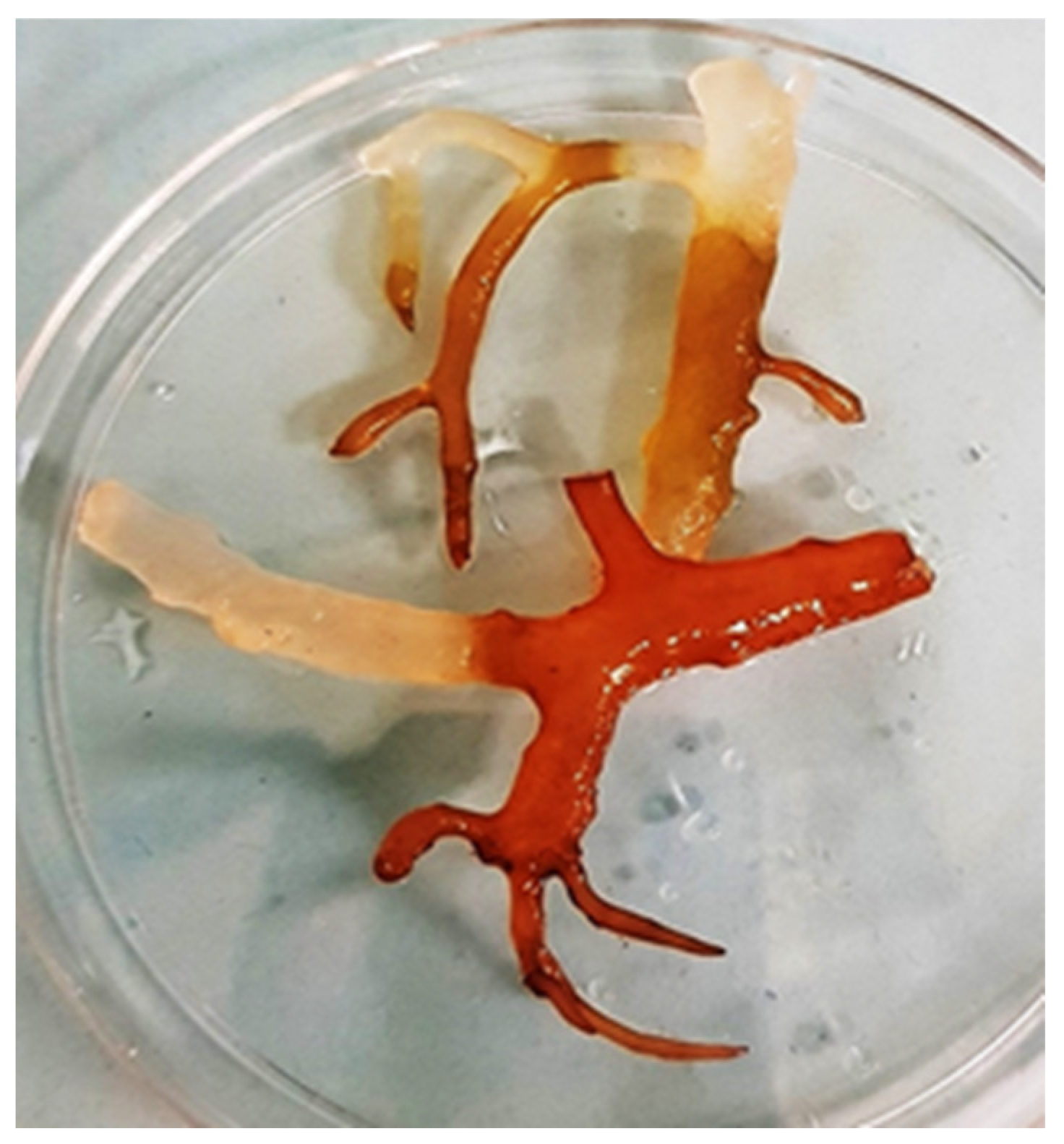
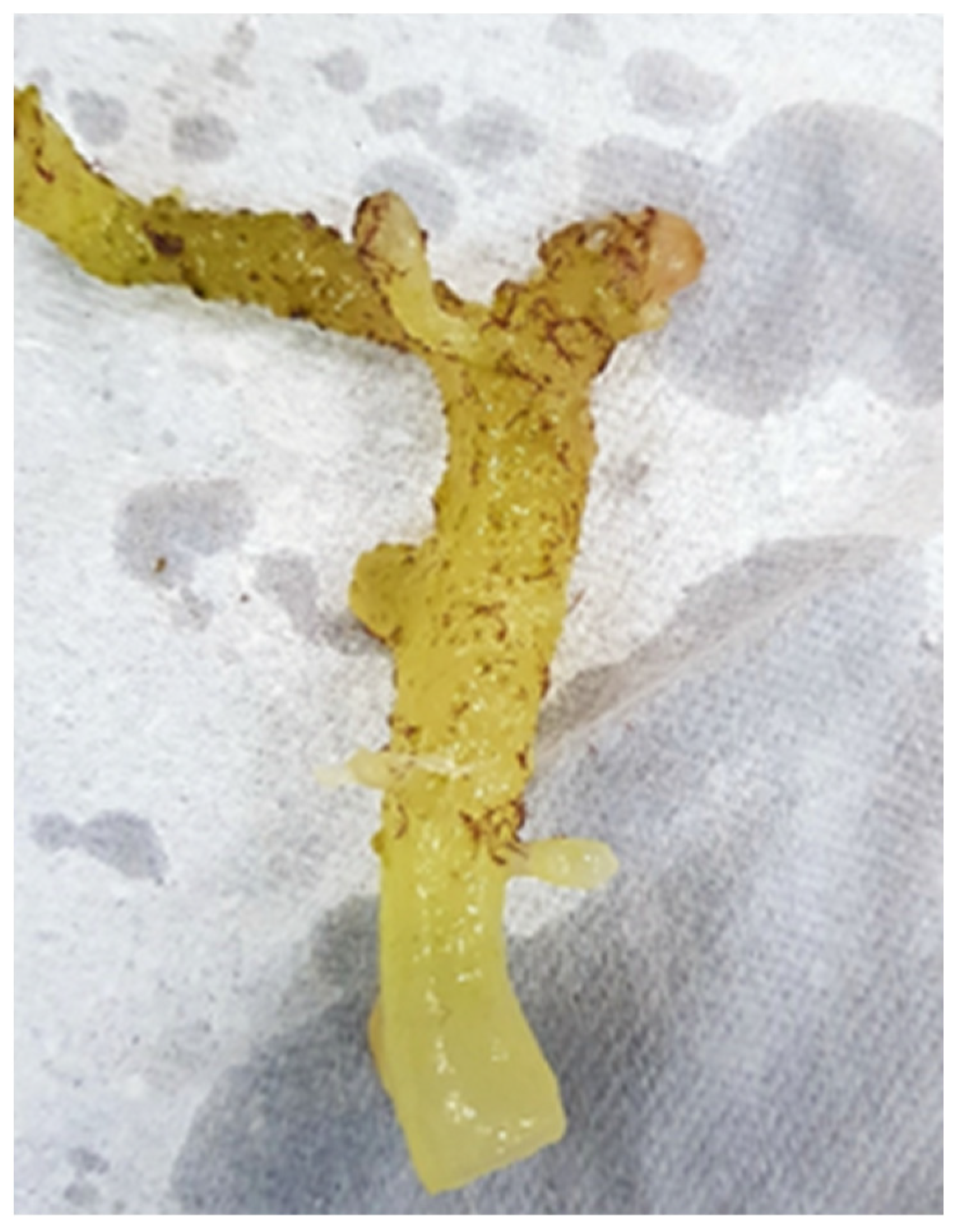
| Seaweed Species (Seaweed per Color) | Production Method | References |
|---|---|---|
| Porphyra sp./Pyropia (red algae) | Commercial production offshore and nearshore using fixed poles, nets, semi-floating rafts, or floating rafts | [8,128,130] |
| Kappaphycus alvarezii and eucheumatoids (red algae) |
| [131,132] |
| Gracilaria/Gracilariopsis (red algae) |
| [128,133] |
| Caulerpa lentillifera, Caulerpa racemosa (green algae) |
| [132,134] |
| Ulva sp. (green algae) |
| [135,136,137] |
| Saccharina latissima, Saccharina japonica (brown algae) | Offshore longline horizontal and vertical methods for commercial-scale production | [120,128,130,138] |
| Sargassum fusiforme, Sargassum fulvellum (brown algae) | Commercial-scale offshore farming using the longline method | [139] |
| Undaria pinnatifida (brown algae) | Commercial-scale offshore farming using longline, vertical hanging methods | [8,140] |
| Laminaria sp. (brown algae) |
| [130,141,142] |
| Ascophyllum nodosum (brown algae) | Commercial-scale sustainable harvesting by mechanical and hand cutting on seashore beds | [143,144] |
| Seaweed Species (Seaweed per Color) | Effluent Source | Effect of Growth and Quality of Seaweed | Reference |
|---|---|---|---|
| Inland cultivation | |||
| Kappaphycus alvarezii (red algae) | Litopenaeus vannamei cultivation in a biofloc system | Significant growth rate of 1.70% day−1 compared with control; increase in total phenolics, flavonoids, and carotenoids; ice-ice disease observed in some samples | [155] |
| Gracilaria verru-cosa (red algae) | Mytilus galloprovin-cialis (Mediterra-nean mussels) co-cultivation | Maximum growth rate of 4.45% on day-1 during spring; reduction in water ammo-nium and phosphate concentration | [156] |
