Do They Really Work? Quantifying Fabric Mask Effectiveness to Improve Public Health Messaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Face Barrier Selection
2.2. Face Barrier Characteristics
2.3. Face Mount Apparatus
2.4. Filtration Testing Methods
2.5. Data Analysis and Statistics
3. Results
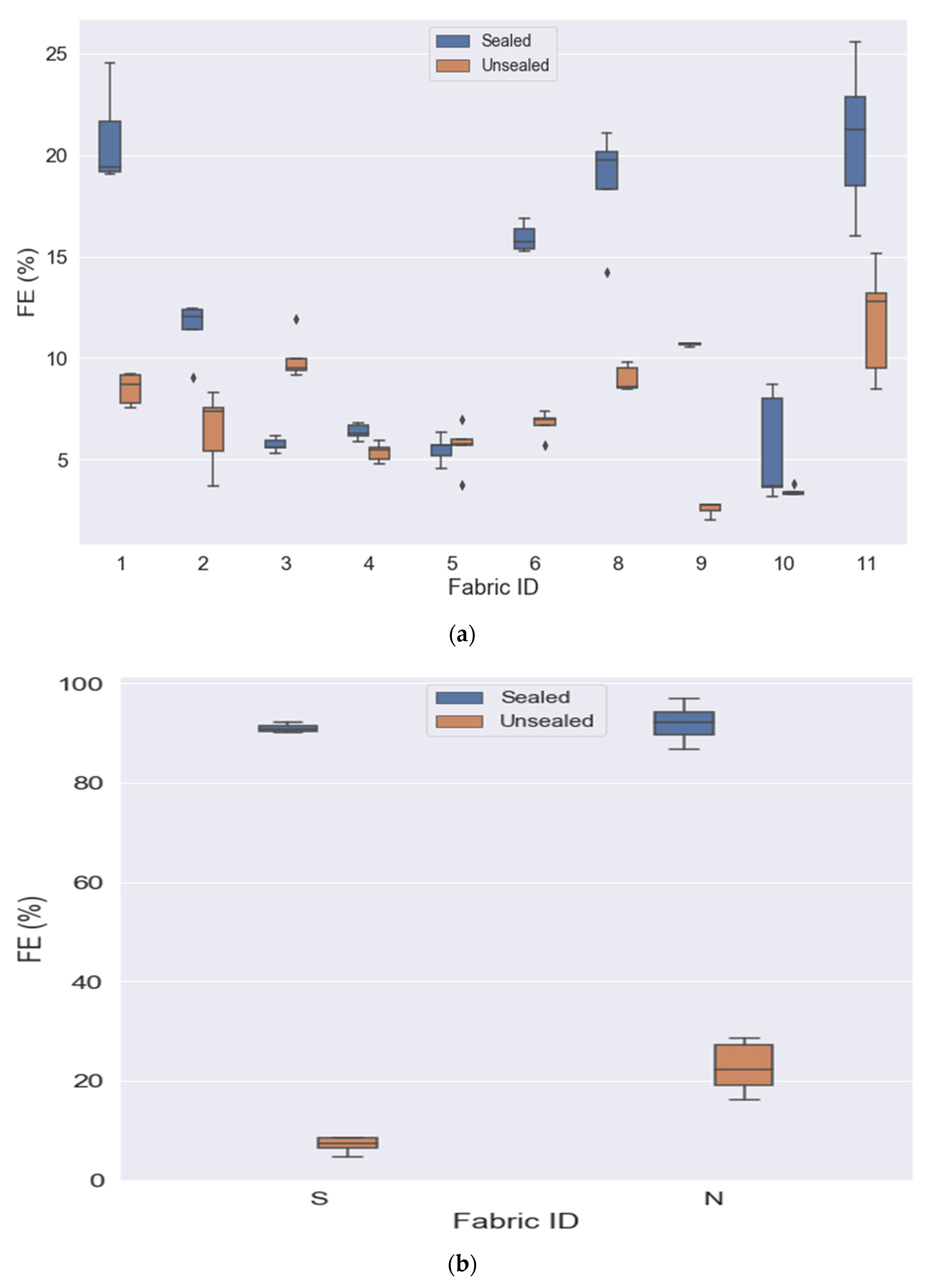
3.1. Overall Mask Comparison—Sealed Mask Setting
3.2. Sealed vs. Unsealed
3.3. Outflow vs. Inflow
3.4. Comparison by Fabric Mask Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. COVID-19 Archived Webpage. Centers for Disease Control and Prevention, 11 February 2022. Available online: https://www.cdc.gov/coronavirus/2019-nCoV/index.html (accessed on 12 April 2022).
