Emergency Craniotomy and Burr-Hole Trephination in a Low-Resource Setting: Capacity Building at a Regional Hospital in Cambodia
Abstract
1. Introduction
- Five 150-h training courses in primary trauma surgery
- Instructors from UNN conducted five consecutive 150 h training courses in primary trauma surgery at six regional hospitals in rural Cambodia from 2002 to 2005. The teaching program proved successful, with a significant reduction in trauma morbidity in the study hospitals [9]. The intervention was built on the Mozambique experience [10] and the previous experience of UNN’s “village university” teaching model [7,11,12].
- Cranial neurosurgical capacity-building program
- The training included a four-week in-hospital skill training at the neurosurgery department at UNN for a selected surgeon followed by teaching and surgical supervision for the surgeon’s team.
- In September 2013, senior surgeon Vannara Sokh from the Military Hospital underwent four weeks of in-hospital skill training at the neurosurgical department at UNN to enhance the quality of operative and postoperative care. Since October 2013, the hospital in Battambang had performed 14 craniotomies in TBI cases and 11 craniotomies in patients with cerebral hemorrhage. During this period, the neurosurgeon instructors at UNN guided the Cambodian surgical team online by evaluating CT scans and other patient information. They also made several visits to the study hospital, where they performed surgeries together with the local surgical team to provide hands-on teaching and supervision. The training program in emergency cranial neurosurgery did not introduce new techniques or procedures but controlled the quality of medical procedures already in use in the study hospital in Cambodia.
2. Materials and Methods
2.1. Eligibility Criteria for Intervention and Data Sources
2.2. Data Collection
2.3. Statistical Analysis
3. Results
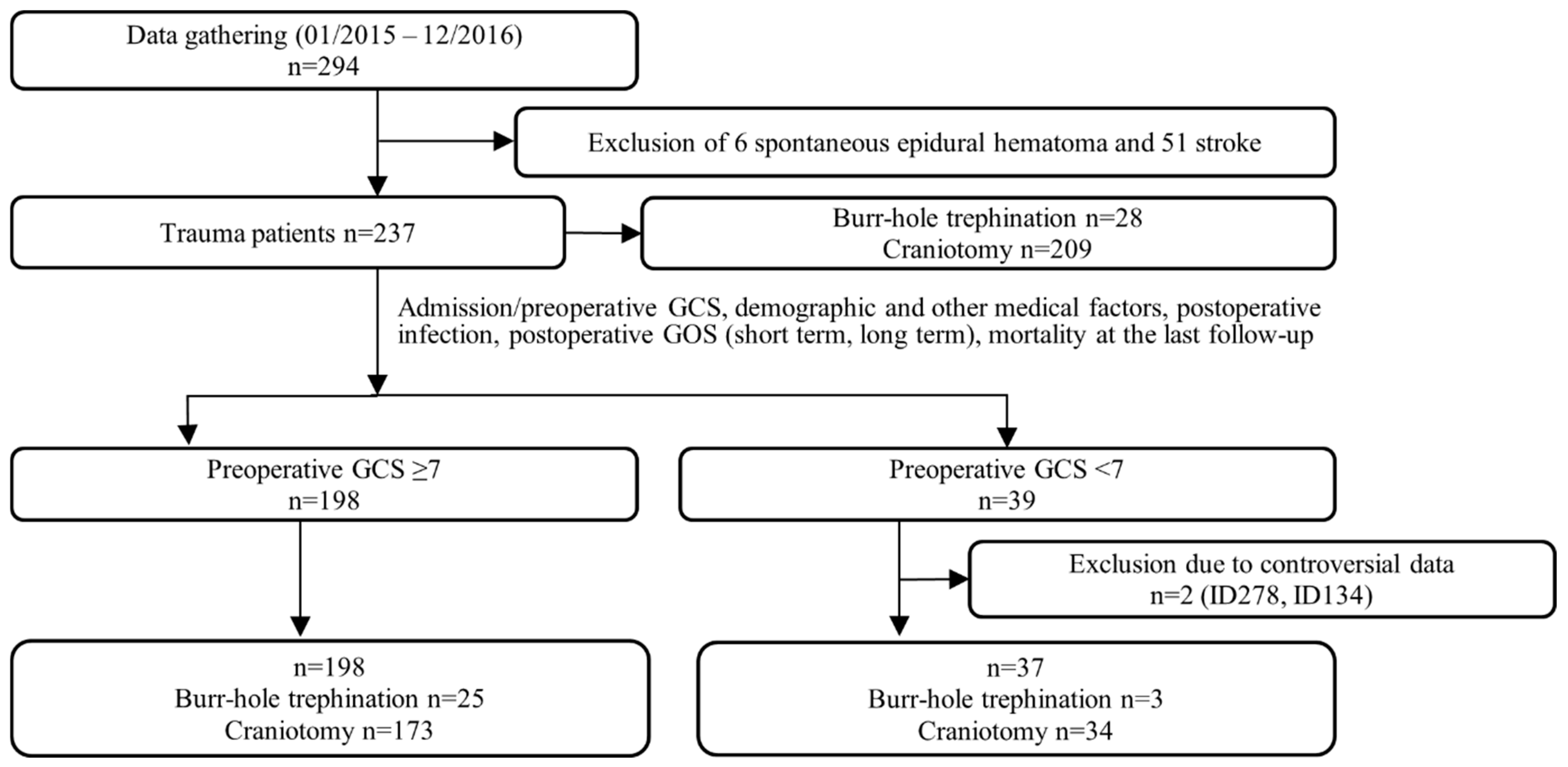
3.1. Data Inclusion/Exclusion for Statistical Analysis
3.2. Patient Demographics and Clinical Status
3.3. Outcome Measures
3.4. Risk Factors for Unfavorable Outcomes
4. Discussion
4.1. Surgical Outcomes
4.2. Patient Characteristics: Mainly Young Males, High Incidence of Acute EDH, Late Admission
4.3. Risk Factors of Unfavorable Outcomes
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Review. Available online: https://worldpopulationreview.com/countries/cambodia-population (accessed on 14 May 2021).
