Effectiveness of Curcumin in Reducing Self-Rated Pain-Levels in the Orofacial Region: A Systematic Review of Randomized-Controlled Trials
Abstract
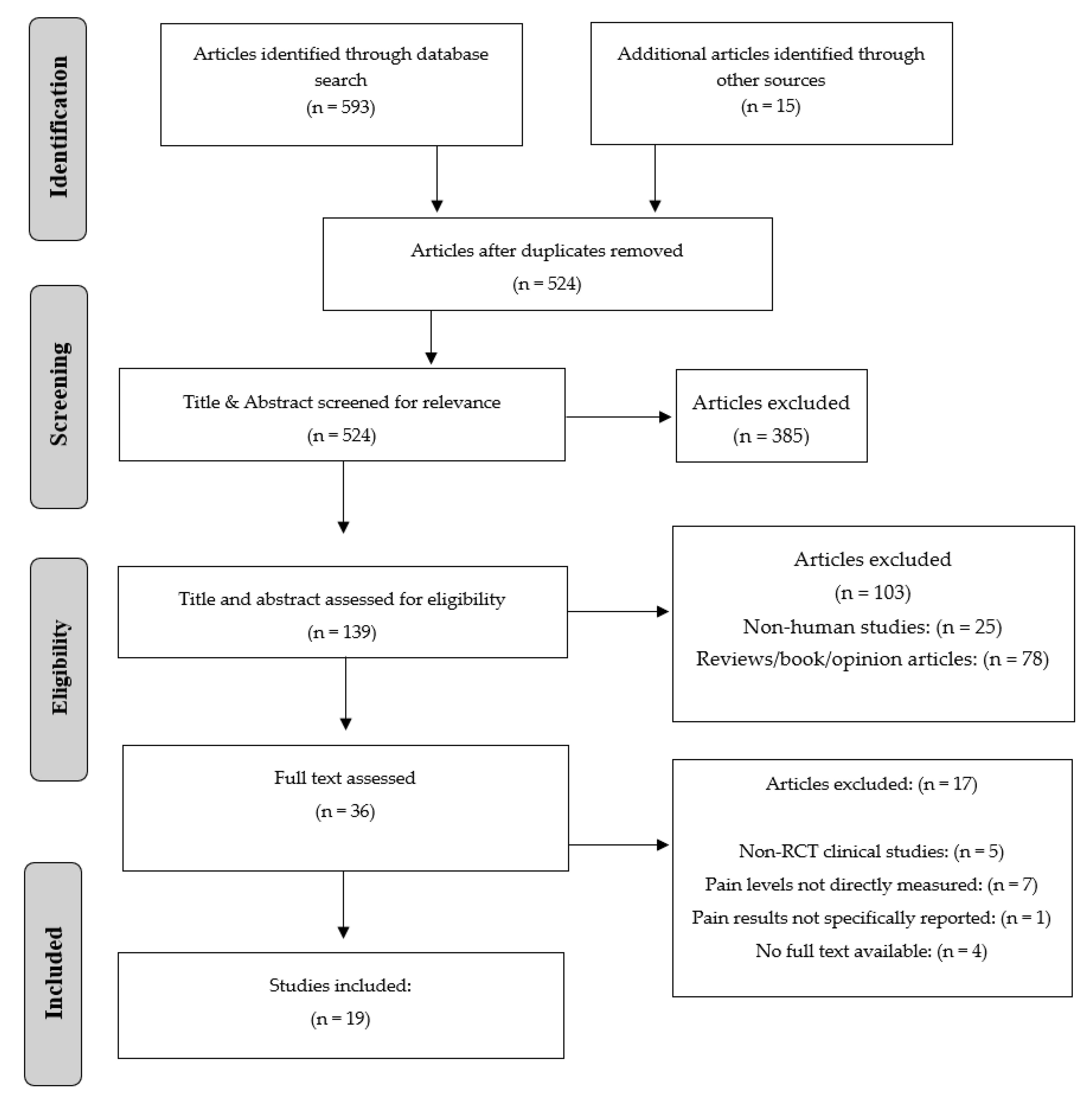
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Focused Question, PICO, and PRISMA
2.3. Eligibility Criteria
2.4. Data Sources and Search Strategy
2.5. Data Extraction
2.6. Risk of Bias Assessment
3. Results
3.1. General Characteristics of the Studies Included
3.2. Curcumin-Related Parameters
3.2.1. Outcomes of Included Studies
3.2.2. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crandall, J.A. An Introduction to Orofacial Pain. Dent. Clin. N. Am. 2018, 62, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Bertossi, D.; Barone, A.; Iurlaro, A.; Marconcini, S.; De Santis, D.; Finotti, M.; Procacci, P. Odontogenic Orofacial Infections. J. Craniofacial Surg. 2017, 28, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Christidis, N.; Al-Moraissi, E.A. Editorial: Orofacial Pain of Muscular Origin-From Pathophysiology to Treatment. Front. Oral Health 2021, 2, 825490. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, J. Neuropathic Orofacial Pain. Dent. Clin. N. Am. 2018, 62, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.A.; Ship, J.A.; Larach-Robinson, D. Estimated prevalence and distribution of reported orofacial pain in the United States. J. Am. Dent. Assoc. 1993, 124, 115–121. [Google Scholar] [CrossRef]
- Schiffman, E.L.; Fricton, J.R.; Haley, D.P.; Shapiro, B.L. The prevalence and treatment needs of subjects with temporomandibular disorders. J. Am. Dent. Assoc. 1990, 120, 295–303. [Google Scholar] [CrossRef]
- Martin, W.J.; Forouzanfar, T. The efficacy of anticonvulsants on orofacial pain: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2011, 111, 627–633. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Szydłowski, M.; Kozak, M.; Sobolewska, E. Comparison of Early Effectiveness of Three Different Intervention Methods in Patients with Chronic Orofacial Pain: A Randomized, Controlled Clinical Trial. Pain Res. Manag. 2019, 2019, 7954291. [Google Scholar] [CrossRef] [Green Version]
- Zubrzycki, M.; Stasiolek, M.; Zubrzycka, M. Opioid and endocannabinoid system in orofacial pain. Physiol. Res. 2019, 68, 705–715. [Google Scholar] [CrossRef]
- Bush, F.M. Occlusal therapy in the management of chronic orofacial pain. Anesth. Prog. 1984, 31, 10–16. [Google Scholar]
- Miernik, M.; Wieckiewicz, M.; Paradowska, A.; Wieckiewicz, W. Massage therapy in myofascial TMD pain management. Adv. Clin. Exp. Med. 2012, 21, 681–685. [Google Scholar] [PubMed]
- Javed, F.; Kellesarian, S.V.; Romanos, G.E. Role of diode lasers in oro-facial pain management. J. Biol. Regul. Homeost. Agents 2017, 31, 153–155. [Google Scholar] [PubMed]
- Bauer, B.A.; Tilburt, J.C.; Sood, A.; Li, G.X.; Wang, S.H. Complementary and alternative medicine therapies for chronic pain. Chin. J. Integr. Med. 2016, 22, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Dadjoo, S.; Michelogiannakis, D.; Rossouw, P.E.; Javed, F. Potential adjunct therapies for the management of temporomandibular disorders: An evidence-based review. Cranio 2022, 1–11. Available online: https://www.tandfonline.com/doi/full/10.1080/08869634.2022.2036437 (accessed on 5 March 2022). [CrossRef]
