A Systematic Review and Meta-Analysis of Malaria Test Positivity Outcomes and Programme Interventions in Low Transmission Settings in Southern Africa, 2000–2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
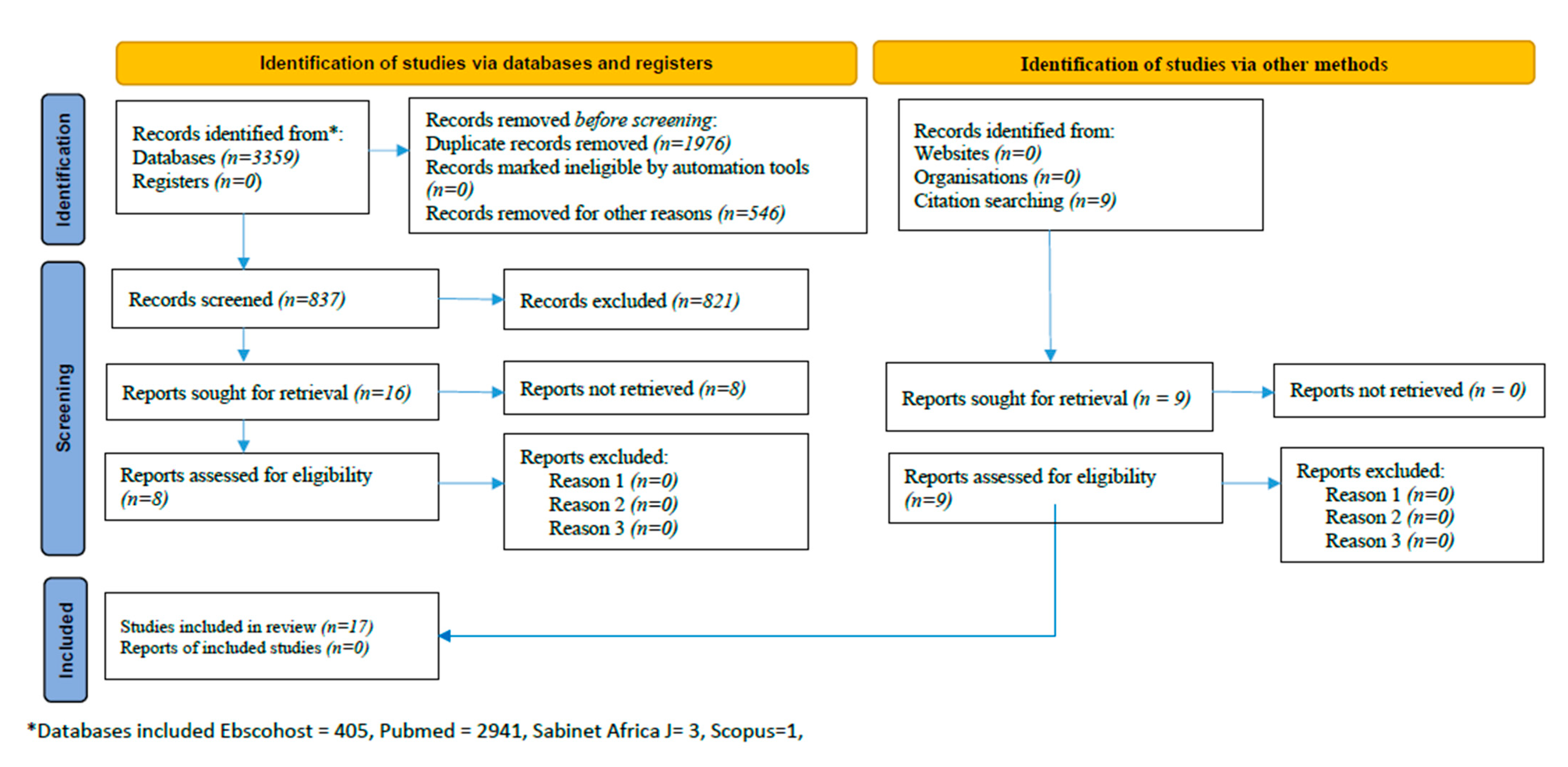
2.2. Search Strategy and Article Suitability
2.3. Data Extraction and Quality Assessment
2.4. Statistical Data Analyses
2.5. Results
2.6. Descriptive Results of Eligible Studies
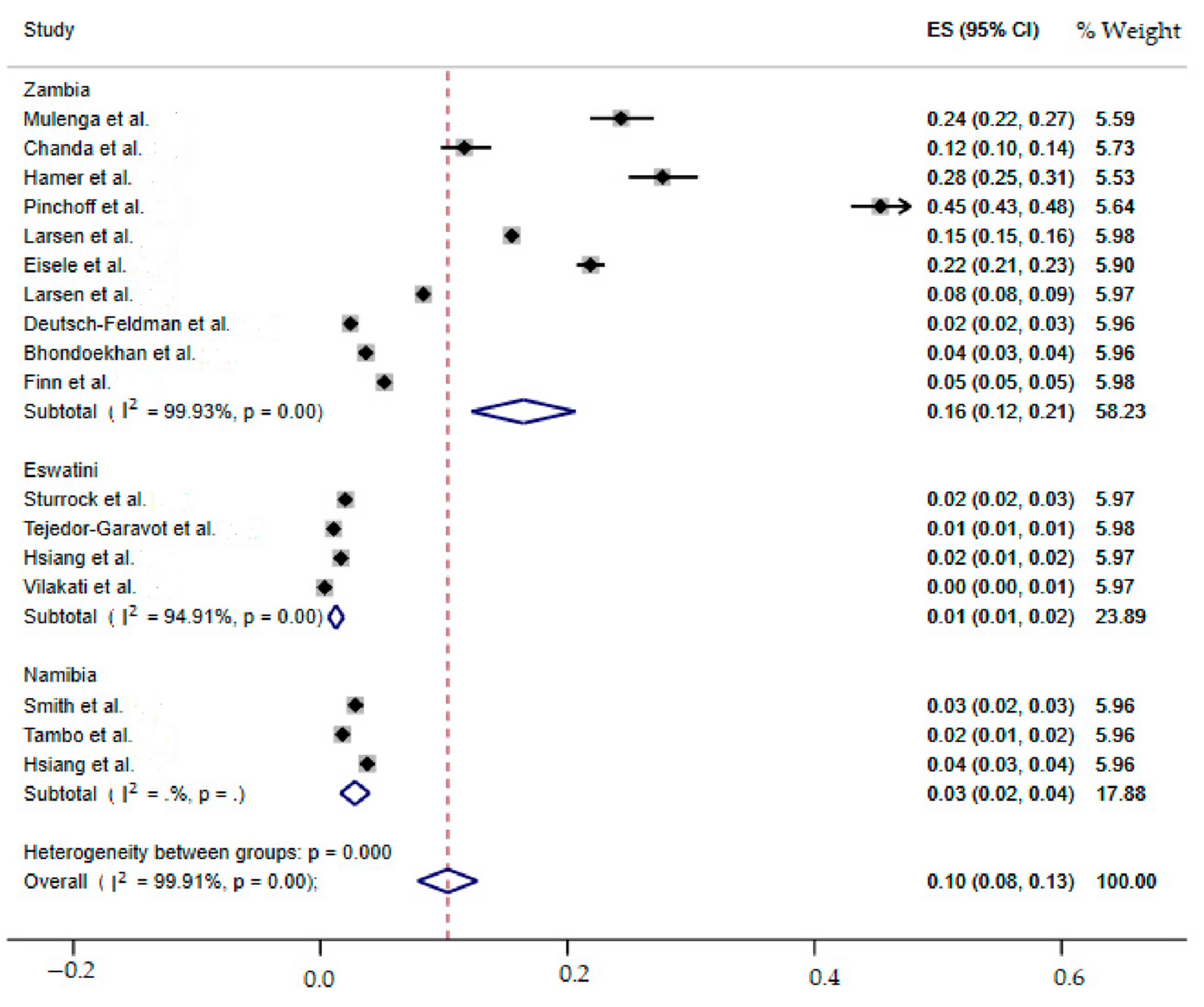
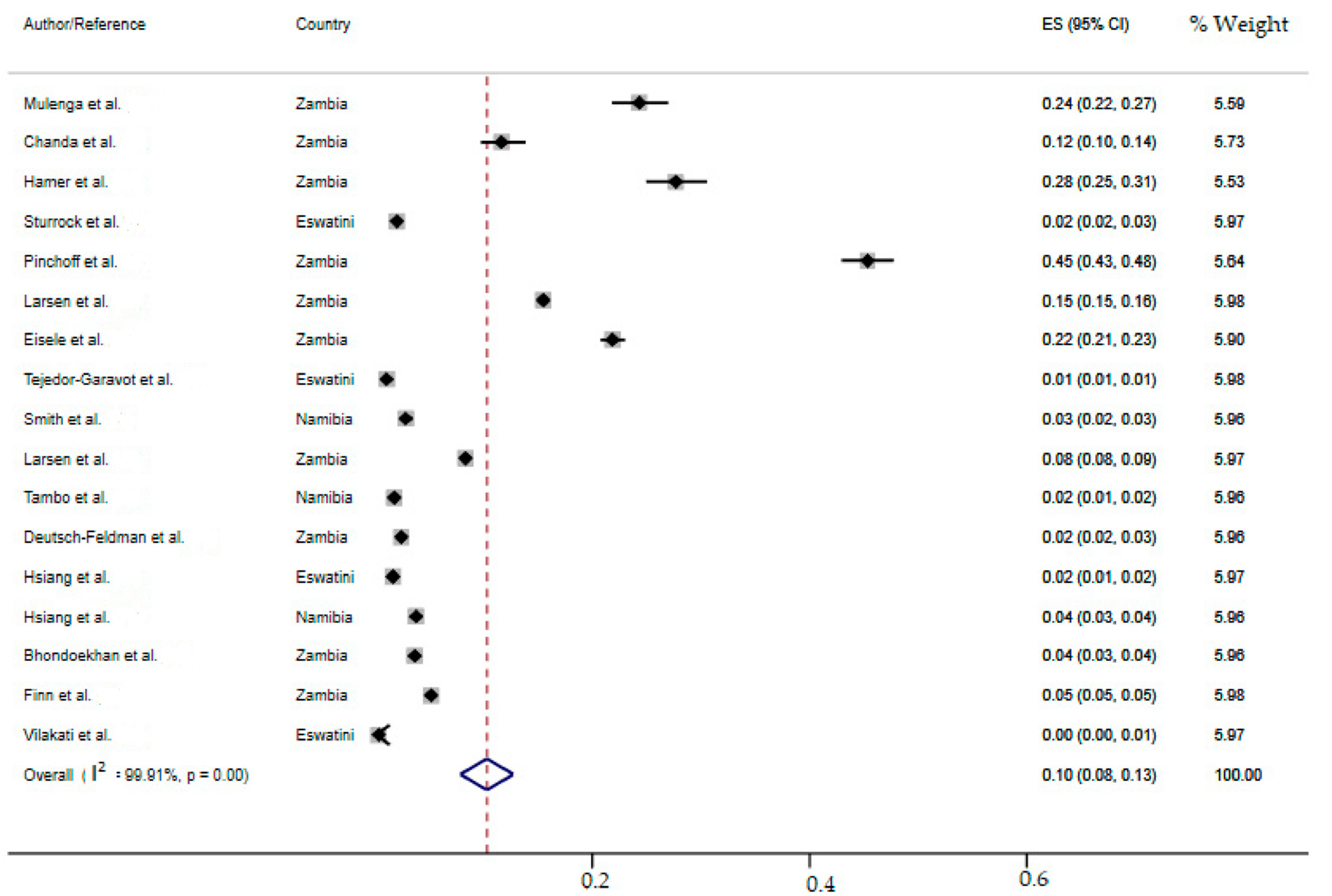
2.7. Meta-Analysis of the Overall Incidence of Malaria Test Positivity in Southern African Countries
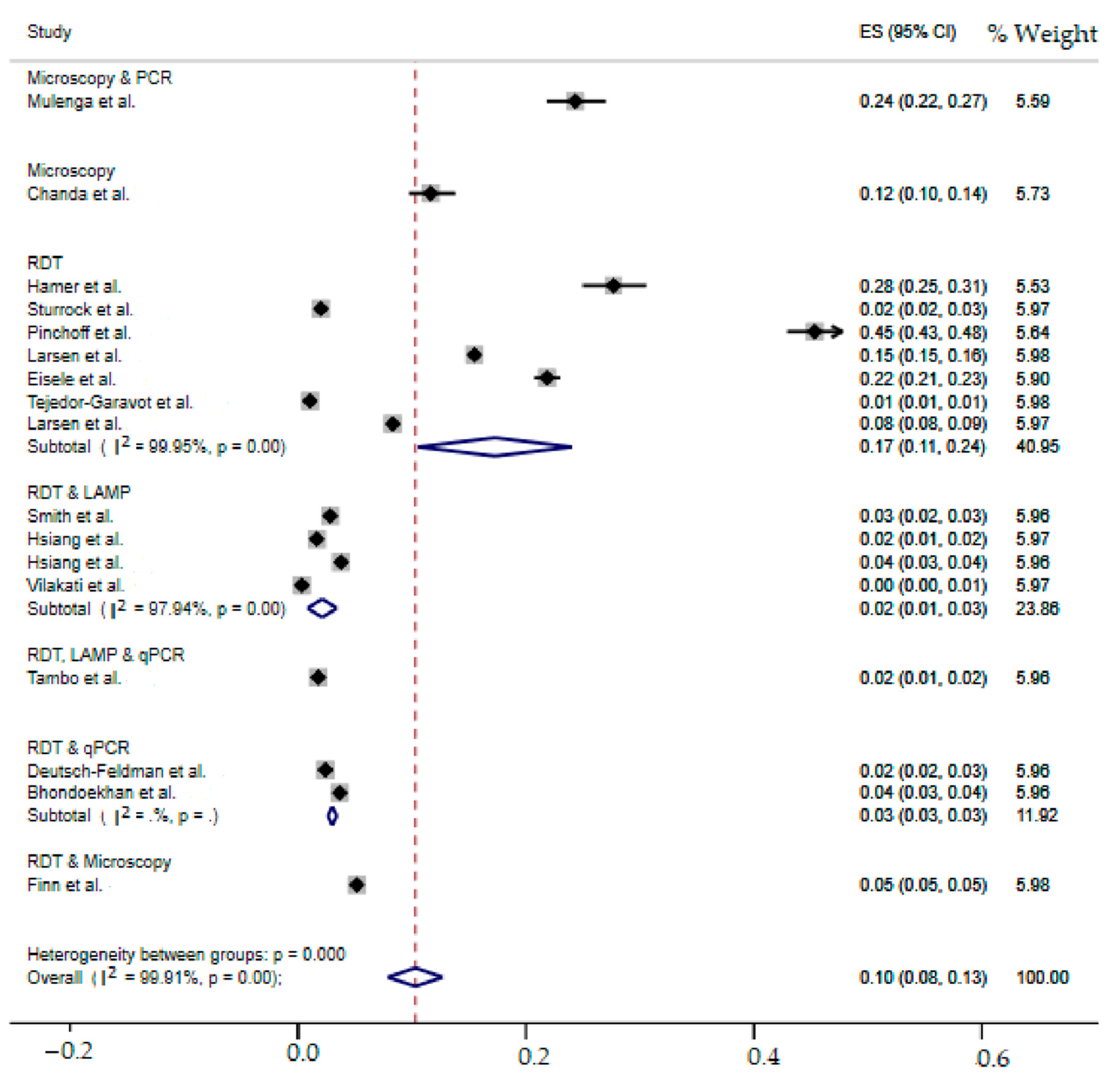
2.8. SubgroupMeta-Analysis
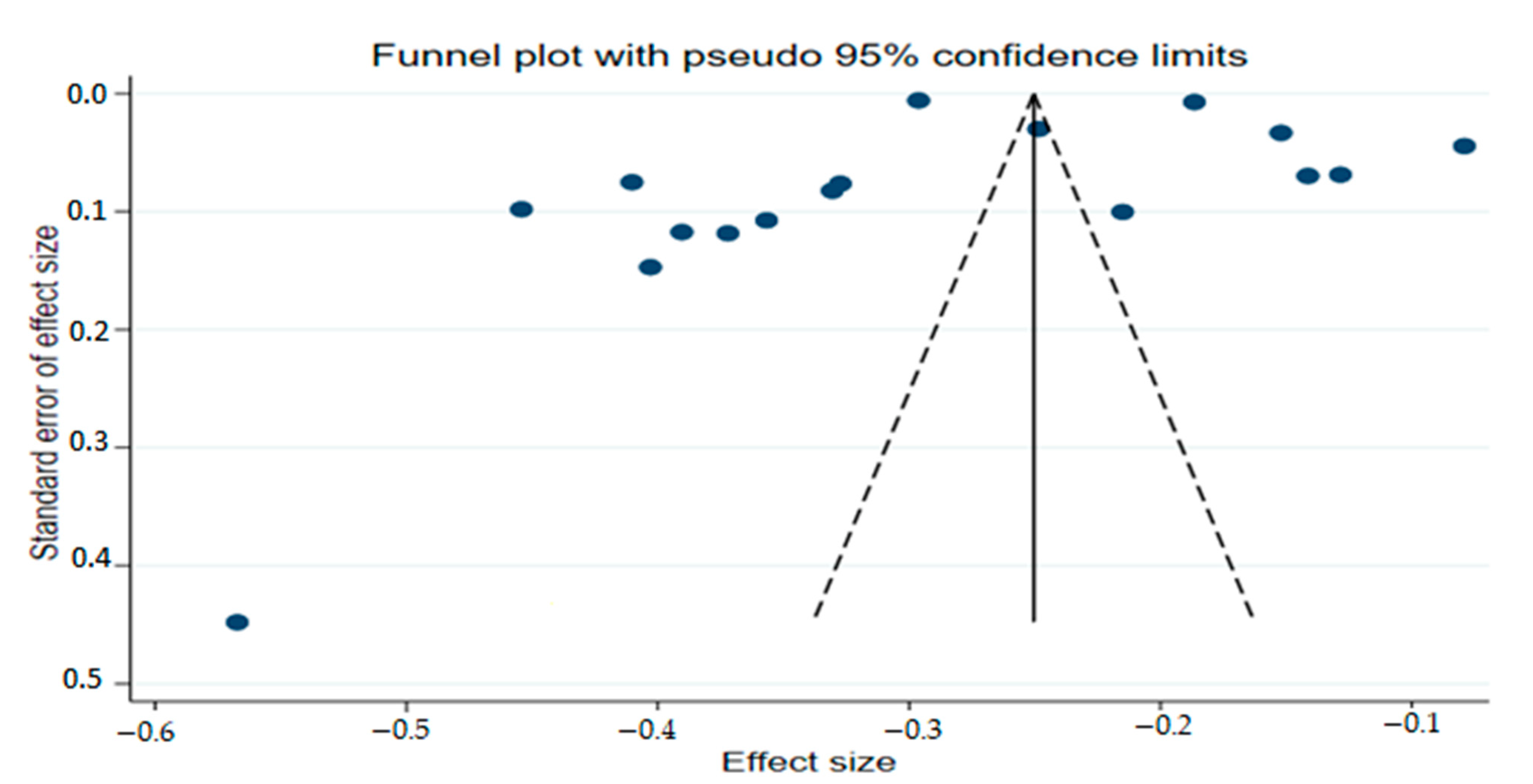
2.9. PublicationBias Assessment
2.10. Meta-Regression Analysis
3. Discussion
4. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barber, B.E.; Rajahram, G.S.; Grigg, M.J.; William, T.; Anstey, N.M. World Malaria Report: Time to acknowledge Plasmodium knowlesi malaria. Malar. J. 2017, 16, 135. [Google Scholar] [CrossRef]