- World Bank. Cambodia GNI (Current US$). 2019. Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD?view=map (accessed on 9 May 2021).
- WHO. The Kingdom of Cambodia Health System Review; WHO Regional Office for the Western Pacific: Manila, Philippines, 2015. [Google Scholar]
- WHO. Road Safety in Cambodia. Available online: https://www.who.int/violence_injury_prevention/road_traffic/countrywork/khm/en/ (accessed on 8 May 2021).
- Kim, M.; Yoo, C.B.; Lee-Park, O.; Nang, S.; Vuthy, D.; Park, K.B.; Vycheth, I. Patterns of neurosurgical conditions at a major government hospital in Cambodia. Asian J. Neurosurg. 2020, 15, 952. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef] [PubMed]
- Husum, H.; Gilbert, M.; Wisborg, T. Save lives, save limbs. In Life Support to Victims of Mines, Wars, and Accidents; Third World Network: Penang, Malaysia, 2000. [Google Scholar]
- Murad, M.K.; Issa, D.B.; Mustafa, F.M.; Hassan, H.O.; Husum, H. Prehospital trauma system reduces mortality in severe trauma: A controlled study of road traffic casualties in Iraq. Prehospital Disaster Med. 2012, 27, 36–41. [Google Scholar] [CrossRef]
- Van Heng, Y.; Davoung, C.; Husum, H. Non-doctors as trauma surgeons? A controlled study of trauma training for non-graduate surgeons in rural Cambodia. Prehospital Disaster Med. 2008, 23, 483–489. [Google Scholar] [CrossRef]
- Pereira, C.; Bugalho, A.; Bergström, S.; Vaz, F.; Cotiro, M. A comparative study of caesarean deliveries by assistant medical officers and obstetricians in Mozambique. BJOG Int. J. Obstet. Gynaecol. 1996, 103, 508–512. [Google Scholar] [CrossRef]
- Husum, H.; Gilbert, M.; Wisborg, T. Training pre-hospital trauma care in low-income countries: The Village University experience. Med. Teach. 2003, 25, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Husum, H.; Gilbert, M.; Wisborg, T.; Van Heng, Y.; Murad, M. Rural prehospital trauma systems improve trauma outcome in low-income countries: A prospective study from North Iraq and Cambodia. J. Trauma Acute Care Surg. 2003, 54, 1188–1196. [Google Scholar] [CrossRef]
- Ślusarz, R.; Jabłońska, R.; Królikowska, A.; Haor, B.; Barczykowska, E.; Biercewicz, M.; Głowacka, M.; Szrajda, J. Measuring scales used for assessment of patients with traumatic brain injury: Multicenter studies. Patient Prefer. Adherence 2015, 9, 869. [Google Scholar] [CrossRef][Green Version]