- Lavigne, G.J.; Sessle, B.J. Canadian Orofacial Pain Team workshop report on the global year against orofacial pain. Pain Res. Manag. 2015, 20, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Xu, X.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, Y.; Li, H.B. Bioactivity, Health Benefits, and Related Molecular Mechanisms of Curcumin: Current Progress, Challenges, and Perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, T.P. Uses of turmeric in dentistry: An update. Indian J. Dent. Res. 2009, 20, 107–109. [Google Scholar] [CrossRef]
- Maulina, T.; Diana, H.; Cahyanto, A.; Amaliya, A. The efficacy of curcumin in managing acute inflammation pain on the post-surgical removal of impacted third molars patients: A randomised controlled trial. J. Oral Rehabil. 2018, 45, 677–683. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Eke-Okoro, U.J.; Raffa, R.B.; Pergolizzi, J.V., Jr.; Breve, F.; Taylor, R., Jr. Curcumin in turmeric: Basic and clinical evidence for a potential role in analgesia. J. Clin. Pharm. Ther. 2018, 43, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep. 2018, 39, 1523–1531. [Google Scholar] [CrossRef]
- Tsai, I.C.; Hsu, C.W.; Chang, C.H.; Tseng, P.T.; Chang, K.V. The Effect of Curcumin Differs on Individual Cognitive Domains across Different Patient Populations: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 1235. [Google Scholar] [CrossRef]
- Hernández, M.; Wicz, S.; Corral, R.S. Cardioprotective actions of curcumin on the pathogenic NFAT/COX-2/prostaglandin E(2) pathway induced during Trypanosoma cruzi infection. Phytomedicine 2016, 23, 1392–1400. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.Y.; Yang, M.F.; Cao, M.Z.; Li, D.W.; Yang, X.Y.; Sun, J.Y.; Zhang, Z.Y.; Mao, L.L.; Zhang, S.; Wang, F.Z.; et al. Strategy to Suppress Oxidative Damage-Induced Neurotoxicity in PC12 Cells by Curcumin: The Role of ROS-Mediated DNA Damage and the MAPK and AKT Pathways. Mol. Neurobiol. 2016, 53, 369–378. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Lin, H.; Zhou, Y. Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans. BioMed Res. Int. 2018, 2018, 4508709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurup, V.P.; Barrios, C.S. Immunomodulatory effects of curcumin in allergy. Mol. Nutr. Food Res. 2008, 52, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Meghana, M.V.S.; Deshmukh, J.; Devarathanamma, M.V.; Asif, K.; Jyothi, L.; Sindhura, H. Comparison of effect of curcumin gel and noneugenol periodontal dressing in tissue response, early wound healing, and pain assessment following periodontal flap surgery in chronic periodontitis patients. J. Indian Soc. Periodontol. 2020, 24, 54–59. [Google Scholar]
- Brignardello-Petersen, R. Curcumin probably does not reduce pain importantly after impacted mandibular third-molar surgery compared with mefenamic acid. J. Am. Dent. Assoc. 2019, 150, e7. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.D.; Quatman, C.E.; Manring, M.M.; Siston, R.A.; Flanigan, D.C. How to write a systematic review. Am. J. Sports Med. 2014, 42, 2761–2768. [Google Scholar] [CrossRef]
- Deshmukh, R.A.; Bagewadi, A.S. Comparison of effectiveness of curcumin with triamcinolone acetonide in the gel form in treatment of minor recurrent aphthous stomatitis: A randomized clinical trial. Int. J. Pharm. Investig. 2014, 4, 138–141. [Google Scholar] [CrossRef] [Green Version]