| Gracilaria vermiculophylla (red algae) | Fishpond effluent | Increased biomass and mycosporine-like amino acid (MAA) content during the summer months of April and May; MAAs also affected by stocking density | [154] |
| Gracilariopsis longissima (formerly Gracilaria verrucosa) (red algae) | Mytilus galloprovincialis (Mediterranean mussels) co-cultivation system | Maximum growth rate of 4.45% on day−1 during spring; reduction in water ammonium and phosphate concentration | [157] |
| Gracilaria edulis, Gracilaria changii (red algae) | Wastewater recirculation system from shrimp culture | Mean growth rates observed of 4.1–4.3% on day−1; removal of ammonium and nitrate at 71.0–72.5% and 56.8–58.8% | [151] |
| Solieria filiformis (red algae) | Sciaenops ocellatus (red drum fish) and sea cucumber integrated system | Significant increase in growth rates and protein content in the integrated system compared with control; highest growth rate recorded at 16.7% from fish integration on day 70; highest protein content recorded at 20.1% on day 30 in fish and sea cucumber integration | [54] |
| Ulva lactuca | Rachycentron canadum (cobia fish) and Perna perna (brown mussel) | Significant daily growth rate observed over time and inclusion of trophic levels; highest recorded at 4.75% (fish) and 6.32% (fish and mussel) | [157] |
| Ocean-based cultivation | |||
| Kappaphycus alvarezii (red algae) | Fish (not specified) in floating net-cage system | Significant growth rate of 3.33% compared with control | [158] |
| Gracilariopsis lemaneiformis (Gracilaria lemaneiformis) (red algae) | Pseudociena crocea (yellow croaker) in floating cage system | Specific growth rates of 5.82–9.84%; total weight at 35 days was 5.3 times than initial weight | [159] |