- World Health Organization; UNICEF. Global Vector Control Response 2017–2030. 2017. Available online: https://www.who.int/publications/i/item/9789241512978 (accessed on 8 October 2020).
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases 2015; World Health Organization: Geneva, Switzerland, 2015; Volume 3. [Google Scholar]
- Snow, R.W.; Guerra, C.A.; Noor, A.M.; Myint, H.Y.; Hay, S.I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. [Google Scholar] [CrossRef]
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasites Vectors 2010, 3, 5. [Google Scholar] [CrossRef]
- Nankabirwa, J.I.; Yeka, A.; Arinaitwe, E.; Kigozi, R.; Drakeley, C.; Kamya, M.R.; Greenhouse, B.; Rosenthal, P.J.; Dorsey, G.; Staedke, S.G. Estimating malaria parasite prevalence from community surveys in Uganda: A comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar. J. 2015, 14, 528. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, K.; Barik, T.K.; Reddy, B.N.; Sharma, P.; Dash, A.P. Malaria vector control: From past to future. Parasitol. Res. 2011, 108, 757–779. [Google Scholar] [CrossRef]
- Derua, Y.A.; Kweka, E.J.; Kisinza, W.N.; Githeko, A.K.; Mosha, F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: A review of their effectiveness and operational feasibility. Parasites Vectors 2019, 12, 426. [Google Scholar] [CrossRef]
- Meyrowitsch, D.W.; Pedersen, E.M.; Alifrangis, M.; Scheike, T.H.; Malecela, M.N.; Magesa, S.M.; Derua, Y.A.; Rwegoshora, R.T.; Michael, E.; Simonsen, P.E. Is the current decline in malaria burden in sub-Saharan Africa due to a decrease in vector population? Malar. J. 2011, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Ndo, C.; Antonio-Nkondjio, C.; Cohuet, A.; Ayala, D.; Kengne, P.; Morlais, I.; Awono-Ambene, P.H.; Couret, D.; Ngassam, P.; Fontenille, D.; et al. Population genetic structure of the malaria vector Anopheles nili in sub-Saharan Africa. Malar. J. 2010, 9, 161. [Google Scholar] [CrossRef]
- World Malaria Report 2021; Licence: CCBY-NC-SA3.0IGO; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 19 December 2021).
- Wangdi, K.; Furuya-Kanamori, L.; Clark, J.; Barendregt, J.J.; Gatton, M.L.; Banwell, C.; Kelly, G.C.; Doi, S.A.; Clements, A.C. Comparative effectiveness of malaria prevention measures: A systematic review and network meta-analysis. Parasites Vectors 2018, 11, 210. [Google Scholar] [CrossRef]
- World Health Organisation. World Malaria Report 2011. Available online: http://www.who.int/ (accessed on 8 October 2020).
- Hamel, M.J.; Otieno, P.; Bayoh, N.; Kariuki, S.; Were, V.; Marwanga, D.; Laserson, K.F.; Williamson, J.; Slutsker, L.; Gimnig, J. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am. J. Trop. Med. Hyg. 2011, 85, 1080. [Google Scholar] [CrossRef] [PubMed]
- Okumu, F.O.; Moore, S.J. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: A review of possible outcomes and an outline of suggestions for the future. Malar. J. 2011, 10, 208. [Google Scholar] [CrossRef]
- Fullman, N.; Burstein, R.; Lim, S.S.; Medlin, C.; Gakidou, E. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar. J. 2013, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2012; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/malaria/publications/world_malaria_report_2012/report/en/ (accessed on 8 October 2020).