- Lone, P.A.; Ahmed, S.W.; Prasad, V.; Ahmed, B. Role of turmeric in management of alveolar osteitis (dry socket): A randomised clinical study. J. Oral Biol. Craniofacial Res. 2018, 8, 44–47. [Google Scholar] [CrossRef] [Green Version]
- Mansourian, A.; Bahar, B.; Moosavi, M.S.; Amanlou, M.; Babaeifard, S. Comparison of the Efficacy of Topical Triamcinolone in Orabase and Curcumin in Orabase in Oral Graft-Versus-Host Disease. J. Dent. 2017, 14, 313–320. [Google Scholar]
- Nakao, R.; Ueno, T. Effects of oral moisturizing gel containing propolis following head and neck radiotherapy: Randomized controlled pilot trial. BDJ Open 2021, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Kia, S.J.; Basirat, M.; Mortezaie, T.; Moosavi, M.S. Comparison of oral Nano-Curcumin with oral prednisolone on oral lichen planus: A randomized double-blinded clinical trial. BMC Complement. Med. Ther. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of nanomicelle curcumin capsules on prevention and treatment of oral mucosits in patients under chemotherapy with or without head and neck radiotherapy: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 232. [Google Scholar] [CrossRef]
- Kia, S.J.; Mansourian, A.; Basirat, M.; Akhavan, M.; Mohtasham-Amiri, Z.; Moosavi, M.S. New concentration of curcumin orabase in recurrent aphthous stomatitis: A randomized, controlled clinical trial. J. Herb. Med. 2020, 22, 100336. [Google Scholar] [CrossRef]
- Kia, S.J.; Shirazian, S.; Mansourian, A.; Khodadadi Fard, L.; Ashnagar, S. Comparative Efficacy of Topical Curcumin and Triamcinolone for Oral Lichen Planus: A Randomized, Controlled Clinical Trial. J. Dent. 2015, 12, 789–796. [Google Scholar]
- Naik, R.; Nazneen, L.; Dhoble, A.; Thombre, A.S.; Saxena, U.; Kosta, S. Curcumin alone and curcumin with prednisone in management Oral Lichen Planuspatients. Eur. J. Mol. Clin. Med. 2021, 8, 3784–3788. [Google Scholar]
- Nosratzehi, T.; Arbabi-Kalati, F.; Hamishehkar, H.; Bagheri, S. Comparison of the Effects of Curcumin Mucoadhesive Paste and Local Corticosteroid on the Treatment of Erosive Oral Lichen Planus Lesions. J. Natl. Med. Assoc. 2018, 110, 92–97. [Google Scholar] [CrossRef]
- Naik, S.; Gupta, P.; Ashok, L.; Khaitan, T.; Shukla, A. A novel mixture of curcumin paste and prednisolone for treating oral lichen planus: A case controlled comparative study. J. Indian Acad. Oral Med. Radiol. 2019, 31, 286–292. [Google Scholar]
- Amirchaghmaghi, M.; Pakfetrat, A.; Delavarian, Z.; Ghalavani, H.; Ghazi, A. Evaluation of the Efficacy of Curcumin in the Treatment of Oral Lichen Planus: A Randomized Controlled Trial. J. Clin. Diagn. Res. 2016, 10, Zc134–Zc137. [Google Scholar] [CrossRef]
- Anil, A.; Gujjari, S.K.; Venkatesh, M.P. Evaluation of a curcumin-containing mucoadhesive film for periodontal postsurgical pain control. J. Indian Soc. Periodontol. 2019, 23, 461–468. [Google Scholar] [PubMed]
- Raman, P.; Pitty, R.; Krithika, C.L.; Anand, S.P.N.; Subramani, G.P. Topical Curcumin and Triamcinolone Acetonide in Recurrent Minor Aphthous Ulcers: A Pilot Trial. J. Contemp. Dent. Pract. 2020, 21, 884–890. [Google Scholar] [PubMed]
- Halim, D.S.; Khalik, N.I.; Taib, H.; Pohchi, A.; Hassan, A.; Alam, M.K. Novel material in the treatment of minor oral recurrent aphthous stomatitis. Int. Med. J. 2013, 20, 392–394. [Google Scholar]
