Contrast Media Adverse Drug Reactions in Highly Polluted Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patients Group
2.3. Statistical Analysis
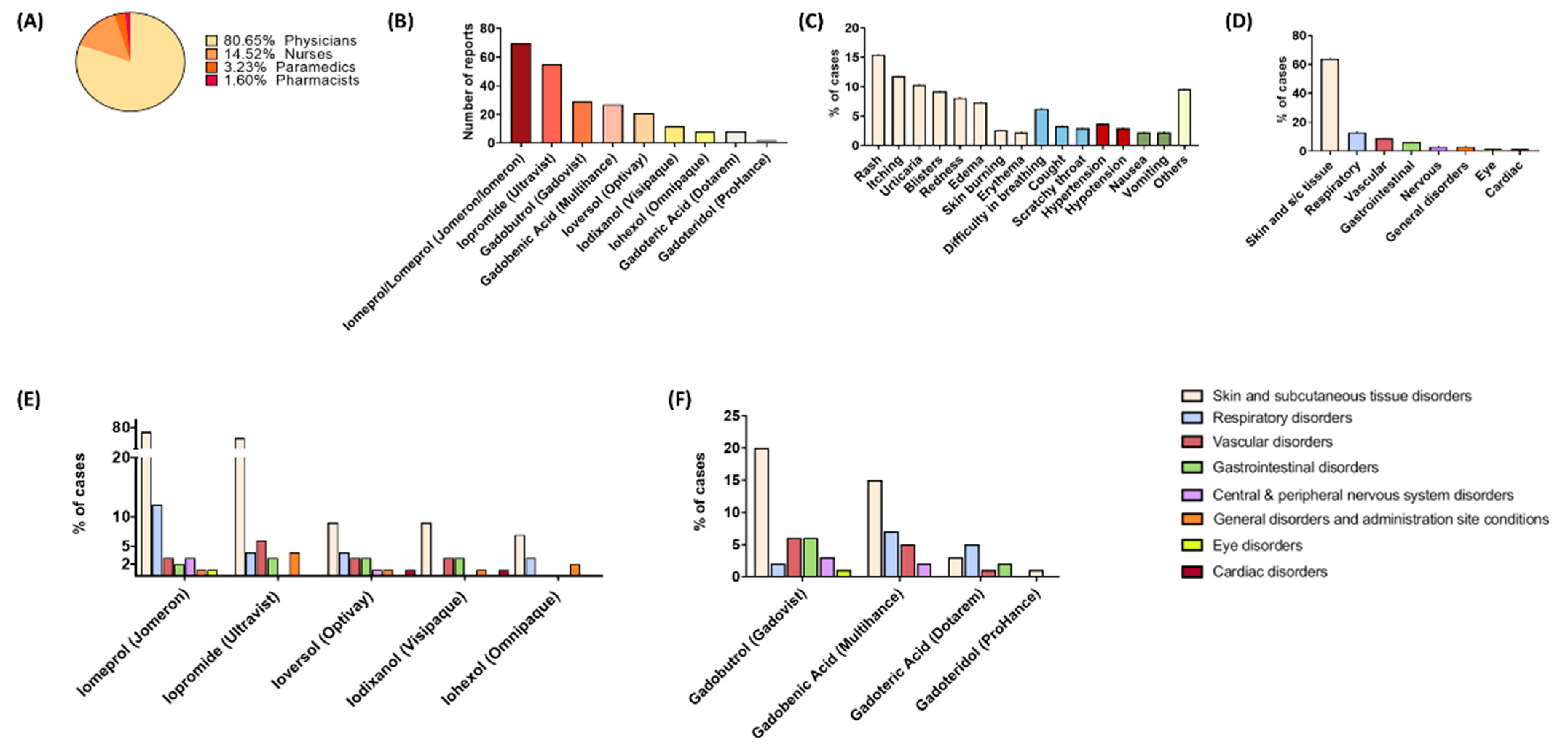
3. Results
3.1. Gadolinium-Based Contrast Media
3.2. Iodine-Based Contrast Media
3.3. Differences in the ADR Profiles between Individual Contrast Media
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side effects of radiographic contrast media: Pathogenesis, risk factors, and prevention. Biomed Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.W.; Stacul, F.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.F.; Clauss, W.; Flaten, H.; Grenier, N.; et al. Late adverse reactions to intravascular iodinated contrast media. Eur. Radiol. 2003, 13, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Liu, S.Z.; Louie, A.Y. Biological effects of MRI contrast agents: Gadolinium retention, potential mechanisms and a role for phosphorus. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20170180. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R. Contrast Media: Are There Differences in Nephrotoxicity among Contrast Media? Biomed Res. Int. 2014, 2014, 934947. [Google Scholar] [CrossRef]
- Laurent, S.; Elst, L.V.; Muller, R.N. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol. Imaging 2006, 1, 128–137. [Google Scholar] [CrossRef]
- Prince, M.R.; Zhang, H.; Zou, Z.; Staron, R.B.; Brill, P.W. Incidence of Immediate Gadolinium Contrast Media Reactions. Am. J. Roentgenol. 2012, 196, W138–W143. [Google Scholar] [CrossRef]
- Murphy, K.J.; Brunberg, J.A.; Cohan, R.H. Adverse reactions to gadolinium contrast media: A review of 36 cases. AJR Am. J. Roentgenol. 2013, 167, 847–849. [Google Scholar] [CrossRef]
- Clough, T.J.; Jiang, L.; Wong, K.L.; Long, N.J. Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef]
- Hiremath, S.; Kayibanda, J.F.; Chow, B.J.W.; Fergusson, D.; Knoll, G.A.; Shabana, W.; Lahey, B.; McBride, O.; Davis, A.; Akbari, A. Drug discontinuation before contrast procedures and the effect on acute kidney injury and other clinical outcomes: A systematic review protocol. Syst. Rev. 2018, 7, 34. [Google Scholar] [CrossRef]
- Kerl, J.M.; Nguyen, S.A.; Lazarchick, J.; Powell, J.W.; Oswald, M.W.; Alvi, F.; Costello, P.; Vogl, T.J.; Schoepf, U.J. Iodinated contrast media: Effect of osmolarity and injection temperature on erythrocyte morphology in vitro. Acta Radiol. 2008, 49, 337–343. [Google Scholar] [CrossRef]
- van der Molen, A.J.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.F.; Flaten, H.; Jakobsen, J.Å.; Krestin, G.P.; Löwe, A.; et al. Effect of iodinated contrast media on thyroid function in adults. Eur. Radiol. 2004, 14, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.L.; Thompson, J.A.; Benyunes, M.C.; Winter, T.C.; Fefer, A. Adverse reactions to intravenous contrast media in patients treated with interleukin-2. J. Immunother. Emphasis Tumor Immunol. 1993, 13, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Krynicka, J.; Drzeniecka-Osiadacz, A. Analysis of Variability in PM10 Concentration in the Wrocław Agglomeration. Pol. J. Environ. Stud. 2013, 22, 4. [Google Scholar]
