Contact Laxative Use and the Risk of Arteriovenous Fistula Maturation Failure in Patients Undergoing Hemodialysis: A Multi-Center Cohort Study
Abstract
:1. Introduction
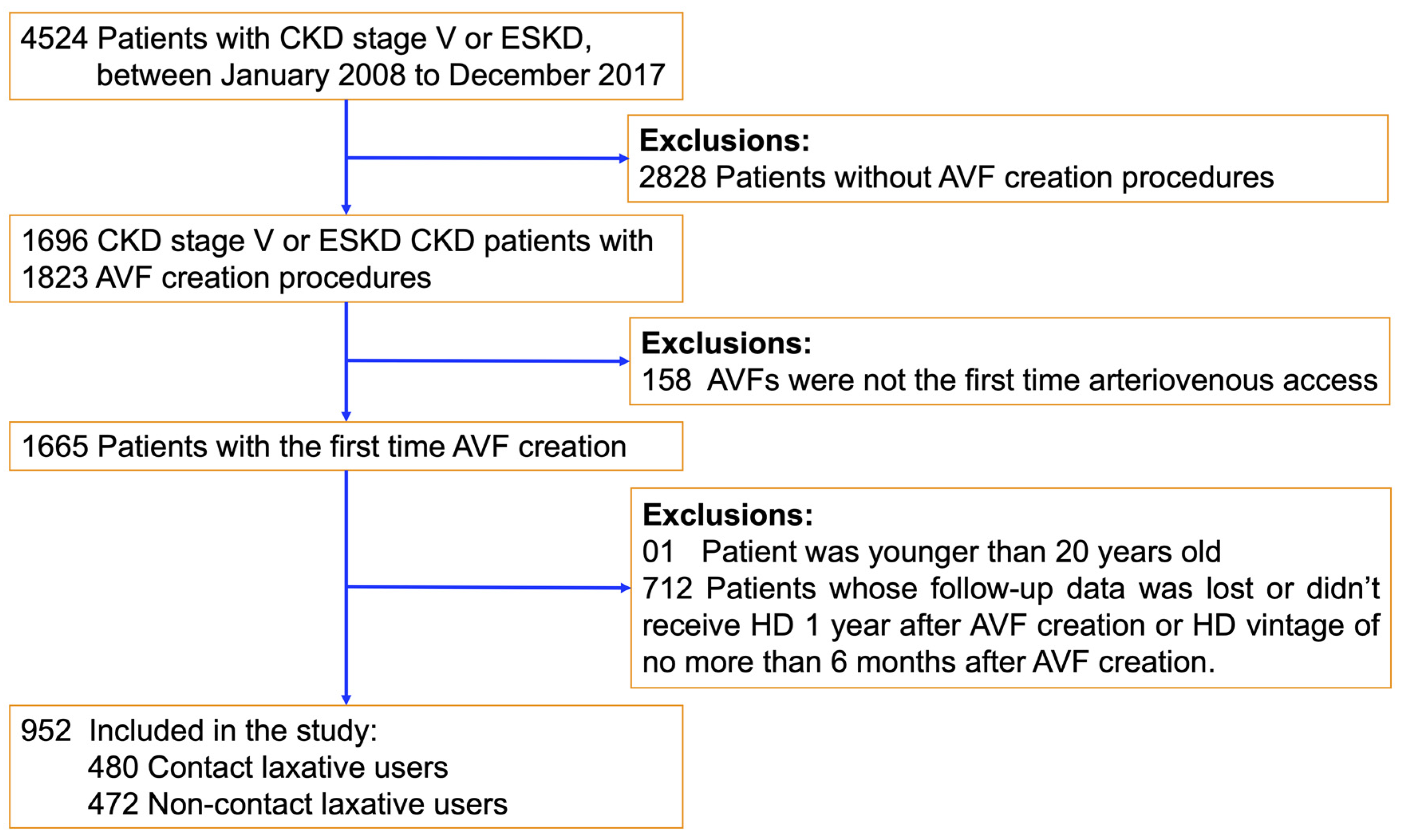
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Contact Laxative Exposures
2.4. Outcome Measurement
2.5. Measurement of Covariates
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. AVF Maturation Outcome in the Contact Laxative Users and Non-Users
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bharucha, A.E.; Pemberton, J.H.; Locke, G.R. American Gastroenterological Association technical review on constipation. Gastroenterology 2013, 144, 218–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avesani, C.M. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transpl. 2012, 27, 2430–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezani, A. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attaluri, A. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuvela, J. Gastrointestinal symptoms in patients receiving dialysis: A systematic review. Nephrology 2018, 23, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumida, K. Laxative use in patients with advanced chronic kidney disease transitioning to dialysis. Nephrol. Dial. Transpl. 2021, 36, 2018–2026. [Google Scholar] [CrossRef]
- Krogh, K.; Chiarioni, G.; Whitehead, W. Management of chronic constipation in adults. United Eur. Gastroenterol. J. 2017, 5, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Heher, E.C. Adverse renal and metabolic effects associated with oral sodium phosphate bowel preparation. Clin. J. Am. Soc. Nephrol. 2008, 3, 1494–1503. [Google Scholar] [CrossRef] [Green Version]
- Jung, G.J. Severe hypermagnesemia causing quadriparesis in a CAPD patient. Perit. Dial. Int. 2008, 28, 206. [Google Scholar] [CrossRef]
- Kubota, Y.; Iso, H.; Tamakoshi, A. Bowel Movement Frequency, Laxative Use, and Mortality From Coronary Heart Disease and Stroke Among Japanese Men and Women: The Japan Collaborative Cohort (JACC) Study. J. Epidemiol. 2016, 26, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, L.K. The Associations of Diuretics and Laxatives Use with Cardiovascular Mortality. An Individual Patient-Data Meta-analysis of Two Large Cohort Studies. Cardiovasc. Drugs Ther. 2019, 33, 567–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, Y. Laxative use and mortality in patients on haemodialysis: A prospective cohort study. BMC Nephrol. 2021, 22, 363. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel. Dis. 2009, 15, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Capasso, F. Laxatives and the production of autacoids by rat colon. J. Pharm. Pharmacol. 1986, 38, 627–629. [Google Scholar] [CrossRef]