- Srivastava, R.; Kundu, A.; Pradhan, D.; Jyoti, B.; Chokotiya, H.; Parashar, P. A Comparative Study to Evaluate the Efficacy of Curcumin Lozenges (TurmNova®) and Intralesional Corticosteroids with Hyaluronidase in Management of Oral Submucous Fibrosis. J. Contemp. Dent. Pract. 2021, 22, 751–755. [Google Scholar] [CrossRef]
- Mugilan, R.; Jayaswal, R.; Sowmya, R.; Vincent, S.S.; Vaishali, K.; Prasad, K. Effect of Curcumin on Healing of Extraction Sockets in Type II Diabetic Patients- A Pilot Study. J. Evol. Med. Dent. Sci. 2020, 9, 1045–1049. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), S240–S252. [Google Scholar]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Martín-Ares, M.; Barona-Dorado, C.; Martínez-Rodríguez, N.; Cortés-Bretón-Brinkmann, J.; Sanz-Alonso, J.; Martínez-González, J.M. Does the postoperative administration of antibiotics reduce the symptoms of lower third molar removal? A randomized double blind clinical study. J. Clin. Exp. Dent. 2017, 9, e1015–e1022. [Google Scholar]
- Halpern, L.R.; Dodson, T.B. Does prophylactic administration of systemic antibiotics prevent postoperative inflammatory complications after third molar surgery? J. Oral Maxillofac. Surg. 2007, 65, 177–185. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Ansari, M.T.; Berkman, N.D.; Chang, S.; Hartling, L.; McPheeters, M.; Santaguida, P.L.; Shamliyan, T.; Singh, K.; Tsertsvadze, A.; et al. AHRQ Methods for Effective Health Care; Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
| Authors et al. | Subjects (n) | Gender | Study Groups | Mean Age | Source of OFP | Scale for Rating Pain | Follow-Up |
|---|---|---|---|---|---|---|---|
| Maulina et al. [24] | 90 subjects | 44 males 46 females | Test group: Individuals using curcumin (45) Control-group: Individuals using MA (45) | Mean age: NR Range: 18 to 40 years | Extraction of impacted third molars | NRS | After 24 h |
| Meghana et al. [35] | 20 subjects (40 quadrants) | Male = 7 Female = 13 | Test group: Individuals receiving curcumin gel (n = 20) Control group: Individuals receiving periodontal dressing (n = 20) | All subjects mean age: 38.3 ± 9.82 | Periodontal flap surgery | Modified VAS | 1 week |
| Deshmukh et al. [39] | 60 subjects | 31 males 29 females | Test group: Individuals using curcumin (n = 30) Control group: Individuals using steroids (n = 30) | Mean age: 32.51 ± 11.797 years Range: 13 to 66 years | RAS | VAS | 7 days |
| Lone et. al. [40] | 178 subjects | NR | Test group: Individuals using curcumin in mustard oil (n = 90) Control group: Individuals using ZOE (n = 88) | NR | Alveolar osteitis | NR | NR |
| Mansourian et al. [41] | 26 subjects | 15 males 11 females | Test group: Individuals using curcumin (n = 13) Control group: Individuals using steroids (n = 13) | Mean age test group: 35.23 ± 7.67 years Mean age control group: 39.15 ± 12.13 years Range: 20 to 58 years | Graft vs. Host Disease * | VAS | 28 days |
| Nakao et al. [42] | 25 subjects ** | 16 males 9 females | Test group: Individuals using turmeric (n = 4) Control group: Individuals using placebo (n = 3) | Mean age test group: 53.4 ± 15.5 years Test group range: 28–55 years Mean age control group: 65.2 ± 9.3 years Control group range: 55–77 years | Head and neck radiotherapy | VAS | 1 month (mean intervention period = 37.5 ± 11.5 days) |