- World Health Organisation. Malaria Surveillance, Monitoring & Evaluation: A Reference Manual; Licence: CC BY-NC-SA3.0IGO; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/272284/9789241565578-eng.pdf?ua=1 (accessed on 8 October 2020).
- Larsen, D.A.; Chisha, Z.; Winters, B.; Mwanza, M.; Kamuliwo, M.; Mbwili, C.; Hawela, M.; Hamainza, B.; Chirwa, J.; Craig, A.S.; et al. Malaria surveillance in low-transmission areas of Zambia using reactive case detection. Malar. J. 2015, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Littrell, M.; Sow, G.D.; Ngom, A.; Ba, M.; Mboup, B.M.; Dieye, Y.; Mutombo, B.; Earle, D.; Steketee, R.W. Case investigation and reactive case detection for malaria elimination in northern Senegal. Malar. J. 2013, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, E.K.; Afrane, Y.A.; Machani, M.G.; Chebore, W.; Lawson, B.W.; Atieli, H.; Kariuki, S.; Lee, M.-C.; Koepfli, C.; Zhou, G.; et al. Reactive case detection of Plasmodium falciparum in western Kenya highlands: Effective in identifying additional cases, yet the limited effect on transmission. Malar. J. 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Bansil, P.; Yeshiwondim, A.K.; Guinovart, C.; Serda, B.; Scott, C.; Tesfay, B.H.; Agmas, A.; Bezabih, B.; Zeleke, M.T.; Guesses, G.S.; et al. Malaria case investigation with reactive focal testing and treatment: Operational feasibility and lessons learned from low and moderate transmission areas in Amhara Region, Ethiopia. Malar. J. 2018, 17, 449. [Google Scholar] [CrossRef]
- Gerardin, J.; Bever, C.A.; Bridenbecker, D.; Hamainza, B.; Silumbe, K.; Miller, J.M.; Eisele, T.P.; Eckhoff, P.A.; Wenger, E.A. Effectiveness of reactive case detection for malaria elimination in three archetypical transmission settings: A modelling study. Malar. J. 2017, 16, 248. [Google Scholar] [CrossRef]
- Southern Africa Development Council Malaria Report. 2013. Available online: https://www.sadc.int/files/6214/1890/8290/000_14_SADC___Malaria_Report_2012.pdf (accessed on 8 October 2020).
- South Africa National Department of Health. Malaria Elimination Strategic Plan for South Africa 2019–2023. 2019. Available online: www.health.gov.za (accessed on 8 October 2020).
- Elimination-8. Available online: https://malariaelimination8.org/ (accessed on 8 October 2020).
- Hansen, K.S.; Ndyomugyenyi, R.; Magnussen, P.; Lal, S.; Clarke, S.E. Cost-effectiveness analysis of malaria rapid diagnostic tests for appropriate treatment of malaria at the community level in Uganda. Health Policy Plan. 2017, 32, 676–689. [Google Scholar] [CrossRef][Green Version]
- Stresman, G.; Whittaker, C.; Slater, H.C.; Bousema, T.; Cook, J. Quantifying Plasmodium falciparum infections clustering within households to inform household-based intervention strategies for malaria control programs: An observational study and meta-analysis from 41 malaria-endemic countries. PLoS Med. 2020, 17, e1003370. [Google Scholar] [CrossRef]
- Hetzel, M.W.; Chitnis, N. Reducing malaria transmission with reactive focal interventions. Lancet 2020, 395, 1317–1319. Available online: www.thelancet.com (accessed on 31 December 2021). [CrossRef]
- Thiam, S.; Thwing, J.; Diallo, I.; Fall, F.B.; Diouf, M.B.; Perry, R.; Ndiop, M.; Diouf, M.L.; Cisse, M.M.; Diaw, M.M.; et al. Scale-up of home-based management of malaria based on rapid diagnostic tests and artemisinin-based combination therapy in a resource-poor country: Results in Senegal. Malar. J. 2012, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.A.; Jennings, L.; Chinyama, M.; Masaninga, F.; Mulholland, K.; Bell, D.R. Improving community health worker use of malaria rapid diagnostic tests in Zambia: Package instructions, job aid and job aid-plus-training. Malar. J. 2008, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Boyce, R.M.; Reyes, R.; Matte, M.; Ntaro, M.; Mulogo, E.; Lin, F.-C.; Siedner, M.J. Practical Implications of the Non-Linear Relationship between the Test Positivity Rate and Malaria Incidence. PLoS ONE 2016, 11, e0152410. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Br. Med. J. 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Yang, G.G.; Kim, D.; Pham, A.; Paul, C.J. A Meta-Regression Analysis of the Effectiveness of Mosquito Nets for Malaria Control: The Value of Long-Lasting Insecticide Nets. Int. J. Environ. Res. Public Health 2018, 15, 546. [Google Scholar] [CrossRef]
- Mulenga, M.; Malunga, F.; Bennett, S.; Thuma, P.E.; Shulman, C.; Fielding, K.; Alloueche, A.; Greenwood, B.M. A randomised, double-blind, placebo-controlled trial of atovaquone–proguanil vs. sulphadoxine–pyrimethamine in the treatment of malarial anaemia in Zambian children. Trop. Med. Int. Health 2006, 11, 1643–1652. [Google Scholar] [CrossRef]
- Chanda, P.; Hawela, M.; Kango, M.; Sipilanyambe, N. Assessment of the therapeutic efficacy of a paediatric formulation of artemether-lumefantrine (Coartesiane®) for the treatment of uncomplicated Plasmodium falciparum in children in Zambia. Malar. J. 2006, 5, 1–5. [Google Scholar] [CrossRef][Green Version]
- Hamer, D.H.; Brooks, E.T.; Semrau, K.; Pilingana, P.; MacLeod, W.B.; Siazeele, K.; Sabin, L.L.; Thea, D.M.; Yeboah-Antwi, K. Quality and safety of integrated community case management of malaria using rapid diagnostic tests and pneumonia by community health workers. Pathog. Glob. Health 2012, 106, 32–39. [Google Scholar] [CrossRef]