- Oster, J.R.; Materson, B.J.; Rogers, A.I. Laxative abuse syndrome. Am. J. Gastroenterol. 1980, 74, 451–458. [Google Scholar]
- Ozeki, T. The Type of Vascular Access and the Incidence of Mortality in Japanese Dialysis Patients. Intern. Med. 2017, 56, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Ravani, P. Associations between hemodialysis access type and clinical outcomes: A systematic review. J. Am. Soc. Nephrol. 2013, 24, 465–473. [Google Scholar] [CrossRef]
- Wilmink, T. Natural History of Common Autologous Arteriovenous Fistulae: Consequences for Planning of Dialysis. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Al-Jaishi, A.A. Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 63, 464–478. [Google Scholar] [CrossRef]
- Smith, G.E.; Gohil, R.; Chetter, I.C. Factors affecting the patency of arteriovenous fistulas for dialysis access. J. Vasc. Surg. 2012, 55, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.H. Antiplatelet agents maintain arteriovenous fistula and graft function in patients receiving hemodialysis: A nationwide case-control study. PLoS ONE 2018, 13, e0206011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, M. Randomized clinical trial of the use of glyceryl trinitrate patches to aid arteriovenous fistula maturation. Br. J. Surg. 2016, 103, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Wärme, A. The association of erythropoietin-stimulating agents and increased risk for AV-fistula dysfunction in hemodialysis patients. A retrospective analysis. BMC Nephrol. 2021, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef] [Green Version]
- Quan, H. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Portalatin, M.; Winstead, N. Medical management of constipation. Clin. Colon Rectal Surg. 2012, 25, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Ford, C.A.; Suares, N.C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut 2011, 60, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Brattich, M. Vascular access thrombosis: Etiology and prevention. ANNA J. 1999, 26, 537–540. [Google Scholar]
- Pandey, S. The effects of preoperative blood pressure on early failure rate of distal arteriovenous fistulas for hemodialysis access. Hemodial. Int. 2019, 23, 314–318. [Google Scholar] [CrossRef]
- Dufour, P.; Gendre, P. Ultrastructure of mouse intestinal mucosa and changes observed after long term anthraquinone administration. Gut 1984, 25, 1358–1363. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z. Anthraquinone Laxative-Altered Gut Microbiota Induces Colonic Mucosal Barrier Dysfunction for Colorectal Cancer Progression; Research Square: Durham, NY, USA, 2020. [Google Scholar]