- Lingsma, H.F.; Roozenbeek, B.; Steyerberg, E.W.; Murray, G.D.; Maas, A.I. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010, 9, 543–554. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of outcome after severe brain damage: A practical scale. Lancet 1975, 305, 480–484. [Google Scholar] [CrossRef]
- Kanyi, J.K.; Ogada, T.V.; Oloo, M.J.; Parker, R.K. Burr-hole craniostomy for chronic subdural hematomas by general surgeons in rural Kenya. World J. Surg. 2018, 42, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Weigel, R.; Schmiedek, P.; Krauss, J. Outcome of contemporary surgery for chronic subdural haematoma: Evidence based review. J. Neurol. Neurosurg. Psychiatry 2003, 74, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Yu, X.; Liu, X.; Jing, Q.; Liu, B.; Liu, W. A Comparative Study of Chronic Subdural Hematoma in Patients With and Without Head Trauma: A Retrospective Cross Sectional Study. Front. Neurol. 2020, 11, 1538. [Google Scholar] [CrossRef] [PubMed]
- Rohde, V.; Graf, G.; Hassler, W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: A retrospective analysis of 376 patients. Neurosurg. Rev. 2002, 25, 89–94. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Chang, C.-K.; Chen, S.-J.; Lin, J.-F.; Tsai, C.-C. Chronic subdural hematoma in elderly Taiwan patients: A retrospective analysis of 342 surgical cases. Int. J. Gerontol. 2014, 8, 37–41. [Google Scholar] [CrossRef][Green Version]
- Karnjanasavitree, W.; Phuenpathom, N.; Tunthanathip, T. The optimal operative timing of traumatic intracranial acute subdural hematoma correlated with outcome. Asian J. Neurosurg. 2018, 13, 1158. [Google Scholar]
- Li, L.M.; Kolias, A.G.; Guilfoyle, M.R.; Timofeev, I.; Corteen, E.A.; Pickard, J.D.; Menon, D.K.; Kirkpatrick, P.J.; Hutchinson, P.J. Outcome following evacuation of acute subdural haematomas: A comparison of craniotomy with decompressive craniectomy. Acta Neurochir. 2012, 154, 1555–1561. [Google Scholar] [CrossRef]
- Kotwica, Z.; Brzeziński, J. Acute subdural haematoma in adults: An analysis of outcome in comatose patients. Acta Neurochir. 1993, 121, 95–99. [Google Scholar] [CrossRef]
- Cheung, P.S.; Lam, J.M.; Yeung, J.H.; Graham, C.A.; Rainer, T.H. Outcome of traumatic extradural haematoma in Hong Kong. Injury 2007, 38, 76–80. [Google Scholar] [CrossRef]
- Onodera, K.; Kamide, T.; Kimura, T.; Tabata, S.; Ikeda, T.; Kikkawa, Y.; Kurita, H. Identification of prognostic factors in surgically treated patients with acute epidural hematoma. Asian J. Neurosurg. 2020, 15, 532. [Google Scholar] [PubMed]