- Piekarska, K.; Karpińska-Smulikowska, J. Mutagenic Activity of Environmental Air Samples from the Area of Wrocław, Poland. Pol. J. Environ. Stud. 2007, 16, 745–752. [Google Scholar]
- Bate, A.; Evans, S.J.W. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef]
- Evans, S.J.W.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Laroche, D.; Namour, F.; Lefrançois, C.; Aimone-Gastin, I.; Romano, A.; Sainte-Laudy, J.; Laxenaire, M.C.; Guéant, J.L. Anaphylactoid and anaphylactic reactions to iodinated contrast material. Allergy 1999, 54 (Suppl. S5), 13–16. [Google Scholar] [CrossRef]
- Cochran, S.T. Anaphylactoid reactions to radiocontrast media. Curr. Allergy Asthma Rep. 2005, 5, 28–31. [Google Scholar] [CrossRef]
- Hunt, C.H.; Hartman, R.P.; Hesley, G.K. Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: Retrospective review of 456,930 doses. AJR Am. J. Roentgenol. 2009, 193, 1124–1127. [Google Scholar] [CrossRef]
- Güner, M.D.; Ekmekci, P.E. Healthcare professionals’ pharmacovigilance knowledge and adverse drug reaction reporting behavior and factors determining the reporting rates. J. Drug Assess. 2019, 8, 13. [Google Scholar] [CrossRef]
- Delio, J.; Catalanotti, J.S.; Marko, K.; Paul, C.; Taffel, M.; Ho, G.; Berger, J. Integrating Adverse Event Reporting Into a Free-Text Mobile Application Used in Daily Workflow Increases Adverse Event Reporting by Physicians. Am. J. Med. Qual. 2020, 35, 374–379. [Google Scholar] [CrossRef]
- Cutroneo, P.; Polimeni, G.; Curcuruto, R.; Calapai, G.; Caputi, A.P. Adverse reactions to contrast media: An analysis from spontaneous reporting data. Pharmacol. Res. 2007, 56, 35–41. [Google Scholar] [CrossRef]
- Wang, C.L.; Cohan, R.H.; Ellis, J.H.; Caoili, E.M.; Wang, G.; Francis, I.R. Frequency, outcome, and appropriateness of treatment of nonionic iodinated contrast media reactions. AJR Am. J. Roentgenol. 2008, 191, 409–415. [Google Scholar] [CrossRef]
- Mortelé, K.J.; Oliva, M.R.; Ondategui, S.; Ros, P.R.; Silverman, S.G. Universal use of nonionic iodinated contrast medium for CT: Evaluation of safety in a large urban teaching hospital. AJR Am. J. Roentgenol. 2005, 184, 31–34. [Google Scholar] [CrossRef]
- Goksel, O.; Aydin, O.; Atasoy, C.; Akyar, S.; Demirel, Y.S.; Misirligil, Z.; Bavbek, S. Hypersensitivity Reactions to Contrast Media: Prevalence, Risk Factors and the Role of Skin Tests in Diagnosis—A Cross-Sectional Survey. Int. Arch. Allergy Immunol. 2011, 155, 297–305. [Google Scholar] [CrossRef]
- Ho, J.; Kingston, R.J.; Young, N.; Katelaris, C.H.; Sindhusake, D. Immediate hypersensitivity reactions to IV non-ionic iodinated contrast in computed tomography. Asia Pac. Allergy 2012, 2, 242–247. [Google Scholar] [CrossRef][Green Version]
- Iordache, A.M.; Docea, A.O.; Buga, A.M.; Mitrut, R.; Albulescu, D.; Zlatian, O.; Ianosi, S.; Ianosi, G.; Neagoe, D.; Sifaki, M.; et al. The incidence of skin lesions in contrast media-induced chemical hypersensitivity. Exp. Ther. Med. 2019, 17, 1113–1124. [Google Scholar] [CrossRef]