- Ponziani, F.R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.L.; Backhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C. Early and late fistula failure. Clin. Nephrol. 2008, 69, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O. The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J. Korean Med. Sci. 2006, 21, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Ashton, J.H. Serotonin as a mediator of cyclic flow variations in stenosed canine coronary arteries. Circulation 1986, 73, 572–578. [Google Scholar] [CrossRef] [Green Version]
- Nemecek, G.M. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc. Natl. Acad. Sci. USA 1986, 83, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Padron, R.I. Intimal Hyperplasia and Arteriovenous Fistula Failure: Looking Beyond Size Differences. Kidney360 2021, 2, 1360–1372. [Google Scholar] [CrossRef]
- Yue, H. CD36 Enhances Vascular Smooth Muscle Cell Proliferation and Development of Neointimal Hyperplasia. Arter. Thromb. Vasc. Biol. 2019, 39, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Janmaat, M.L. Erythropoietin accelerates smooth muscle cell-rich vascular lesion formation in mice through endothelial cell activation involving enhanced PDGF-BB release. Blood 2010, 115, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.Y. Administration of a High-Dose Erythropoietin-Stimulating Agent in Hemodialysis Patients is Associated with Late Arteriovenous Fistula Failure. Yonsei Med. J. 2017, 58, 793–799. [Google Scholar] [CrossRef]
- Fragakis, A. Association between Drug Usage and Constipation in the Elderly Population of Greater Western Sydney Australia. Int. J. Environ. Res. Public Health 2018, 15, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | Overall | Contact Laxative Group (n = 480) | Non-Contact Laxative Group (n = 472) | p-Value a |
|---|---|---|---|---|
| Age, n (%) | <0.0001 | |||
| Age < 65 yr. | 492 (51.7) | 217 (45.2) | 275 (58.3) | |
| Age ≥ 65 yr. | 460 (48.3) | 263 (54.8) | 197 (41.7) | |
| Mean (SD) | 63.6 (13.4) | 66.4 (12.9) | 60.8 (13.4) | <0.0001 |
| Sex, n (%) | 0.163 | |||
| Female | 364 (38.2) | 194 (40.4) | 170 (36.0) | |
| Male | 588 (61.8) | 286 (59.6) | 302 (64.0) | |
| The timing of AVF creation, n (%) | 0.002 | |||
| Before HD | 228 (23.9) | 95 (19.8) | 133 (28.2) | |
| After HD | 724 (76.1) | 385 (80.2) | 339 (71.8) | |
| History of PD, n (%) | 0.082 | |||
| No | 897 (94.2) | 446 (92.9) | 451 (95.6) | |
| Yes | 55 (5.8) | 34 (7.1) | 21 (4.4) | |