- Sturrock, H.J.W.; Novotny, J.M.; Kunene, S.; Dlamini, S.; Zulu, Z.; Cohen, J.M.; Hsiang, M.S.; Greenhouse, B.; Gosling, R.D. Reactive Case Detection for Malaria Elimination: Real-Life Experience from an Ongoing Program in Swaziland. PLoS ONE 2013, 8, e63830. [Google Scholar] [CrossRef]
- Pinchoff, J.; Henostroza, G.; Carter, B.S.; Roberts, S.T.; Hatwiinda, S.; Hamainza, B.; Hawela, M.; Curriero, F.C. Spatial patterns of incident malaria cases and their household contacts in a single clinic catchment area of Chongwe District, Zambia. Malar. J. 2015, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Eisele, T.P.; Bennett, A.; Silumbe, K.; Finn, T.P.; Chalwe, V.; Kamuliwo, M.; Hamainza, B.; Moonga, H.; Kooma, E.; ChizemaKawesha, E.; et al. Short-term impact of mass drug administration with dihydroartemisinin plus piperaquine on malaria in Southern Province Zambia: A cluster-randomized controlled trial. J. Infect. Dis. 2016, 214, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Tejedor-Garavito, N.; Dlamini, N.; Pindolia, D.; Soble, A.; Ruktanonchai, N.W.; Alegana, V.; Le Menach, A.; Ntshalintshali, N.; Dlamini, B.; Smith, D.L.; et al. Travel patterns and demographic characteristics of malaria cases in Swaziland, 2010–2014. Malar. J. 2017, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Auala, J.; Tambo, M.; Haindongo, E.; Katokele, S.; Uusiku, P.; Gosling, R.; Kleinschmidt, I.; Mumbengegwi, D.; Sturrock, H.J. Spatial clustering of patent and sub-patent malaria infections in northern Namibia: Implications for surveillance and response strategies for elimination. PLoS ONE 2017, 12, e0180845. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.A.; Ngwenya-Kangombe, T.; Cheelo, S.; Hamainza, B.; Miller, J.; Winters, A.; Bridges, D.J. Location, location, location: Environmental factors better predict malaria-positive individuals during reactive case detection than index case demographics in Southern Province, Zambia. Malar. J. 2017, 16, 18. [Google Scholar] [CrossRef]
- Tambo, M.; Auala, J.R.; Sturrock, H.J.; Kleinschmidt, I.; Bock, R.; Smith, J.L.; Gosling, R.; Mumbengegwi, D.R. Evaluation of loop-mediated isothermal amplification as a surveillance tool for malaria in reactive case detection moving towards elimination. Malar. J. 2018, 17, 255. [Google Scholar] [CrossRef]
- Deutsch-Feldman, M.; Hamapumbu, H.; Lubinda, J.; Musonda, M.; Katowa, B.; Searle, K.M.; Kobayashi, T.; Shields, T.M.; Stevenson, J.C.; Thuma, P.E.; et al. The efficiency of a Malaria Reactive Test-and-Treat Program in Southern Zambia: A Prospective, Observational Study. Am. J. Trop. Med. Hyg. 2018, 98, 1382–1388. [Google Scholar] [CrossRef]
- Hsiang, M.S.; Ntshalintshali, N.; Kang Dufour, M.-S.; Dlamini, N.; Nhlabathi, N.; Vilakati, S.; Malambe, C.; Zulu, Z.; Maphalala, G.; Novotny, J.; et al. Active Case Finding for Malaria: A 3-Year National Evaluation of Optimal Approaches to Detect Infections and Hotspots Through Reactive Case Detection in the Low-transmission Setting of Eswatini. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 70, 1316–1325. [Google Scholar] [CrossRef]
- Hsiang, M.S.; Ntuku, H.; Roberts, K.W.; Dufour, M.-S.K.; Whittemore, B.; Tambo, M.; McCreesh, P.; Medzihradsky, O.F.; Prach, L.M.; Siloka, G.; et al. Effectiveness of reactive focal mass drug administration and reactive focal vector control to reduce malaria transmission in the low malaria-endemic setting of Namibia: A cluster-randomised controlled, open-label, two-by-two factorial design trial. Lancet 2020, 395, 1361–1373. [Google Scholar] [CrossRef]
- Bhondoekhan, F.R.P.; Searle, K.M.; Hamapumbu, H.; Lubinda, M.; Matoba, J.; Musonda, M.; Katowa, B.; Shields, T.M.; Kobayashi, T.; Norris, D.E.; et al. Improving the efficiency of reactive case detection for malaria elimination in southern Zambia: A cross-sectional study. Malar. J. 2020, 19, 175. [Google Scholar] [CrossRef]
- Finn, T.P.; Porter, T.R.; Moonga, H.; Silumbe, K.; Daniels, R.F.; Volkman, S.K.; Yukich, J.O.; Keating, J.; Bennett, A.; Steketee, R.W.; et al. Adherence to Mass Drug Administration with Dihydroartemisinin–Piperaquine and Plasmodium falciparum Clearance in Southern Province, Zambia. Am. J. Trop. Med. Hyg. 2020, 103 (Suppl. 2), 37. [Google Scholar] [CrossRef] [PubMed]
- Vilakati, S.; Mngadi, N.; Benjamin-Chung, J.; Dlamini, N.; Dufour, M.S.K.; Whittemore, B.; Bhangu, K.; Prach, L.M.; Baltzell, K.; Nhlabathi, N.; et al. Effectiveness and safety of reactive focal mass drug administration (rfMDA) using dihydroartemisinin–piperaquine to reduce malaria transmission in the very low-endemic setting of Eswatini: A pragmatic cluster randomised controlled trial. BMJ Glob. Health 2021, 6, e005021. [Google Scholar] [CrossRef] [PubMed]
- Rajvanshi, H.; Bharti, P.K.; Nisar, S.; Jain, Y.; Jayswar, H.; Mishra, A.K.; Sharma, R.K.; Saha, K.B.; Shukla, M.M.; Das, A.; et al. Study design and operational framework for a community-based Malaria Elimination Demonstration Project (MEDP) in 1233 villages of district Mandla, Madhya Pradesh. Malar. J. 2020, 19, 410. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, S.P.; Kigozi, R.N.; Sserwanga, A.; Nankabirwa, J.I.; Staedke, S.G.; Kamya, M.R.; Pullan, R.L. Malaria burden through routine reporting: The relationship between incidence and test positivity rates. Am. J. Trop. Med. Hyg. 2019, 101, 137. [Google Scholar] [CrossRef] [PubMed]
- Severe Malaria Observatory. Zambia Malaria Facts. 2020. Available online: https://www.severemalaria.org/countries/zambia (accessed on 30 December 2021).