| Kia et al. [43] | 57 subjects | 48 males 9 females | Test group: Individuals using nano-curcumin (n = 29) Control group: Individuals using prednisolone (n = 28) | Mean age test group: 51.86 ± 9.94 Mean age control group: 53.67 ± 8.90 Range: NR | OLP | VAS | Four weeks |
| Kia et al. [44] | 50 subjects | 28 males 22 females | Test group: Individuals receiving curcumin (n = 25) Control group: Individuals receiving placebo (n = 25) | Mean age test group: 54.98 Mean age control group: 56.94 Mean age all subjects: 55.96 ± 1.10 | Chemotherapy-induced oral mucositis with and without head and neck radiotherapy | NRS | 7 weeks |
| Kia et al. [45] | 58 subjects | 36 males 22 females | Test group: Individuals receiving curcumin (n = 29) Control group: Individuals receiving triamcinolone (n = 29) | Mean age test group: 9.62 ± 43.72 Mean age control group: 45.05 ± 8.9 | RAS | VAS | 10 days |
| Kia et al. [46] | 50 subjects | 14 males 36 females | Test group: Individuals using curcumin (n = 25) Control group: Individuals using triamcinolone (n = 25) | Mean age test group: 49.24 ± 8.17 Mean age control group: 52.08 ± 9.20 All subjects’ range: 38–73 | OLP | VAS | Four weeks |
| Naik et al. [47] | 68 subjects | NR | Test group: Individuals using curcumin gel (n = 34) Control group: Individuals using curcumin gel and prednisone (n = 34) | NR | OLP | Modified VAS | 20 days |
| Nosratzehi et al. [48] | 40 subjects | 26 females 14 males; subjects matched for age and gender and divided into 2 groups | Test group: Individuals using curcumin (n = 20) Control group: Individuals using betamethasone with nystatin suspension (n = 20) | Mean age test group: 41.9 ± 11.22 Mean age control group: 38.5 ± 7.03 All subjects: 28–60 years | OLP | VAS | 12 weeks |
| Naik et al. [49] | 60 subjects | Males: 30 Females: 30 | Test group: Individuals using curcumin gel only (n = 30) Control group: Individuals using curcumin gel and prednisolong (n = 30) | Range test group: 13–61 Range control group: 21–65 Total range: 13–65 Mean age: NR | OLP | VAS | 20 days |
| Amirchaghmaghi et al. [50] | 20 subjects | 7 male 13 female | Test group: Individuals using curcumin tablets and dexamethasone/nystatin mouthwash (n = 12) Control group: Individuals using placebo and dexamethasome/nystatin mouthwash (n = 8) | Mean test group: 49.42 ± 11.22 Mean control group: 52.75 ± 9.43 | OLP | VAS | Four weeks |
| Anil et al. [51] | 15 subjects (30 sites) | 7 males 8 females | Test group: Individuals receiving curcumin (n = 15) Control group: Individuals receiving placebo (n = 15) | All subjects mean age: 42.27 ± 6.55 | Periodontal flap surgery | NRS | 48 h *** |
| Raman et al. [52] | 60 subjects | 19 males 41 females | Test group: Individuals receiving curcumin (n = 30) Control group: Individuals receiving triamcinolone (n = 30) | All subjects range: 18–30 Mean age test group: 21.3 Mean age control group: 21.6 | RAS | NRS | 8 days **** |
| Halim et al. [53] | 20 subjects | NR | Test group: Individuals receiving turmeric (n = 10) Control group: Individuals receiving triamcinolone (n = 10) | NR | RAS | VAS | 5 days |
| Srivastava et al. [54] | 80 subjects | 71 males 9 females | Test group: Individuals receiving curcumin with clove oil (n = 40) Control group: Individuals receiving dexamethasone with hyaluronidase (n = 40) | Mean age all subjects: 33.5 ± 9.5. Range all subjects: 31–40. | Oral submucous fibrosis | VAS | 3 months |