| History of the long-term catheter, n (%) | 0.130 | |||
| No | 660 (69.3) | 322(67.1) | 338 (71.6) | |
| Yes | 292 (30.7) | 158 (32.9) | 134 (28.4) | |
| Age-unadjusted CCI scores, n (%) b | <0.0001 | |||
| CCI < 3 | 276 (29.0) | 108 (22.5) | 168 (35.6) | |
| CCI ≥ 3 | 676 (71.0) | 372 (77.5) | 304 (64.4) | |
| Mean (SD) | 3.37 (1.34) | 3.63 (1.44) | 3.10 (1.17) | <0.0001 |
| Comorbidities, n (%) | ||||
| Septicemia | 83 (8.7) | 56 (11.7) | 27 (5.7) | 0.001 |
| Malignant neoplasms | 65 (6.8) | 39 (8.1) | 26 (5.5) | 0.110 |
| Diabetes mellitus | 578 (60.7) | 325 (67.7) | 253 (53.6) | <0.0001 |
| Disorders of lipid metabolism | 280 (29.4) | 150 (31.3) | 130 (27.5) | 0.209 |
| Hypertension | 767 (80.6) | 401 (83.5) | 366 (77.5) | 0.019 |
| Ischemic heart disease | 299 (31.4) | 166 (34.6) | 133 (28.2) | 0.033 |
| Cardiac dysrhythmias | 111 (11.7) | 59 (12.3) | 52 (11.0) | 0.540 |
| Congestive heart failure | 326 (34.2) | 197 (41.0) | 129 (27.3) | <0.0001 |
| Cerebral vascular disease | 114 (12.0) | 75 (15.6) | 39 (8.3) | <0.0001 |
| Peripheral vascular disease | 42 (4.4) | 28 (5.8) | 14 (3.0) | 0.031 |
| Chronic pulmonary disease | 91 (9.6) | 55 (11.5) | 36 (7.6) | 0.044 |
| Liver diseases | 54 (5.7) | 29 (6.0) | 25 (5.3) | 0.619 |
| Peptic ulcer disease | 160 (16.8) | 96 (20.0) | 64 (13.6) | 0.008 |
| Anxiety and depression | 52 (5.5) | 35 (7.3) | 17 (3.6) | 0.012 |
| Laboratory data, Mean (SD) | ||||
| HGB (g/dL) | 9.48 (1.09) | 9.46 (1.07) | 9.50 (1.11) | 0.561 |
| WBC (103/uL) | 7.43 (2.35) | 7.56 (2.31) | 7.29 (2.38) | 0.077 |
| PLT (103/uL) | 187.8 (63.1) | 187.4 (62.8) | 188.1 (63.5) | 0.875 |
| BUN (mg/dL) | 79.0 (26.5) | 78.1 (26.22) | 79.9 (26.7) | 0.318 |
| Creatinine (mg/dL) | 9.10 (2.96) | 8.62 (2.76) | 9.59 (3.08) | <0.0001 |
| Ca (mg/dL) | 8.33 (0.77) | 8.36 (0.79) | 8.30 (0.75) | 0.169 |
| P (mg/dL) | 5.48 (1.47) | 5.32 (1.43) | 5.64 (1.50) | 0.001 |
| Na (mmol/L) | 136.3 (3.12) | 136.2 (3.22) | 136.4 (3.01) | 0.257 |
| K (mmol/L) | 4.47 (0.62) | 4.38 (0.62) | 4.55 (0.60) | <0.0001 |
| Medications, n (%) | ||||
| Antiplatelets | 419 (44.0) | 226 (47.1) | 193 (40.9) | 0.054 |
| ESAs | 783 (82.5) | 389 (81.0) | 396 (83.9) | 0.247 |
| Organic nitrates | 318 (33.4) | 170 (35.4) | 148 (31.4) | 0.184 |
| Beta blocking agents | 546 (57.4) | 269 (56.0) | 277 (58.7) | 0.409 |
| Calcium channel blockers | 643 (67.5) | 323 (67.3) | 320 (67.3) | 0.868 |
| ACE inhibitors and ARBs | 368 (38.7) | 190 (39.6) | 178 (37.7) | 0.553 |
| Statins | 284 (29.8) | 148 (30.8) | 136 (28.8) | 0.496 |
| Loop diuretics | 647 (68.0) | 347 (72.3) | 300 (63.6) | 0.004 |