- World Health Organization [WHO]. Advice on the Use of Masks in the Community, during Home Care and in Healthcare Settings in the Context of the Novel Coronavirus (COVID-19) Outbreak. Available online: https://www.who.int/publications-detail-redirect/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak (accessed on 12 April 2022).
- Seeger, M. Crisis Communication Researcher Shares 5 Key Principles that Officials Should Use in Coronavirus, The Conversation. Available online: http://theconversation.com/crisis-communication-researcher-shares-5-key-principles-that-officials-should-use-in-coronavirus-133046 (accessed on 12 April 2022).
- Wang, Y.; Hao, H.; Platt, L.S. Examining risk and crisis communications of government agencies and stakeholders during early-stages of COVID-19 on Twitter. Comput. Hum. Behav. 2021, 114, 106568. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, G.; Hui, D.S.; Ntoumi, F.; Maeurer, M.; Zumla, A. Toning down the 2019-nCoV media hype—and restoring hope. Lancet Respir. Med. 2020, 8, 230–231. [Google Scholar] [CrossRef] [Green Version]
- Standard Specification for Barrier Face Coverings; ASTM International: West Conshohocken, PA, USA, 2020.
- Klompas, M.; Morris, C.A.; Sinclair, J.; Pearson, M.; Shenoy, E.S. Universal masking in hospitals in the COVID-19 era. N. Engl. J. Med. 2020, 382, e63. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Jammalamadaka, U.; Meacham, K.W.; Hoegger, M.J.; Burke, B.A.; Morris, J.A.; Scott, A.R.; O’Connor, Z.; Gan, C.; Hu, J.; et al. Quantitative fit tested N95 respirator-Alternatives generated with CT imaging and 3D printing: A response to potential shortages during the COVID-19 pandemic. Acad. Radiol. 2021, 28, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Suen, L.K.P.; Guo, Y.P.; Ho, S.S.K.; Au-Yeung, C.H.; Lam, S.C. Comparing mask fit and usability of traditional and nanofibre N95 filtering facepiece respirators before and after nursing procedures. J. Hosp. Infect. 2020, 104, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Drewnick, F.; Pikmann, J.; Fachinger, F.; Moormann, L.; Sprang, F.; Borrmann, S. Aerosol filtration efficiency of household materials for homemade face masks: Influence of material properties, particle size, particle electrical charge, face velocity, and leaks. Aerosol Sci. Technol. 2021, 55, 63–79. [Google Scholar] [CrossRef]
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Assessment of N95 Respirator Decontamination and Re-Use for SARS-CoV-2. Infectious Diseases (Except HIV/AIDS). MedRxiv, 2020; preprint. [Google Scholar] [CrossRef] [Green Version]
- Clase, C.M.; Fu, E.L.; Joseph, M.; Beale, R.C.L.; Dolovich, M.B.; Jardine, M.; Mann, J.F.E.; Pecoits-Filho, R.; Winkelmayer, W.C.; Carrero, J.J. Cloth masks may prevent transmission of COVID-19: An evidence-based, risk-based approach. Ann. Intern. Med. 2020, 173, 489–491. [Google Scholar] [CrossRef]