- Islam, M.; Saha, S.; Elahy, M.; Islam, K.; Ahamed, S. Factors influencing the outcome of patients with acute extradural haematomas undergoing surgery. Bangladesh J. Med. Sci. 2011, 10, 112–120. [Google Scholar] [CrossRef]
- Ruff, L.M.; Mendelow, A.D.; Lecky, F.E. Improving mortality after extradural haematoma in England and Wales. Br. J. Neurosurg. 2013, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rehman, L.; Karachi, N.J. Association of outcome of traumatic extradural hematoma with Glasgow Coma Scale and hematoma size. Ann. Pak. Inst. Med. Sci. 2010, 6, 133–138. [Google Scholar]
- Siddique, M.S.; Gregson, B.A.; Fernandes, H.M.; Barnes, J.; Treadwell, L.; Wooldridge, T.D.; Mendelow, A.D. Comparative study of traumatic and spontaneous intracerebral hemorrhage. J. Neurosurg. 2002, 96, 86–89. [Google Scholar] [CrossRef]
- Mendelow, A.D.; Gregson, B.A.; Rowan, E.N.; Francis, R.; McColl, E.; McNamee, P.; Chambers, I.R.; Unterberg, A.; Boyers, D.; Mitchell, P.M. Early surgery versus initial conservative treatment in patients with traumatic intracerebral hemorrhage (STITCH [Trauma]): The first randomized trial. J. Neurotrauma 2015, 32, 1312–1323. [Google Scholar] [CrossRef]
- Fernandes, Y.; Borges, G.; Ramina, R.; Carvalho, F.; Cançado, B.; Morais, J. Minimally invasive approach to traumatic intracerebral hematomas. Min-Minim. Invasive Neurosurg. 2001, 44, 221–225. [Google Scholar] [CrossRef]
- Aromatario, M.; Torsello, A.; D’Errico, S.; Bertozzi, G.; Sessa, F.; Cipolloni, L.; Baldari, B. Traumatic Epidural and Subdural Hematoma: Epidemiology, Outcome, and Dating. Medicina 2021, 57, 125. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chen, Y.; Tseng, S.-H. Chronic encapsulated intracerebral haematoma. J. Clin. Neurosci. 2007, 14, 58–61. [Google Scholar] [CrossRef]
- Nomura, M.; Miyashita, K.; Tamase, A.; Kamide, T.; Mori, K.; Kitamura, Y.; Seki, S.; Shima, H.; Yanagimoto, K. A chronic intracerebral fluid hematoma. Neuroradiol. J. 2014, 27, 191–194. [Google Scholar] [CrossRef]
- Cepeda, S.; Gómez, P.A.; Castaño-Leon, A.M.; Martínez-Pérez, R.; Munarriz, P.M.; Lagares, A. Traumatic intracerebral hemorrhage: Risk factors associated with progression. J. Neurotrauma 2015, 32, 1246–1253. [Google Scholar] [CrossRef]