- WHO. Disease Surveillance for Malaria Elimination: An Operational Manual; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Centre for Disease Control (CDC). How Can Malaria Cases and Deaths Be Reduced? 2018. Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/index.html (accessed on 30 December 2021).
- Burkot, T.R.; Farlow, R.; Min, M.; Espino, E.; Mnzava, A.; Russell, T.L. A global analysis of National Malaria Control Programme vector surveillance by elimination and control status in 2018. Malar. J. 2019, 18, 399. [Google Scholar] [CrossRef]
- Russell, T.L.; Farlow, R.; Min, M.; Espino, E.; Mnzava, A.; Burkot, T.R. Capacity of National Malaria Control Programmes to implement vector surveillance: A global analysis. Malar. J. 2020, 19, 422. [Google Scholar] [CrossRef]
- Newby, G.; Hwang, J.; Koita, K.; Chen, I.; Greenwood, B.; Von Seidlein, L.; Shanks, G.D.; Slutsker, L.; Kachur, S.P.; Wegbreit, J.; et al. Review of mass drug administration for malaria and its operational challenges. Am. J. Trop. Med. Hyg. 2015, 93, 125. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Yasuoka, J.; Poudel, K.C.; Poudel-Tandukar, K.; Nguon, C.; Ly, P.; Socheat, D.; Jimba, M. Assessing the quality of service of village malaria workers to strengthen community-based malaria control in Cambodia. Malar. J. 2010, 9, 109. [Google Scholar] [CrossRef]
- Druetz, T.; Ridde, V.; Kouanda, S.; Ly, A.; Diabaté, S.; Haddad, S. Utilization of community health workers for malaria treatment: Results from a three-year panel study in the districts of Kaya and Zorgho, Burkina Faso. Malar. J. 2015, 14, 71. [Google Scholar] [CrossRef]
- Grossenbacher, B.; Holzschuh, A.; Hofmann, N.E.; Omar, K.A.; Stuck, L.; Fakih, B.S.; Ali, A.; Yukich, J.; Hetzel, M.W.; Felger, I. Molecular methods for tracking residual Plasmodium falciparum transmission in a close-to-elimination setting in Zanzibar. Malar. J. 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Caldera, A.; Wickremasinghe, A.R. Reactive Case Detection (RACD) and foci investigation strategies in malaria control and elimination: A review. Malar. J. 2020, 19, 401. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria Rapid Diagnostic Test Performance: Results of Product Testing of Malaria RDTs: Round 5. 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/128678/9789241507554_eng.pdf (accessed on 16 November 2020).
- Manning, L.; Laman, M.; Rosanas-Urgell, A.; Turlach, B.; Aipit, S.; Bona, C.; Warrell, J.; Siba, P.; Mueller, I.; Davis, T.M.E. Rapid antigen detection tests for malaria diagnosis in severely ill Papua New Guinean children: A comparative study using Bayesian latent class models. PLoS ONE 2012, 7, e48701. [Google Scholar] [CrossRef]
- Zhao, J.; Lama, M.; Korenromp, E.; Aylward, P.; Shargie, E.; Filler, S.; Komatsu, R.; Atun, R. Adoption of rapid diagnostic tests for the diagnosis of malaria, a preliminary analysis of the Global Fund program data, 2005 to 2010. PLoS ONE 2012, 7, e43549. [Google Scholar] [CrossRef]
- Boadu, N.Y.; Amuasi, J.; Ansong, D.; Einsiedel, E.; Menon, D.; Yanow, S.K. Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: A qualitative study. Malar. J. 2016, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Stuck, L.; Fakih, B.S.; Al-mafazy, A.-W.H.; Hofmann, N.E.; Holzschuh, A.; Grossenbacher, B.; Bennett, A.; Cotter, C.; Reaves, E.; Ali, A.; et al. Malaria infection prevalence and sensitivity of reactive case detection in Zanzibar. Int. J. Infect. Dis. 2020, 97, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, G.; Melaku, Z.; Abreha, T.; Alemayehu, B.; Girma, S.; Tadesse, Y.; Gadisa, T.; Lulseged, S.; Balcha, T.T.; Hoos, D.; et al. The burden of malaria among adult patients attending general medical outpatient department and HIV care and treatment clinics in Oromia, Ethiopia: A comparative cross-sectional study. Malar. J. 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Deress, T.; Girma, M. Plasmodium falciparum and Plasmodium vivax prevalence in Ethiopia: A systematic review and meta-analysis. Malar. Res. Treat. 2019, 2019, 7065064. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J. How should meta-regression analyses be undertaken and interpreted. Int. J. Environ. Res. Public Health 2018, 21, 1559–1573. [Google Scholar] [CrossRef]


| Author | Year | Country | Diagnostic Method | Study Type | Screened | Malaria Positive |
|---|---|---|---|---|---|---|