- Sodagari, F.; Mozaffary, A.; Wood, C.G.; Schmitz, B.; Miller, F.H.; Yaghmai, V. Reactions to Both Nonionic Iodinated and Gadolinium-Based Contrast Media: Incidence and Clinical Characteristics. Am. J. Roentgenol. 2018, 210, 715–719. [Google Scholar] [CrossRef]
- Kirchin, M.A.; Runge, V.M. Contrast agents for magnetic resonance imaging: Safety update. Top. Magn. Reson. Imaging 2003, 14, 426–435. [Google Scholar] [CrossRef]
- Katayama, H.; Yamaguchi, K.; Kozuka, T.; Takashima, T.; Seez, P.; Matsuura, K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990, 175, 621–628. [Google Scholar] [CrossRef]
- Stacul, F. Current iodinated contrast media. Eur. Radiol. 2001, 11, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.M.; Choi, N.K.; Lee, J.; Chang, Y.; Kim, Y.J.; Yang, B.R.; Jin, X.M.; Kim, J.Y.; Park, B.J. Comparison of the Safety of Seven Iodinated Contrast Media. J. Korean Med. Sci. 2013, 28, 1703. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.S.; Hunt, C.H.; Kolbe, A.B.; Schmitz, J.J.; Hartman, R.P.; Maddox, D.E.; Kallmes, D.F.; McDonald, R.J. Acute adverse events following gadolinium-based contrast agent administration: A single-center retrospective study of 281,945 injections. Radiology 2019, 292, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, A.H.; Zhao, Y.; Farooq, Z.; Prince, M.R. Immediate allergic reactions to gadolinium-based contrast agents: A systematic review and meta-analysis. Radiology 2018, 286, 471–482. [Google Scholar] [CrossRef]
- An, J.; Jung, H.; Kwon, O.Y.; Kang, Y.; Lee, J.H.; Won, H.K.; Song, W.J.; Kwon, H.S.; Cho, Y.S.; Moon, H.B.; et al. Differences in Adverse Reactions Among Iodinated Contrast Media: Analysis of the KAERS Database. J. Allergy Clin. Immunol. Pract. 2019, 7, 2205–2211. [Google Scholar] [CrossRef]
- Iyer, R.S.; Schopp, J.G.; Swanson, J.O.; Thapa, M.M.; Phillips, G.S. Safety essentials: Acute reactions to iodinated contrast media. Can. Assoc. Radiol. J. 2013, 64, 193–199. [Google Scholar] [CrossRef]
- Liu, J.; Gordin, V. Gadolinium Encephalopathy After Intrathecal Gadolinium Injection. Pain Physician 2010, 13, E321–E326. [Google Scholar] [CrossRef]
- Park, J.; Byun, I.H.; Park, K.H.; Lee, J.H.; Nam, E.J.; Park, J.W. Acute Respiratory Distress Syndrome after the Use of Gadolinium Contrast Media. Yonsei Med. J. 2015, 56, 1155. [Google Scholar] [CrossRef]
- Demirhan, A.; Tekelioglu, U.Y.; Akkaya, A.; Dagistan, E.; Ayhan, S.S.; Ozturk, S.; Yildiz, I.; Kocoglu, H. PP-358 magnetic resonance imaging contrast agent related pulmonary edema: A case report. Int. J. Cardiol. 2012, 155, S216. [Google Scholar] [CrossRef]
- Lee, Y.; Chung, T.Y.; Liu, H.C. A rare case of acute respiratory distress syndrome caused by use of gadolinium-based magnetic resonance imaging contrast media. Respirol. Case Rep. 2019, 7, 483. [Google Scholar] [CrossRef]
- İzmir, A.; Özen, N.; Yerli, H.; Uzun, B.; Pekcan, İ.; Yurteri, Y.; Osmanoğlu, S. Contrast media induced acute pulmonary edema during computed tomographic examination: A case report bilgisayarli tomografi çekiminde kontrast maddenin indüklediği pulmoner ödem: Olgu sunumu. İzmir Göğüs Hastan. Derg. 2016, 30, 59–62. [Google Scholar]