- Sturiale, C.L.; De Bonis, P.; Rigante, L.; Calandrelli, R.; D’Arrigo, S.; Pompucci, A.; Mangiola, A.; D’Apolito, G.; Colosimo, C.; Anile, C. Do traumatic brain contusions increase in size after decompressive craniectomy? J. Neurotrauma 2012, 29, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Labor Force Participation Rate by Sex; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Peeters, S.; Blaine, C.; Vycheth, I.; Nang, S.; Vuthy, D.; Park, K.B. Epidemiology of Traumatic Brain Injuries at a Major Government Hospital in Cambodia. World Neurosurg. 2017, 97, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, B.; Mazurek, M.; Rams, Ł.; Nogalski, A. Acute epidural and subdural hematomas after head injury: Clinical distinguishing features. Indian J. Surg. 2020, 83, 96–104. [Google Scholar] [CrossRef]
- Bir, S.C.; Maiti, T.K.; Ambekar, S.; Nanda, A. Incidence, hospital costs and in-hospital mortality rates of epidural hematoma in the United States. Clin. Neurol. Neurosurg. 2015, 138, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rosyidi, R.M.; Priyanto, B.; Al Fauzi, A.; Sutiono, A.B. Toward zero mortality in acute epidural hematoma: A review in 268 cases problems and challenges in the developing country. Interdiscip. Neurosurg. 2019, 17, 12–18. [Google Scholar] [CrossRef]
- Crimmins, D.W.; Palmer, J.D. Snapshot view of emergency neurosurgical head injury care in Great Britain and Ireland. J. Neurol. Neurosurg. Psychiatry 2000, 68, 8–13. [Google Scholar] [CrossRef]
- Vyvyan, H.; Kee, S.; Bristow, A. A survey of secondary transfers of head injured patients in the south of England. Anaesthesia 1991, 46, 728–731. [Google Scholar] [CrossRef]
- Kulesza, B.; Nogalski, A.; Kulesza, T.; Prystupa, A. Prognostic factors in traumatic brain injury and their association with outcome. J. Pre-Clin. Clin. Res. 2015, 9, 163–166. [Google Scholar] [CrossRef]
- Hanif, S.; Abodunde, O.; Ali, Z.; Pidgeon, C. Age related outcome in acute subdural haematoma following traumatic head injury. Ir. Med. J. 2009, 102, 255. [Google Scholar]
- Kulesza, B.; Litak, J.; Mazurek, M.; Nogalski, A. Initial Factors Affecting 6-month Outcome of Patients Undergoing Surgery for Acute Post-traumatic Subdural and Epidural Hematoma. Folia Med. 2020, 62, 94. [Google Scholar] [CrossRef]
- Deverill, J.; Aitken, L.M. Treatment of extradural haemorrhage in Queensland: Interhospital transfer, preoperative delay and clinical outcome. Emerg. Med. Australas. 2007, 19, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Leach, P.; Childs, C.; Evans, J.; Johnston, N.; Protheroe, R.; King, A. Transfer times for patients with extradural and subdural haematomas to neurosurgery in Greater Manchester. Br. J. Neurosurg. 2007, 21, 11–15. [Google Scholar] [CrossRef] [PubMed]