| Mugilan et al. [55] | 11 subjects | NR | Test group: Individuals receiving curcumin dressing (n = 6) | NR | Extraction socket healing in Type II diabetics | VAS | 7 days |
| Authors et al. | Condition | Curcumin (Mode of Use) | Control (Mode of Use) | Curcumin (Concentration) | Control (Concentration) | Curcumin (Frequency of Use) | Control (Frequency of Use) |
|---|---|---|---|---|---|---|---|
| Deshmukh et al. [39] | RAS | Oral gel | Oral gel | 10 mg curcumin/gram | 0.1% triamcinolone | Three × daily for 7 days | Three × daily for 7 days |
| Nakao et al. [42] | Head and neck radiotherapy | Oral gel | Oral gel | 160 μg/mL in oral moisturizing gel | Placebo | Once daily for 1 month | Once daily for 1 month |
| Kia et al. [43] | OLP | Capsule | Capsule | 80 mg nano-curcumin | 10 mg prednisolone | 1 cap daily for 4 weeks | 1 cap daily for 4 weeks |
| Kia et al. [44] | Chemotherapy-induced oral mucositis | Capsule | Capsule | 80 mg nanomicelle curcumin | Placebo | 2 × daily | 2 × daily |
| Kia et al. [45] | RAS | Oral gel | Oral gel | 5% curcumin | 0.1% triamcinolone | 3 × daily | 3 × daily |
| Kia et al. [46] | OLP | Oral paste | Oral paste | 5% curcumin | 0.1% triamcinolone | 3 × daily for four weeks | 3 × daily for four weeks |
| Naik et al. [47] | OLP | Oral gel | Paste of crushed tablet | Curcumin-concentration NR | Curcumin with Prednisone– concentration NR | 3 × daily for 15 min | 3 × daily for 15 min |
| Nosratzehi et al. [48] | VAS | Mucoadhesive paste | Lotion/Suspension | Curcumin-concentration NR | 0.1% Betamethasone and nystatin suspension (concentration NR) | 3 × daily | 3 × daily |
| Naik et al. [49] | OLP | Oral gel | Paste of crushed tablet/oral gel mix | Curcumin–concentration NR | 10 mg/tab prednisolone with | 3 × daily for 15 min | 3 × daily for 15 min |
| Raman et al. [52] | RAS | Oral gel | Oral paste | 2% Curcuma longa-10 mg | 0.1% triamcinolone | 3 × daily | 3 × daily |
| Halim et al. [53] | RAS | Powder | NR | NR | 0.1% triamcinolone | 2 × daily for 5 min | 2 × daily for 5 min |
| Authors et al. | Condition | Curcumin (Mode of Use) | Control (Mode of Use) | Curcumin (Concentration) | Control (Concentration) | Curcumin (Frequency of Use) | Control (Frequency of Use) |
|---|---|---|---|---|---|---|---|
| Maulina et al. [24] | Extraction of impacted third molars | Capsule | Capsule | 500 mg amoxicillin and 200 mg curcumin | 500 mg amoxicillin and 500 g mefenamic acid | 2 caps every 8 h for 24 h | 1 cap every 8 h for 24 h |
| Meghana et al. [35] | Periodontal flap surgery | Oral gels and ibuprofen tablet | Periodontal dressing and ibuprofen tablet | Curcumin-concentration NR 600 mg ibuprofen | COE-pak-concentration N/A 600 mg ibuprofen | Curcumin: Twice daily for 1 week Ibuprofen: 1 tablet every 8 h for 24 h and as needed thereafter | COE-pak: N/A Ibuprofen: 1 tablet every 8 h for 24 h an D as needed thereafter |
| Lone et al. [40] | Alveolar osteitis | Topical dressing | Topical dressing | Fresh ground turmeric in mustard oil-concentration NR | ZOE-concentration NR | Changed on alternate days until symptoms subsided | Changed on alternate days until symptoms subsided |
| Mansourian et al. [41] | Graft vs. Host Disease * | Oral gel | Oral gel | Curcumin in orabase-concentration NR Systemic prednisone and cyclosporine-concentration NR | Triamcinolone-concentration NR Systemic prednisone and cyclosporine-concentration NR | 28 days; frequency NR | 28 days; frequency NR |