- Borish, L.; Matloff, S.M.; Findlay, S.R. Case report Radiographic contrast media-induced noncardiogenic pulmonary edema: Case report and review of the literature. J. Allergy Clin. Immunol. 1984, 74, 104–107. [Google Scholar] [CrossRef]
- Pincet, L.; Lecca, G. Acute pulmonary edema induced by non-ionic low-osmolar radiographic contrast media. Open Access Emerg. Med. 2018, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Kanal, E. Gadolinium based contrast agents (GBCA): Safety overview after 3 decades of clinical experience. Magn. Reson. Imaging 2016, 34, 1341–1345. [Google Scholar] [CrossRef]
- Voeltz, M.D.; Nelson, M.A.; McDaniel, M.C.; Manoukian, S.V. The Important Properties of Contrast Media: Focus on Viscosity—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17592180/ (accessed on 7 March 2022).
- Nakano, S.; Tsushima, Y.; Taketomi-Takahashi, A.; Higuchi, T.; Amanuma, M.; Oriuchi, N.; Endo, K. Hypertensive crisis due to contrast-enhanced computed tomography in a patient with malignant pheochromocytoma. Jpn. J. Radiol. 2011, 29, 449–451. [Google Scholar] [CrossRef]
- John, A.M.; Yadav, S. Effect of bolus administration of non-ionic radiopaque contrast media on blood pressure variation. Radiography 2019, 25, 346–348. [Google Scholar] [CrossRef]
- Beckett, K.R.; Moriarity, A.K.; Langer, J.M. Safe use of contrast media: What the radiologist needs to know. Radiographics 2015, 35, 1738–1750. [Google Scholar] [CrossRef]
- Balzer, J.O.; Loewe, C.; Davis, K.; Goyen, M.; Leiner, T.; Meaney, J.F.M.; Pöckler-Schöniger, C.; Schulte-Altedorneburg, G.; Tombach, B.; Vosshenrich, R.; et al. Safety of contrast-enhanced MR angiography employing gadobutrol 1.0 M as contrast material. Eur. Radiol. 2002, 13, 2067–2074. [Google Scholar] [CrossRef]
- Liss, P.; Hansell, P.; Carlsson, P.O.; Fasching, A.; Palm, F. Iodinated Contrast Media Decrease Renomedullary Blood Flow. Adv. Exp. Med. Biol. 2009, 645, 213–218. [Google Scholar] [CrossRef]
- Sook Hong, H.; Ho Kim, D.; Kyung Lee, H.; Chan Chung, M.; Lin Choi, D.; Hyang Kwon, K.; Jung Kim, K. Clinical application of intravascular administration of non-ionic, low osmolar contrast agent, ioversol(Optiray320) and its side effects comparison with meglumine iothalamate. J. Korean Radiol. Soc. 2016, 26, 1284–1290. [Google Scholar] [CrossRef][Green Version]
- Singh, J.; Daftary, A. Iodinated Contrast Media and Their Adverse Reactions. J. Nucl. Med. Technol. 2008, 36, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvan, V.; Sharma, S.; Singh, G.N. Adverse reactions to contrast media: An analysis of spontaneous reports in the database of the pharmacovigilance programme of India. Drug Saf. 2014, 37, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Morcos, S.K.; Thomsen, H.S. Adverse reactions to iodinated contrast media. Eur. Radiol. 2001, 11, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Takahashi, O.; Ueda, T.; Deshpande, G.A.; Arioka, H.; Fukui, T. Risk factors for adverse reactions from contrast agents for computed tomography. BMC Med. Inform. Decis. Mak. 2013, 13, 18. [Google Scholar] [CrossRef]