| Rating | Interpretation |
|---|---|
| 1 | Dead |
| 2 | No response, but alive |
| 3 | Severe disability, conscious but needs support for all daily activities |
| 4 | Patient is independent but has some disability |
| 5 | Full recovery, no disability |
| Overall | Trauma Diagnosis | |||||
|---|---|---|---|---|---|---|
| Characteristic | N = 235 | Epidural Hematoma | Subdural Hematoma | Intracerebral Hematoma | Chronic | p-Value |
| n = 100 | n = 74 | n = 35 | n = 26 | |||
| Age | 34.9 ± 17.3 | 26.3 ± 11.3 | 37.2 ± 16.8 | 36.2 ± 15.3 | 59.7 ± 13.7 | <0.001 a |
| Time from injury to admission (h) | 83 ± 213.5 | 25.3 ± 33.3 | 37.1 ± 99 | 58.5 ± 91.3 | 484.2 ± 455.6 | <0.001 a |
| Time from admission to surgery (h) | 21.4 ± 36.4 | 17.2 ± 25.1 | 19.3 ± 28 | 24.5 ± 29.6 | 39.3 ± 77.5 | 0.7 a |
| Injury Severity Score | 23.2 ± 3.6 | 23.5 ± 3.4 | 23.5 ± 3.3 | 22.7 ± 4 | 22.2 ± 4.2 | 0.279 a |
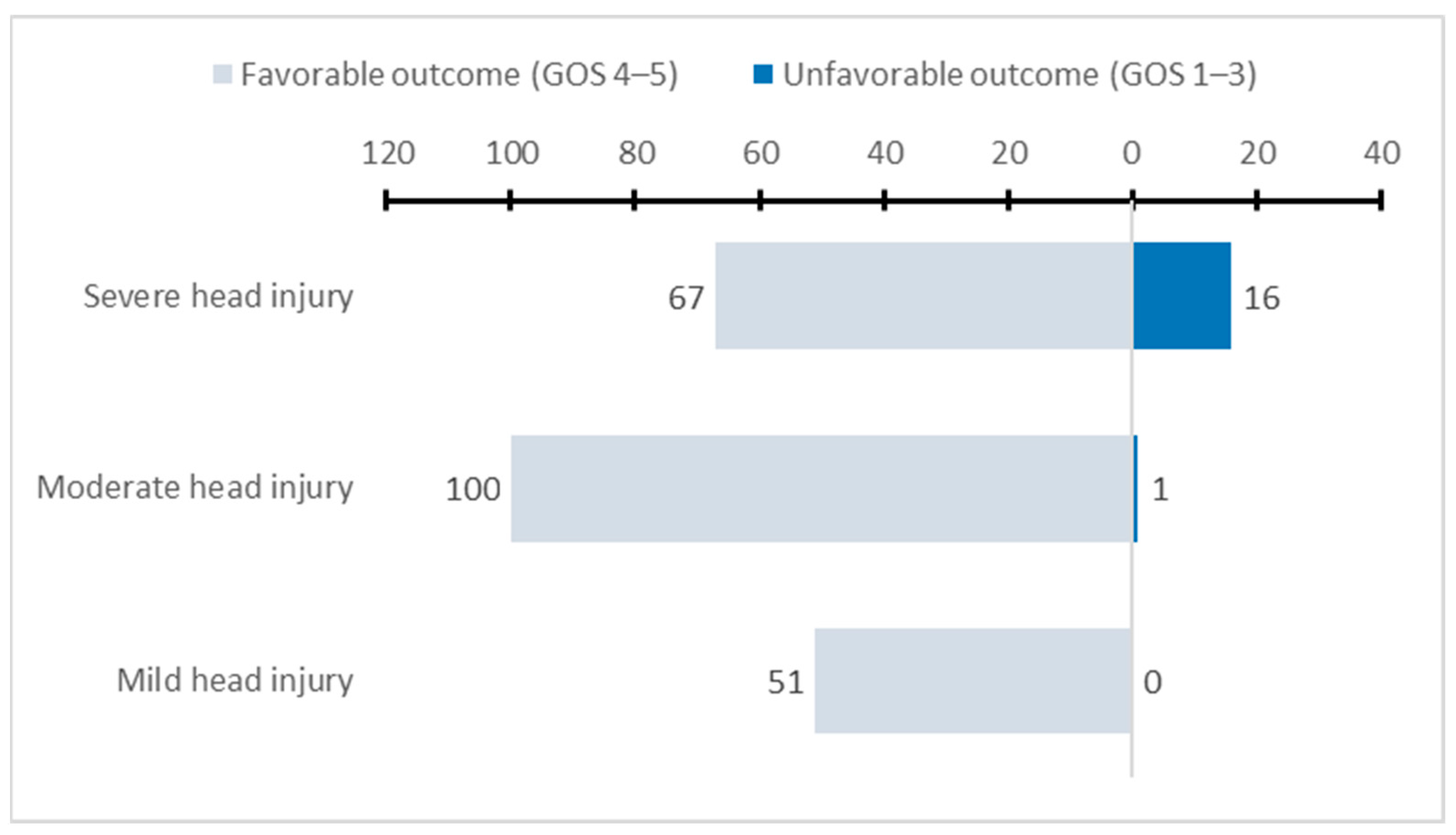
| Head injury severity | <0.001 b | |||||
| Severe (GCS 3–8) | 83 (35.3%) | 29 (29%) | 33 (44.6%) | 15 (42.9%) | 6 (23.1%) | |
| Moderate (GCS 9–12) | 101 (43.0%) | 50 (50%) | 33 (44.6%) | 12 (32.3%) | 6 (23.1%) | |
| Mild (GCS 13–15) | 51 (21.7%) | 21 (21%) | 8 (10.8%) | 8 (22.9%) | 14 (53.8%) | |
| Gender | 0.353 c | |||||
| Female | 29 (12.3%) | 11 (11%) | 13 (17.6%) | 2 (5.7%) | 3 (11.5%) | |
| Male | 206 (87.7%) | 89 (89%) | 61 (82.4%) | 33 (94.3%) | 23 (88.5%) | |
| Age groups | <0.001 c | |||||
| <20 y | 41 (17.4%) | 31 (31%) | 5 (6.8%) | 5 (14.3%) | 0 (0%) | |
| 20–34 y | 105 (44.7%) | 53 (53%) | 38 (51.4%) | 12 (34.3%) | 2 (7.7%) | |
| 35–49 y | 29 (12.3%) | 8 (8%) | 9 (12.2%) | 10 (28.6%) | 2 (7.7%) | |
| 50–64 y | 40 (17%) | 7 (7%) | 15 (20.3%) | 7 (20%) | 11 (42.3%) | |
| > or =65 y | 20 (8.5%) | 1 (1%) | 7 (9.5%) | 1 (2.9%) | 11 (42.3%) | |
| Type of fracture | <0.001 c | |||||
| Close fracture | 130 (55.3%) | 98 (98%) | 16 (21.6%) | 15 (42.9%) | 1 (3.8%) | |
| Open fracture | 8 (3.4%) | 2 (2%) | 3 (4.1%) | 3 (8.6%) | 0 (0%) | |
| Without fracture | 97 (41.3%) | 0 (0%) | 55 (74.3%) | 17 (48.6%) | 25 (96.2%) | |
| Polytrauma | <0.001 b | |||||
| No other injuries | 68 (28.9%) | 20 (20%) | 13 (17.6%) | 9 (25.7%) | 26 (100%) | |
| Other moderate injury no need for surgery | 162 (68.9%) | 77 (77%) | 60 (81.1%) | 25 (71.4%) | 0 (0%) | |