- Viola, I.M.; Peterson, B.; Pisetta, G.; Pavar, G.; Akhtar, H.; Menoloascina, F.; Mangano, E.; Dunn, K.E.; Gabl, R.; Nila, A.; et al. Face coverings, aerosol dispersion and mitigation of virus transmission risk. IEEE Open J. Eng. Med. Biol. 2021, 2, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Burch, R.; Strawderman, L.; Black, C.; Saucier, D.; Rickert, J.; Wilson, J.; Bealor, S.A.; Ratledge, M.; Fava, S.; et al. Preliminary evaluation of filtration efficiency and differential pressure ASTM F3502 testing methods of non-medical masks using a face filtration mount. Int. J. Environ. Res. Public. Health 2021, 18, 4124. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, A.; Brochot, C.; Layne, B. Leakage versus material filtration in barrier facemask efficiency. Health 2021, 13, 439–453. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, L.; Xiao, W.; Yu, X.; Wang, H.; Wang, Q.; Lin, Y.L.; Kilinc-Balci, F.S.; Price, A.; Chu, L.; et al. Household materials selection for homemade cloth face coverings and their filtration efficiency enhancement with triboelectric charging. Nano Lett. 2020, 20, 5544–5552. [Google Scholar] [CrossRef] [PubMed]
- Kähler, C.J.; Hain, R. Fundamental protective mechanisms of face masks against droplet infections. J. Aerosol Sci. 2020, 148, 105617. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Blachere, F.M.; Beezhold, D.H.; Law, B.F.; Derk, R.C.; Hettick, J.M.; Woodfork, K.; Goldsmith, W.T.; Harris, J.R.; Duling, M.G.; et al. A comparison of performance metrics for cloth masks as source control devices for simulated cough and exhalation aerosols. Aerosol Sci. Technol. 2021, 55, 1125–1142. [Google Scholar] [CrossRef]
- Chiera, S.; Cristoforetti, A.; Benedetti, L.; Nollo, G.; Borro, L.; Mazzei, L.; Tessarolo, F. A Simple Method to Quantify Outward Leakage of Medical Face Masks and Barrier Face Coverings: Implication for the Overall Filtration Efficiency. Int. J. Environ. Res. Public. Health 2022, 19, 3548. [Google Scholar] [CrossRef] [PubMed]
- National Personal Protective Technology Laboratory. Determination of Particulate Filter Efficiency Level for N95 Series Filters Against Solid Particulates for Non-Powered, Air-Purifying Respirators Standard Testing Procedure (STP); National Institute of Occupational Safety & Health: Washington DC, USA, 2018; p. 9. [Google Scholar]
- Tso, R.V.; Cowling, B.J. Importance of face masks for COVID-19: A call for effective public education. Clin. Infect. Dis. 2020, 71, 2195–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.D.; Young Leslie, H.; Sharafaddin-zadeh, Y.; Noels, K.; Lou, N.M. Public health messages about face masks early in the COVID-19 pandemic: Perceptions of and impacts on canadians. J. Community Health 2021, 46, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Standard Test Method for End (Warp) and Pick (Filling) Count of Woven Fabrics. Available online: https://www.astm.org/d3775-17e01.html (accessed on 12 April 2022).
- Standard Test Method for Wale and Course Count of Weft Knitted Fabrics. Available online: https://www.astm.org/d8007-15r19.html (accessed on 12 April 2022).
- Standard Test Methods for Mass per Unit Area (Weight) of Fabric. Available online: https://www.astm.org/d3776_d3776m-20.html (accessed on 12 April 2022).
- Standard Test Method for Thickness of Textile Materials. Available online: https://www.astm.org/d1777-96r19.html (accessed on 12 April 2022).