- Morcos, S.K.; Thomsen, H.S.; Webb, J.A.W. Prevention of generalized reactions to contrast media: A consensus report and guidelines. Eur. Radiol. 2001, 11, 1720–1728. [Google Scholar] [CrossRef]
- Bettmann, M.A.; Heeren, T.; Greenfield, A.; Goudey, C. Adverse events with radiographic contrast agents: Results of the SCVIR Contrast Agent Registry. Radiology 1997, 203, 611–620. [Google Scholar] [CrossRef]
- Lang, D.M.; Alpern, M.B.; Visintainer, P.F.; Smith, S.T. Increased risk for anaphylactoid reaction from contrast media in patients on β-adrenergic blockers or with asthma. Ann. Intern. Med. 1991, 115, 270–276. [Google Scholar] [CrossRef]
- Koenig, J.Q. Air pollution and asthma. J. Allergy Clin. Immunol. 1999, 104, 717–722. [Google Scholar] [CrossRef]
- Breysse, P.N.; Diette, G.B.; Matsui, E.C.; Butz, A.M.; Hansel, N.N.; McCormack, M.C. Indoor air pollution and asthma in children. Proc. Am. Thorac. Soc. 2010, 7, 102–106. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Esposito, S.; Tenconi, R.; Lelii, M.; Preti, V.; Nazzari, E.; Consolo, S.; Patria, M.F. Possible molecular mechanisms linking air pollution and asthma in children. BMC Pulm. Med. 2014, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Kariyanna, P.T.; Aurora, L.; Jayarangaiah, A.; Das, S.; Gonzalez, J.C.; Hegde, S.; McFarlane, I.M. Neurotoxicity Associated with Radiological Contrast Agents Used during Coronary Angiography: A Systematic Review. Am. J. Med. Case Rep. 2020, 8, 60. [Google Scholar] [CrossRef]
- Ruffmann, C.; Bogliun, G.; Beghi, E. Epileptogenic drugs: A systematic review. Expert Rev. Neurother. 2014, 6, 575–589. [Google Scholar] [CrossRef]
- Leong, S.; Fanning, N.F. Persistent neurological deficit from iodinated contrast encephalopathy following intracranial aneurysm coiling. A case report and review of the literature. Interv. Neuroradiol. 2012, 18, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, H.S.; Morcos, S.K. Radiographic contrast media. BJU Int. 2000, 86 (Suppl. S1), 1–10. [Google Scholar] [CrossRef] [PubMed]
- Junck, L.; Marshall, W.H. Neurotoxicity of radiological contrast agents. Ann. Neurol. 1983, 13, 469–484. [Google Scholar] [CrossRef]
- Donepudi, B.; Trottier, S. A Seizure and Hemiplegia following Contrast Exposure: Understanding Contrast-Induced Encephalopathy. Case Rep. Med. 2018, 2018, 9278526. [Google Scholar] [CrossRef]
- Mitsuyama, T.; Sato, S.; Ishii, A.; Kawamata, T. Contrast Medium-Induced Seizures and Prolonged Motor Weakness After Cerebral Angiography. Neurosurgery 2010, 67, E1460–E1463. [Google Scholar] [CrossRef]
- Law, S.; Panichpisal, K.; Demede, M.; John, S.; Marmur, J.D.; Nath, J.; Baird, A.E. Contrast-induced neurotoxicity following cardiac catheterization. Case Rep. Med. 2012, 2012, 267860. [Google Scholar] [CrossRef]
- Sadiq, M.A.; Al Habsi, M.S.; Nadar, S.K.; Shaikh, M.M.; Baomar, H.A. Transient contrast induced neurotoxicity after coronary angiography: A contrast re-challenge case. Pak. J. Med. Sci. 2020, 36, 1140. [Google Scholar] [CrossRef] [PubMed]