| Other injury in need for surgery | 5 (2.1%) | 3 (3%) | 1 (1.4%) | 1 (2.9%) | 0 (0%) | |
| Type of operation | <0.001 c | |||||
| Burr-hole trephination | 28 (11.9%) | 1 (1%) | 0 (0%) | 1 (2.9%) | 26 (100%) | |
| Craniotomy | 207 (88.1%) | 99 (99%) | 74 (100%) | 34 (97.1%) | 0 (0%) | |
| Overall | Trauma Diagnosis | |||||
|---|---|---|---|---|---|---|
| Characteristic | N = 235 | Epidural Hematoma | Subdural Hematoma | Intracerebral Hematoma | Chronic | p-Value a |
| n = 100 | n = 74 | n = 35 | n = 26 | |||
| Dead | 0.540 | |||||
| Yes | 17 (7.2%) | 7 (7%) | 8 (10.8%) | 1 (2.9%) | 1 (3.8%) | |
| No | 218 (92.8%) | 93 (93%) | 66 (89.2%) | 34 (97.1%) | 25 (96.2%) | |
| Postoperative GOS (3 months post-injury) | 0.540 | |||||
| Unfavorable (GOS 1–3) | 17 (7.2%) | 7 (7%) | 8 (10.8%) | 1 (2.9%) | 1 (3.8%) | |
| Favorable (GOS 4–5) | 218 (92.8%) | 93 (93%) | 66 (89.2%) | 34 (97.1%) | 25 (96.2%) | |
| Unfavorable Outcome (GOS 1–3) at 3 Months after Injury | ||||
|---|---|---|---|---|
| Variable | p-Value | OR | 95% CI for OR | |
| Lower | Upper | |||
| Moderate head injury (GCS 9–12) | - | ref | - | - |
| Severe head injury (GCS 3–8) | 0.002 | 23.88 | 3.09 | 184.36 |
| Mild head injury (GCS 13–15) | 0.998 | 0 a | 0 | - |
| Female | - | ref | - | - |
| Male | 0.156 | 0.42 | 0.13 | 1.39 |
| Type of fracture (Close fracture) | - | ref | - | - |
| Type of fracture (Open fracture) | 0.76 | 1.41 | 0.16 | 12.4 |
| Type of fracture (No fracture) | 0.147 | 0.42 | 0.13 | 1.35 |
| Trauma diagnosis (Epidural hematoma) | - | - | - | - |
| Trauma diagnosis (Subdural hematoma) | 0.379 | 1.61 | 0.56 | 4.66 |
| Trauma diagnosis (Intracerebral hematoma) | 0.388 | 0.39 | 0.05 | 3.29 |
| Trauma diagnosis (Chronic) | 0.563 | 0.53 | 0.06 | 4.52 |
| Referral admission (No) | - | ref | - | - |
| Referral admission (Yes) | 0.849 | 1.11 | 0.39 | 3.1 |
| Polytrauma (No) | - | ref | - | - |
| Polytrauma (Yes) | 0.295 | 1.98 | 0.55 | 7.13 |
| Age group (<20 y) | - | ref | - | - |
| Age group (20–34 y) | 0.177 | 4.21 | 0.52 | 33.99 |
| Age group (35–49 y) | 0.385 | 2.96 | 0.26 | 34.32 |
| Age group (> or =50 y) | 0.356 | 2.86 | 0.31 | 26.53 |
| Surgery type (Burr-hole trephination) | - | ref | - | - |
| Surgery type (Craniotomy) | 0.437 | 2.26 | 0.29 | 17.75 |
| ISS groups (ISS < 25) | - | ref | - | - |
| ISS groups (ISS ≥ 25) | 0.172 | 4.16 | 0.54 | 32.22 |
| Time from injury to admission (Hours) | 0.589 | 1 | 1 | 1 |