| Mulenga et al. [36] | 2006 | Zambia | Microscopy and PCR | Double-blind randomized control trial | 1048 | 255 |
| Chanda et al. [37] | 2006 | Zambia | Microscopy | Prospective study | 953 | 111 |
| Hamer et al. [38] | 2012 | Zambia | RDT | Cluster randomized control trial | 975 | 270 |
| Sturrock et al. [39] | 2013 | Eswatini | RDT | Cohort study | 3671 | 74 |
| Pinchoff et al. [40] | 2015 | Zambia | RDT | Cohort study | 1621 | 735 |
| Larsen et al. [19] | 2015 | Zambia | RDT | Descriptive cross-sectional study | 143,295 | 22,201 |
| Eisele et al. [41] | 2016 | Zambia | RDT | Cluster randomized control trial | 5018 | 1097 |
| Tejedor-Garavot et al. [42] | 2017 | Eswatini | RDT | Ecological study | 9859 | 105 |
| Smith et al. [43] | 2017 | Namibia | RDT and LAMP | Prospective case-control study | 3151 | 89 |
| Larsen et al. [44] | 2017 | Zambia | RDT | Retrospective cohort study | 14,409 | 1200 |
| Tambo et al. [45] | 2018 | Namibia | RDT, LAMP and qPCR | Prospective case-control study | 2642 | 47 |
| Deutsch-Feldman et al. [46] | 2018 | Zambia | RDT and qPCR | Prospective observational study | 3016 | 73 |
| Hsiang et al. [47] | 2019 | Eswatini | RDT and LAMP | Prospective observational study | 10,890 | 180 |
| Hsiang et al. [48] | 2020 | Namibia | RDT and LAMP | Cluster randomized control trial | 4701 | 178 |
| Bhondoekhan et al. [49] | 2020 | Zambia | RDT and qPCR | Cross sectional study | 4170 | 153 |
| Finn et al. [50] | 2020 | Zambia | RDT and Microscopy | Cluster randomized control trial | 597,631 | 30,898 |
| Vilakati et al. [51] | 2021 | Eswatini | RDT and LAMP | Cluster randomized control trial | 1455 | 5 |
| Country | Incidence (95%CI) | I2(%) | Heterogeneity Statistic | Heterogeneity Test | |
|---|---|---|---|---|---|
| df | p-Value | ||||
| Zambia | 16(12–21) | 99.93 | 13,227.55 | 9 | 0.00 |
| Eswatini | 1(1–2) | 94.91 | 58.92 | 3 | 0.00 |
| Namibia | 3(2–4) | - | - | 2 | - |
| Overall | 10(8–13) | 99.91 | 18,143.95 | 16 | 0.00 |
| Diagnostic Method | Incidence (95%CI) | I2(%) | Heterogeneity Statistic | Heterogeneity Test | |
|---|---|---|---|---|---|
| df | p-Value | ||||
| Microscopy and PCR | 24(22–27) | - | - | 0 | - |
| Microscopy | 12(10–14) | - | - | 0 | - |
| RDT | 17(11–24) | 99.95 | 12,896.94 | 6 | 0.00 |
| RDT and LAMP | 2(1–3) | 97.94 | 145.72 | 3 | 0.00 |
| RDT, LAMP and qPCR | 2(1–2) | - | - | 0 | - |
| RDT and qPCR | 3(2–3) | - | - | 1 | 0.00 |
| RDT and microscopy | 5(5–5) | - | - | 0 | - |
| Overall | 10(8–13) | 99.91 | 18,143.95 | 16 | 0.00 |
| Variables | Coefficient | p-Value | 95% Confidence Interval |
|---|---|---|---|
| Year of publication | −0.184 | 0.013 | (−0.322, −0.045) |
| Population screened | 1.70 × 10−6 | 0.403 | (−2.51 × 10 −6, 5.90 × 10−6) |
| Constant | 367.042 | 0.013 | (88.482, 645.602) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyegoke, O.O.; Akoniyon, O.P.; Ogunsakin, R.E.; Ogunlana, M.O.; Adeleke, M.A.; Maharaj, R.; Okpeku, M. A Systematic Review and Meta-Analysis of Malaria Test Positivity Outcomes and Programme Interventions in Low Transmission Settings in Southern Africa, 2000–2021. Int. J. Environ. Res. Public Health 2022, 19, 6776. https://doi.org/10.3390/ijerph19116776
Oyegoke OO, Akoniyon OP, Ogunsakin RE, Ogunlana MO, Adeleke MA, Maharaj R, Okpeku M. A Systematic Review and Meta-Analysis of Malaria Test Positivity Outcomes and Programme Interventions in Low Transmission Settings in Southern Africa, 2000–2021. International Journal of Environmental Research and Public Health. 2022; 19(11):6776. https://doi.org/10.3390/ijerph19116776
Chicago/Turabian StyleOyegoke, Olukunle O., Olusegun P. Akoniyon, Ropo E. Ogunsakin, Michael O. Ogunlana, Matthew A. Adeleke, Rajendra Maharaj, and Moses Okpeku. 2022. "A Systematic Review and Meta-Analysis of Malaria Test Positivity Outcomes and Programme Interventions in Low Transmission Settings in Southern Africa, 2000–2021" International Journal of Environmental Research and Public Health 19, no. 11: 6776. https://doi.org/10.3390/ijerph19116776
APA StyleOyegoke, O. O., Akoniyon, O. P., Ogunsakin, R. E., Ogunlana, M. O., Adeleke, M. A., Maharaj, R., & Okpeku, M. (2022). A Systematic Review and Meta-Analysis of Malaria Test Positivity Outcomes and Programme Interventions in Low Transmission Settings in Southern Africa, 2000–2021. International Journal of Environmental Research and Public Health, 19(11), 6776. https://doi.org/10.3390/ijerph19116776