| Saccharina latissima (brown algae) | Integrated farming with Salmo salar (salmon) | Increased macroalgae yield by 60% with potentially higher protein content compared with nonintegrated systems for a 25-hectare farm. | [160] |
| Saccharina japonica (brown algae) | Integrated farming with Mugil cephalus (striped mullet) and Patinopecten yessoensis (yesso scallop) | Daily growth rates ranged from 0.03–1.9 mm/day; highly affected by seasonal water temperature | [161] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugumaran, R.; Padam, B.S.; Yong, W.T.L.; Saallah, S.; Ahmed, K.; Yusof, N.A. A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects. Int. J. Environ. Res. Public Health 2022, 19, 7087. https://doi.org/10.3390/ijerph19127087
Sugumaran R, Padam BS, Yong WTL, Saallah S, Ahmed K, Yusof NA. A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects. International Journal of Environmental Research and Public Health. 2022; 19(12):7087. https://doi.org/10.3390/ijerph19127087
Chicago/Turabian StyleSugumaran, Rajeena, Birdie Scott Padam, Wilson Thau Lym Yong, Suryani Saallah, Kamruddin Ahmed, and Nur Athirah Yusof. 2022. "A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects" International Journal of Environmental Research and Public Health 19, no. 12: 7087. https://doi.org/10.3390/ijerph19127087
APA StyleSugumaran, R., Padam, B. S., Yong, W. T. L., Saallah, S., Ahmed, K., & Yusof, N. A. (2022). A Retrospective Review of Global Commercial Seaweed Production—Current Challenges, Biosecurity and Mitigation Measures and Prospects. International Journal of Environmental Research and Public Health, 19(12), 7087. https://doi.org/10.3390/ijerph19127087