- Sansone, V.; Piazza, L.; Butera, G.; Meola, G.; Fontana, A. Contrast-Induced Seizures After Cardiac Catheterization in a 6-Year-Old Child. Pediatr. Neurol. 2007, 36, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Olchowy, C.; Cebulski, K.; Łasecki, M.; Chaber, R.; Olchowy, A.; Kałwak, K.; Zaleska-Dorobisz, U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—A systematic review. PLoS ONE 2017, 12, e0171704. [Google Scholar] [CrossRef] [PubMed]
- Hirshfeld, J.W. Cardiovascular effects of iodinated contrast agents. Am. J. Cardiol. 1990, 66, F9–F17. [Google Scholar] [CrossRef]
- Ten Dam, M.A.G.J.; Wetzels, J.F.M. Toxicity of contrast media: An update. Neth. J. Med. 2008, 66, 416–422. [Google Scholar]
- Pradubpongsa, P.; Dhana, N.; Jongjarearnprasert, K.; Janpanich, S.; Thongngarm, T. Adverse reactions to iodinated contrast media: Prevalence, risk factors and outcome-the results of a 3-year period. Asian Pac. J. Allergy Immunol. 2013, 31, 299. [Google Scholar] [CrossRef]
- Akre, B.T.; Dunkel, J.A.; Hustvedt, S.O.; Refsum, H. Acute cardiotoxicity of gadolinium-based contrast media: Findings in the isolated rat heart. Acad. Radiol. 1997, 4, 283–291. [Google Scholar] [CrossRef]


| Age Range | Number of Patients | The Percentage of Reports in the Database |
|---|---|---|
| <18 | 7 | 5.65% |
| 19–45 | 48 | 38.71% |
| 46–60 | 26 | 20.97% |
| 61–75 | 37 | 29.84% |
| 76–90 | 4 | 3.23% |
| Unknown age | 2 | 1.61% |
| Total | 124 | 100% |
| Specified Group (SOC) of ADRs | All Other Groups (SOCs) of ADRs | Total | |
|---|---|---|---|
| Contrast medium of interest | a | b | a + b |
| All other contrast media | c | d | c + d |
| Total | a + c | b + d | a + b + c + d |
| Contrast Media | Total Reports | ADR | ||
|---|---|---|---|---|
| N | % | N | % | |
| Iomeprol (Jomeron) | 44 | 35.48 | 91 | 32.16 |
| Iopromide (Ultravist) | 31 | 25.00 | 67 | 23.67 |
| Ioversol (Optivay) | 7 | 5.65 | 22 | 7.77 |
| Iodixanol (Visipaque) | 5 | 4.03 | 12 | 4.24 |
| Iohexol (Omnipaque) | 4 | 3.23 | 12 | 4.24 |
| Gadobutrol (Gadovist) | 15 | 12.10 | 38 | 13.43 |
| Gadobenic Acid (Multihance) | 10 | 8.06 | 29 | 10.25 |
| Gadoteric Acid (Dotarem) | 7 | 5.64 | 11 | 3.89 |
| Gadoteridol (Prohance) | 1 | 0.81 | 1 | 0.35 |
| Total | 124 | 100 | 283 | 100 |
| Contrast Media | System Organ Class | PRR | % of ADRs |
|---|---|---|---|
| Gadobutrol (Gadovist) | Skin and subcutaneous tissue disorders | 1.12 | 51.28% |
| Respiratory disorders | 0.37 | 14.29% | |
| Vascular disorders | 1.13 | 50.00% | |
| Gastrointestinal disorders | 1.63 | 75.00% | |
| Central & peripheral nervous system disorders | 1.68 | 60.00% | |
| Eye disorders | 2.23 | 100% | |
| Gadobenic Acid (Multihance) | Skin and subcutaneous tissue disorders | 0.93 | 38.46% |
| Respiratory disorders | 1.13 | 50.00% | |
| Vascular disorders | 0.97 | 41.67% | |
| Central & peripheral nervous system disorders | 0.93 | 40.00% | |
| Gadoteric Acid (Dotarem) | Skin and subcutaneous tissue disorders | 0.25 | 7.69% |