| Amirchaghmaghi et al. [50] | OLP | Tablet and mouthwash | Tablet and mouthwash | 1000 mg curcumin and 0.5 mg dexamethasone with nystatin suspension 100,000 units | Placebo and 0.5 mg dexamethasone with nystatin suspension 100,000 units | Two 500 mg tablets, twice daily Mouthwash three times daily | Four tablets, twice daily Mouthwash three times daily |
| Anil et al. [51] | Periodontal flap surgery | Curcumin mucoadhesive film | Placebo mucoadhesive film | 0.5% curcumin Amoxicillin 500 mg Diclofenac 100 mg | Placebo Amoxicillin 500 mg Diclofenac 100 mg | Placed under COE-pak for 7 days | Placed under COE-pak for 7 days |
| Srivastava et al. [54] | Oral submucous fibrosis | Lozenge | Intralesional infiltration | 100 mg curcumin, 10 mg clove oil | 8 mg dexamethasone, 1500IU hyaluronidase, 0.5 mL 2% lignocaine | 3 times daily | 2 times per week |
| Mugilan et al. [55] | Extraction socket healing in Type II diabetics | Dressing | None | NR (Abbott Curenext gel) Hifenac (analgesic) concentration NR and novamox 500 mg | Hifenac (analgesic) concentration NR and novamox 500 mg | Placement immediately after extraction | N/A |
| Authors et al. | Main Outcomes | Conclusions |
|---|---|---|
| Deshmukh et al. [39] |
| Curcumin gel showed a similar efficacy to triamcinolone gel in the treatment of minor RAS. |
| Nakao et al. [42] |
| Turmeric in oral gel does not effectively relieve oral pain after head and neck radiotherapy. |
| Kia et. al. [43] |
| Systemic curcumin showed a similar efficacy to systemic prednisone in the treatment of OLP. |
| Kia et. al. [46] |
| Topical curcumin showed a similar outcome to topical triamcinolone in the treatment of OLP. |
| Naik et al. [47] |
| Topical curcumin with prednisone is more effective than topical curcumin alone in the treatment of OLP. |
| Nosratzehi et al. [48] |
| Topical curcumin showed a similar outcome to topical betamethasone with nystatin suspension in the treatment of OLP. |
| Naik et al. [49] |
| Topical curcumin with prednisolone is significantly more effective in reducing pain compared to topical curcumin alone in the treatment of OLP. |
| Raman et al. [52] |
| Triamcinolone paste reduces self-rated pain scores from recurrent aphthous ulcers more rapidly as compared to curcumin gel. |
| Kia et al. [45] |
| 5% Curcumin in orabase is as effective as 0.1% triamcinolone in reducing pain from aphthous ulcers. |
| Halim et al. [53] |
| Turmeric powder and 0.1% triamcinolone had similar efficacy in reducing pain from aphthous ulcers. |
| Kia et al. [44] |
| Curcumin capsules were effective in decreasing pain in patients undergoing chemotherapy either with or without head and neck radiotherapy. |
| Authors et al. | Main Outcome | Conclusions |
|---|---|---|
| Maulina et al. [24] |
| Curcumin with amoxicillin is more effective for pain management after exodontia than mefenamic acid with amoxicillin. |
| Lone et. al. [40] |
| Curcumin dressing with mustard oil showed greater efficacy at subsiding symptoms of alveolar osteitis compared to ZOE dressing. |
| Amirchaghmaghi et al. [50] |
| Systemic curcumin had no detectable effect in the treatment of OLP in the presence of corticosteroid therapy with dexamethasone and nystatin suspension mouth rinse. |
| Meghana et al. [35] |