- Stannard, C.; Freeman, C.; Strawderman, L.; Moore, M.; Newhauser, W.; Burch, R.; Black, C.; Saucier, D. Evaluating the fit-effectiveness of fabric-based reusable face masks on 3D printed NIOSH headforms. Text. Res. J. 2022, 004051752210892. [Google Scholar] [CrossRef]

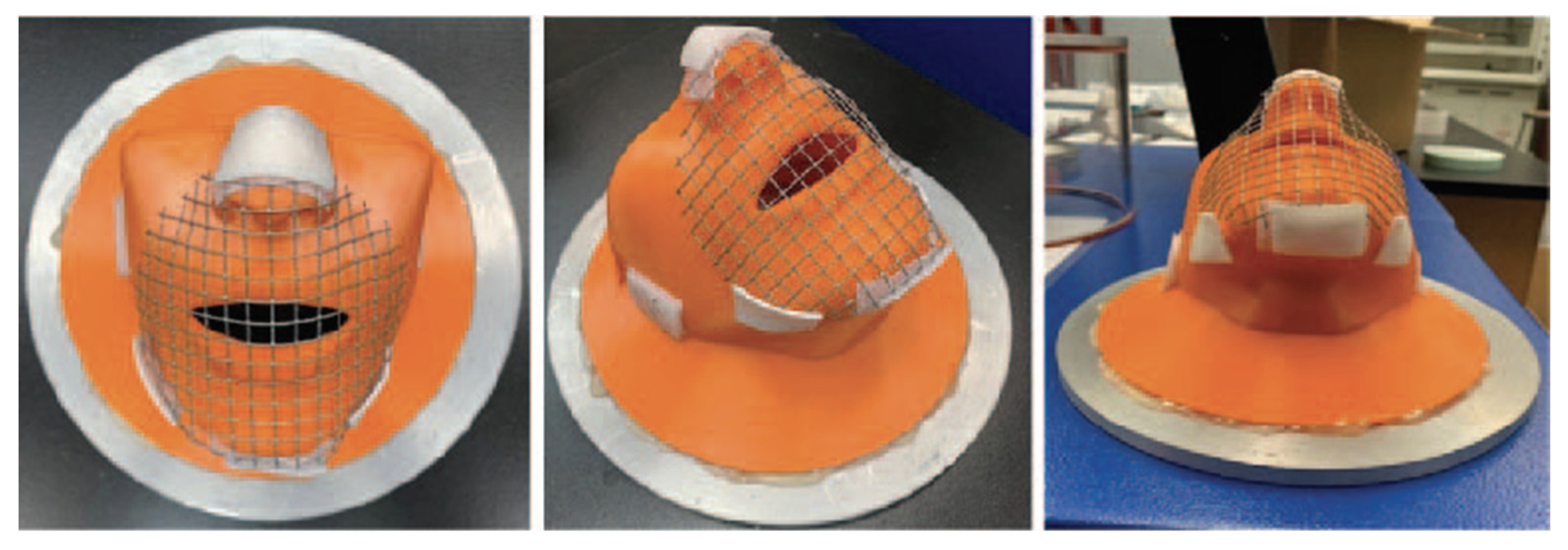
| Mask ID | Available to Purchase | Mask Layer | Fabric Structure | Fiber Content | Thread/ Loop (Per 10 cm2) | Mass (g/m2) | Thickness (mm) |
|---|---|---|---|---|---|---|---|
| F1 | N 1 | Outer | Tricot | 82% nylon 18% spandex | 293 | 296 | 1.05 |
| F1 | - | Lining | Single knit | 87% cotton 13% polyester | 153 | - | - |
| F2 | Y 1 | Outer | Double knit | 93% polyester 7% spandex | 135 | 431 | 1.50 |
| F2 | - | Lining | Raschel warp knit | 92% polyester 8% spandex | 217 | - | - |
| F3 | Y | Outer | Double weft knit | 100% polyester | 195 | 278 | 0.97 |
| F3 | - | Lining | Single knit | 50% viscose 50% cotton | 170 | - | - |
| F4 | Y | Outer | Raschel warp knit | 100% polyester | 170 | 281 | 1.21 |
| F4 | - | Inter-lining | Non-woven | 100% polyester | N/A | - | - |
| F4 | - | Lining | Raschel warp knit | 100% polyester | 209 | - | - |
| F5 | Y | Outer | Plain Weave | 100% cotton | 310 | 277 | 0.62 |
| F5 | - | Lining | Single Knit | 96% polyester 4% spandex | 192 | - | |
| F6 | N | Outer | Single Knit | 89% cotton 11% spandex | 229 | 322 | 1.25 |
| F6 | - | Lining | Single knit | 87% cotton 13% polyester | 153 | - | |
| F7 | Y | Outer | Single knit | 83% polyester 17% spandex | 195 | 192 | 0.46 |
| F8 | Y | Outer | Double weft knit | 100% polyester | 212 | 320 | 0.99 |
| F8 | - | Lining | Double weft knit | 100% polyester | 211 | - | - |
| F9 | Y | Outer | Plain weave | 100% cotton | 245 | 241 | 0.61 |
| F9 | - | Lining | Plain weave | 100% cotton | 276 | - | - |
| F10 | Y | Outer | Plain weave | 100% cotton | 324 | 206 | 0.40 |
| F10 | - | Lining | Plain weave | 100% cotton | 324 | - | - |
| F11 | Y | Outer | Double knit | 100% polyester | 144 | 439 | 2.01 |
| F11 | - | Inter-lining | Open cell foam | 100% polyurethane | N/A | - | - |