| Respiratory disorders | 0.85 | 35.71% | |
| Vascular disorders | 0.21 | 8.33% | |
| Gastrointestinal disorders | 0.60 | 25.00% | |
| Gadoteridol (Prohance) | Skin and subcutaneous tissue disorders | 0.09 | 2.56% |
| Contrast Media | System Organ Class | PRR | % of ADRs |
|---|---|---|---|
| Iomeprol (Iomeron) | Skin and subcutaneous tissue disorders | 1.03 | 47.89% |
| Respiratory disorders | 1.12 | 52.17% | |
| Vascular disorders | 0.52 | 23.08% | |
| Gastrointestinal disorders | 0.45 | 20.00% | |
| Central & peripheral nervous system disorders | 1.62 | 75.00% | |
| General disorders and administration site conditions | 0.25 | 11.11% | |
| Eye disorders | 2.17 | 100.00% * | |
| Cardiac disorders | 1.09 | 50.00% | |
| Iopromide (Ultravist) | Skin and subcutaneous tissue disorders | 1.02 | 35.21% |
| Respiratory disorders | 0.54 | 17.39% | |
| Vascular disorders | 1.32 | 46.15% | |
| Gastrointestinal disorders | 0.88 | 30.00% | |
| General disorders and administration site conditions | 1.28 | 44.44% | |
| Ioversol (Optivay) | Skin and subcutaneous tissue disorders | 0.72 | 6.34% |
| Respiratory disorders | 1.54 | 17.39% | |
| Vascular disorders | 2.03 | 23.08% | |
| Gastrointestinal disorders | 2.61 | 30.00% | |
| Central & peripheral nervous system disorders | 2.30 | 25.00% | |
| General disorders and administration site conditions | 1.05 | 11.11% | |
| Cardiac disorders | 4.56 | 50.00% | |
| Iodixanol (Visipaque) | Skin and subcutaneous tissue disorders | 0.96 | 5.63% |
| Vascular disorders | 1.25 | 7.69% | |
| Gastrointestinal disorders | 2.99 | 20.00% | |
| General disorders and administration site conditions | 1.78 | 11.11% | |
| Iohexol (Omnipaque) | Skin and subcutaneous tissue disorders | 0.88 | 4.93% |
| Respiratory disorders | 1.94 | 13.04% | |
| General disorders and administration site conditions | 3.30 | 22.22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauer, N.; Szlasa, W.; Jonderko, L.; Głowacka, K.; Karłowicz-Bodalska, K.; Wiela-Hojeńska, A. Contrast Media Adverse Drug Reactions in Highly Polluted Environment. Int. J. Environ. Res. Public Health 2022, 19, 7077. https://doi.org/10.3390/ijerph19127077
Sauer N, Szlasa W, Jonderko L, Głowacka K, Karłowicz-Bodalska K, Wiela-Hojeńska A. Contrast Media Adverse Drug Reactions in Highly Polluted Environment. International Journal of Environmental Research and Public Health. 2022; 19(12):7077. https://doi.org/10.3390/ijerph19127077
Chicago/Turabian StyleSauer, Natalia, Wojciech Szlasa, Laura Jonderko, Krystyna Głowacka, Katarzyna Karłowicz-Bodalska, and Anna Wiela-Hojeńska. 2022. "Contrast Media Adverse Drug Reactions in Highly Polluted Environment" International Journal of Environmental Research and Public Health 19, no. 12: 7077. https://doi.org/10.3390/ijerph19127077
APA StyleSauer, N., Szlasa, W., Jonderko, L., Głowacka, K., Karłowicz-Bodalska, K., & Wiela-Hojeńska, A. (2022). Contrast Media Adverse Drug Reactions in Highly Polluted Environment. International Journal of Environmental Research and Public Health, 19(12), 7077. https://doi.org/10.3390/ijerph19127077