| AVF Maturation Failure, n (%) | AVF Maturation Success, n (%) | Adjusted OR (95% CI) a | p-Value | |
|---|---|---|---|---|
| Contact laxatives (aDD, mg) | ||||
| Non-users (ref.) | 88 (18.6) | 384 (81.4) | 1.00 | - |
| aDD < 15 | 60 (26.0) | 171 (74.0) | 1.36 (0.91–2.03) | 0.135 |
| aDD ≥ 15 | 84 (33.7) | 165 (66.3) | 1.91 (1.31–2.80) | 0.001 |
| Contact laxatives (cTD, days) | ||||
| Non-users (ref.) | 88 (18.6) | 384 (81.4) | 1.00 | - |
| cTD < 60 | 31 (23.1) | 103 (76.9) | 1.13 (0.69–1.86) | 0.615 |
| 60 ≤ cTD < 90 | 33 (30.6) | 75 (69.4) | 1.80 (1.09–2.97) | 0.021 |
| cTD ≥ 90 | 80 (33.6) | 158 (66.4) | 1.89 (1.28–2.80) | 0.001 |
| Contact laxatives (aDD, mg; and cTD, days) | ||||
| Non-users (ref.) | 88 (18.6) | 384 (81.4) | 1.00 | |
| aDD < 15 and cTD < 90 | 29 (23.8) | 93 (76.2) | 1.30 (0.79–2.15) | 0.297 |
| aDD < 15 and cTD ≥ 90 | 31 (28.4) | 78 (71.6) | 1.42 (0.85–2.39) | 0.182 |
| aDD ≥ 15 and cTD < 90 | 35 (29.2) | 85 (70.8) | 1.51 (0.92–2.46) | 0.101 |
| aDD ≥ 15 and cTD ≥ 90 | 49 (38.0) | 80 (62.0) | 2.38 (1.50–3.79) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Anh, T.; Nguyen, P.-A.; Duong, A.; Chiu, I.-J.; Chou, C.-L.; Ko, Y.-C.; Chang, T.-H.; Huang, C.-W.; Wu, M.-S.; Liao, C.-T.; et al. Contact Laxative Use and the Risk of Arteriovenous Fistula Maturation Failure in Patients Undergoing Hemodialysis: A Multi-Center Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 6842. https://doi.org/10.3390/ijerph19116842
Hoang Anh T, Nguyen P-A, Duong A, Chiu I-J, Chou C-L, Ko Y-C, Chang T-H, Huang C-W, Wu M-S, Liao C-T, et al. Contact Laxative Use and the Risk of Arteriovenous Fistula Maturation Failure in Patients Undergoing Hemodialysis: A Multi-Center Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(11):6842. https://doi.org/10.3390/ijerph19116842
Chicago/Turabian StyleHoang Anh, Trung, Phung-Anh Nguyen, Anh Duong, I-Jen Chiu, Chu-Lin Chou, Yu-Chen Ko, Tzu-Hao Chang, Chih-Wei Huang, Mai-Szu Wu, Chia-Te Liao, and et al. 2022. "Contact Laxative Use and the Risk of Arteriovenous Fistula Maturation Failure in Patients Undergoing Hemodialysis: A Multi-Center Cohort Study" International Journal of Environmental Research and Public Health 19, no. 11: 6842. https://doi.org/10.3390/ijerph19116842
APA StyleHoang Anh, T., Nguyen, P.-A., Duong, A., Chiu, I.-J., Chou, C.-L., Ko, Y.-C., Chang, T.-H., Huang, C.-W., Wu, M.-S., Liao, C.-T., & Hsu, Y.-H. (2022). Contact Laxative Use and the Risk of Arteriovenous Fistula Maturation Failure in Patients Undergoing Hemodialysis: A Multi-Center Cohort Study. International Journal of Environmental Research and Public Health, 19(11), 6842. https://doi.org/10.3390/ijerph19116842