| F11 | - | Lining | Double weft knit | 77% nylon/23% spandex | 234 | - | - |
| Surgical | Y | N/A | Spun bond non-woven | 100% polypropylene | N/A | 25 | 0.4 |
| N95 | Y | N/A | Spun bond/ melt-blown non-woven | 100% polypropylene | N/A | 75 | 2.14 |
| Mask ID | FE (%) (M(SD)) | Comparison | Effect Size (Cohen’s D) | |
|---|---|---|---|---|
| Sealed | Unsealed | |||
| F1 | 20.840 (1.738) | 8.482 (0.769) | t(4) = 13.32, p < 0.001 | 5.917 |
| F2 | 11.592 (1.880) | 6.486 (1.886) | t(4) = 8.552, p = 0.001 | 3.825 |
| F3 | 6.931 (0.528) | 9.987 (1.114) | t(4) = −5.111, p = 0.007 | −2.286 |
| F4 | 7.174 (0.486) | 5.359 (0.458) | t(4) = 29.283, p < 0.001 | 13.096 |
| F5 | 6.186 (1.339) | 5.635 (1.168) | t(4) = 0.494, p = 0.647 | 0.221 |
| F6 | 19.962 (5.532) | 6.757 (0.628) | t(4) = 4.851, p = 0.008 | 2.170 |
| F8 | 18.230 (2.758) | 8.992 (0.621) | t(4) = 8.543, p = 0.001 | 2.418 |
| F9 | 10.874 (1.284) | 2.568 (0.332) | t(2) = 8.796, p = 0.013 | 5.079 |
| F10 | 5.725 (3.120) | 3.429 (0.237) | t(4) = 1.561, p = 0.194 | 3.290 |
| F11 | 21.967 (2.997) | 11.808 (2.755) | t(4) = 4.713, p = 0.009 | 4.820 |
| S | 91.304 (0.788) | 7.064 (1.617) | t(4) = 97.157, p = <0.001 | 43.450 |
| N95 | 90.427 (2.812) | 22.598 (5.229) | t(4) = 20.871, p = <0.001 | 9.334 |
| Mask ID | dP mm H2O (M(SD)) | Comparison | Effect Size (Cohen’s D) | |
|---|---|---|---|---|
| Sealed | Unsealed | |||
| F1 | 6.760 (0.462) | 3.040 (0.428) | t(4) = 10.11, p = 0.001 | 4.521 |
| F2 | 3.280 (0.335) | 2.140 (0.114) | t(4) = 7.421, p = 0.002 | 3.319 |
| F3 | 1.500 (0.122) | 2.360 (0.445) | t(4) = −3.984, p = 0.016 | −1.782 |
| F4 | 1.640 (0.230) | 1.340 (0.207) | t(4) = 1.604, p = 0.184 | 0.717 |
| F5 | 2.920 (0.286) | 1.840 (0.089) | t(4) = 8.703, p = 0.001 | 3.892 |
| F6 | 5.680 (0.427) | 1.640 (0.114) | t(4) = 21.719, p < 0.001 | 9.713 |
| F8 | 4.100 (0.548) | 1.880 (0.228) | t(4) = 7.929, p = 0.001 | 0.626 |
| F9 | 11.633 (0.551) | 1.300 (0.332) | t(2) = 39.307, p = 0.001 | 22.694 |
| F10 | 5.860 (2.900) | 1.180 (0.259) | t(4) = 3.749, p = 0.02 | 2.791 |