| Time from admission to surgery (Hours) | 0.453 | 0.99 | 0.97 | 1.02 |
| Trauma Diagnosis | Surgical Outcomes in the Study Hospital | Surgical Outcomes * in the Literature on the General Population | Surgical Outcomes * in the Literature on a Specific Population |
|---|---|---|---|
| Chronic subdural hematoma | Favorable outcome at 3 months 96.2% Mortality 3.8% | Favorable outcome 90.8% [16] Mortality 0–32% [16,17,18,19] | Age ≥ 65 years [20]: Favorable outcome 83.3% Mortality 2.34% |
| Traumatic acute subdural hematoma | Favorable outcome at 3 months 89.2% Mortality 10.8% | Favorable outcome 42–51% [21,22] Mortality 32–35.2% [21,22] | Comatose patients (GCS < 10) [23]: Favorable outcome 23% Mortality 57% |
| Traumatic acute epidural hematoma | Favorable outcome at 3 months 93% Mortality 7% | Favorable outcome 50–76.7% [24,25,26] Mortality 2–15.7% [24,26,27,28] | Not available |
| Traumatic intracerebral hematoma | Favorable outcome at 3 months 97.1% Mortality 2.9% | Favorable outcome 62–63% [29,30] Mortality 10–15% [30,31] | Not available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Sokh, V.; Nguon, S.; Heng, Y.V.; Husum, H.; Kloster, R.; Odland, J.Ø.; Xu, S. Emergency Craniotomy and Burr-Hole Trephination in a Low-Resource Setting: Capacity Building at a Regional Hospital in Cambodia. Int. J. Environ. Res. Public Health 2022, 19, 6471. https://doi.org/10.3390/ijerph19116471
Hu J, Sokh V, Nguon S, Heng YV, Husum H, Kloster R, Odland JØ, Xu S. Emergency Craniotomy and Burr-Hole Trephination in a Low-Resource Setting: Capacity Building at a Regional Hospital in Cambodia. International Journal of Environmental Research and Public Health. 2022; 19(11):6471. https://doi.org/10.3390/ijerph19116471
Chicago/Turabian StyleHu, Jingjing, Vannara Sokh, Sophy Nguon, Yang Van Heng, Hans Husum, Roar Kloster, Jon Øyvind Odland, and Shanshan Xu. 2022. "Emergency Craniotomy and Burr-Hole Trephination in a Low-Resource Setting: Capacity Building at a Regional Hospital in Cambodia" International Journal of Environmental Research and Public Health 19, no. 11: 6471. https://doi.org/10.3390/ijerph19116471
APA StyleHu, J., Sokh, V., Nguon, S., Heng, Y. V., Husum, H., Kloster, R., Odland, J. Ø., & Xu, S. (2022). Emergency Craniotomy and Burr-Hole Trephination in a Low-Resource Setting: Capacity Building at a Regional Hospital in Cambodia. International Journal of Environmental Research and Public Health, 19(11), 6471. https://doi.org/10.3390/ijerph19116471