| Both periodontal dressing and curcumin have a positive effect on pain control after periodontal flap surgery. |
| Srivastava et al. [54] |
| Curcumin with clove oil is an effective alternative treatment with similar pain outcomes when compared to intralesional dexamethasone and hyaluronidase infiltration in the treatment of oral submucous fibrosis. |
| Anil et al. [51] |
| Curcumin mucoadhesive film showed greater analgesic properties in the presence of an amoxicillin regimen as compared to placebo for periodontal post-surgical pain control. |
| Mugilan et al. [55] |
| Curcumin oral gel dressing post-extraction in diabetic patients showed slightly greater potential for pain reduction in the presence of a hifenac and novamox regimen. |
| Mansourian et al. [41] |
| Curcumin gel showed a similar efficacy to triamcinolone gel in the presence of systemic prednisone and cyclosporine for the treatment of GVHD. |
| Domain | Maulina et al. [24] | Meghana et al. [35] | Deshmukh et al. [39] | Lone et al. [40] | Mansourian et al. [41] | Nakao et al. [42] | Kia et al. [43] | Kia et al. [46] | Naik et al. [47] | Nosratzehi et al. [48] | Naik et al. [49] | Amirchaghmaghi et al. [50] | Anil et al. [51] | Raman et al. [52] | Kia et al. [45] | Halim et al. [53] | Kia et al. [44] | Srivastava et al. [54] | Mugilan et al. [55] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ○ | ○ | ○ | ‡ | ○ | ○ | ○ | ○ | ‡ | ● | ‡ | ○ | ○ | ○ | ○ | ‡ | ○ | ○ | ○ |
| 2 | ○ | ● | ○ | ‡ | ○ | ○ | ○ | ‡ | ‡ | ● | ‡ | ○ | ○ | ‡ | ‡ | ‡ | ○ | ● | ‡ |
| 3 | ● | ● | ‡ | ● | ○ | ○ | ○ | ○ | ‡ | ● | ● | ○ | ● | ● | ‡ | ● | ○ | ● | ● |
| 4 | ‡ | ● | ‡ | ‡ | ‡ | ‡ | ○ | ○ | ‡ | ● | ‡ | ‡ | ● | ● | ● | ● | ○ | ● | ● |
| 5 | ● | ○ | ○ | ‡ | ○ | ● | ‡ | ○ | ○ | ○ | ‡ | ○ | ○ | ● | ● | ○ | ○ | ○ | ○ |
| 6 | ● | ○ | ‡ | ● | ‡ | ○ | ○ | ○ | ● | ○ | ○ | ○ | ● | ● | ○ | λ | ○ | ○ | ○ |
| 7 | ● | ● | ● | ○ | ● | ● | ○ | ● | ● | ● | ● | ○ | ● | ● | ○ | ● | ○ | ● | ○ |
| Summary | ● | ◗ | ◗ | ● | ◗ | ◗ | ○ | ◗ | ● | ● | ● | ○ | ◗ | ● | ◗ | ● | ○ | ◗ | ◗ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterniczuk, B.; Rossouw, P.E.; Michelogiannakis, D.; Javed, F. Effectiveness of Curcumin in Reducing Self-Rated Pain-Levels in the Orofacial Region: A Systematic Review of Randomized-Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 6443. https://doi.org/10.3390/ijerph19116443
Sterniczuk B, Rossouw PE, Michelogiannakis D, Javed F. Effectiveness of Curcumin in Reducing Self-Rated Pain-Levels in the Orofacial Region: A Systematic Review of Randomized-Controlled Trials. International Journal of Environmental Research and Public Health. 2022; 19(11):6443. https://doi.org/10.3390/ijerph19116443
Chicago/Turabian StyleSterniczuk, Barbara, Paul Emile Rossouw, Dimitrios Michelogiannakis, and Fawad Javed. 2022. "Effectiveness of Curcumin in Reducing Self-Rated Pain-Levels in the Orofacial Region: A Systematic Review of Randomized-Controlled Trials" International Journal of Environmental Research and Public Health 19, no. 11: 6443. https://doi.org/10.3390/ijerph19116443
APA StyleSterniczuk, B., Rossouw, P. E., Michelogiannakis, D., & Javed, F. (2022). Effectiveness of Curcumin in Reducing Self-Rated Pain-Levels in the Orofacial Region: A Systematic Review of Randomized-Controlled Trials. International Journal of Environmental Research and Public Health, 19(11), 6443. https://doi.org/10.3390/ijerph19116443