| F11 | 2.940 (0.537) | 1.360 (0.456) | t(4) = 4.427, p = 0.011 | 0.798 |
| S | 11.340 (0.888) | 0.760 (0.182) | t(4) = 28.136, p = <0.001 | 12.583 |
| N95 | 12.480 (1.602) | 3.460 (0.760) | t(4) = 10.303, p = 0.001 | 4.608 |
| Mask ID | FE (%) (M(SD)) | Comparison | Effect Size (Cohen’s D) | |
|---|---|---|---|---|
| Outflow | Inflow | |||
| F1 | 20.840 (1.738) | 20.766 (2.348) | t(4) = 0.091, p = 0.932 | 0.041 |
| F2 | 11.592 (1.880) | 11.483 (1.412) | t(4) = 0.104, p = 0.992 | 0.046 |
| F3 | 6.931 (0.528) | 5.717 (0.332) | t(4) = 6.388, p = 0.003 | 2.857 |
| F4 | 7.174 (0.486) | 6.362 (0.383) | t(4) = 2.419, p = 0.073 | 1.082 |
| F5 | 6.186 (1.339) | 5.500 (0.654) | t(4) = 1.881, p = 0.133 | 0.841 |
| F6 | 19.962 (5.532) | 15.930 (0.698) | t(4) = 1.623, p = 0.180 | 0.726 |
| F8 | 18.230 (2.758) | 18.709 (2.686) | t(4) = −1.62, p = 0.180 | 0.661 |
| F9 | 10.874 (1.284) | 10.675 (0.118) | t(2) = 0.27, p = 0.812 | 1.272 |
| F10 | 5.725 (3.120) | 5.443 (2.666) | t(4) = 0.578, p = 0.594 | 1.093 |
| F11 | 21.967 (2.997) | 20.822 (3.713) | t(4) = 1.219, p = 0.290 | 2.100 |
| S | 91.304 (0.788) | 90.521 (0.404) | t(4) = 3.205, p = 0.033 | 1.433 |
| N95 | 90.427 (2.812) | 93.558 (2.874) | t(4) = −6.933, p = 0.002 | −3.100 |
| Mask ID | dP mm H2O (M(SD)) | Comparison | Effect Size (Cohen’s D) | |
|---|---|---|---|---|
| Outflow | Inflow | |||
| F1 | 6.760 (0.462) | 15.220 (1.994) | t(4) = −10.595, p < 0.001 | −4.738 |
| F2 | 3.280 (0.335) | 5.820 (0.773) | t(4) = −10.772, p < 0.001 | −4.817 |
| F3 | 1.500 (0.122) | 2.580 (0.084) | t(4) = −28.864, p < 0.001 | −12.908 |
| F4 | 1.640 (0.230) | 2.840 (0.297) | t(4) = −12.649, p < 0.001 | −5.657 |
| F5 | 2.920 (0.286) | 4.820 (0.536) | t(4) = −11.355, p < 0.001 | −5.078 |
| F6 | 5.680 (0.427) | 8.840 (0.702) | t(4) = −18.685, p < 0.001 | −8.356 |
| F8 | 4.100 (0.548) | 6.860 (0.623) | t(4) = −13.069, p < 0.001 | 0.472 |
| F9 | 11.633 (0.551) | 15.267 (0.379) | t(2) = −12.503, p = 0.006 | 0.503 |
| F10 | 5.860 (2.900) | 7.800 (3.621) | t(4) = −3.29, p = 0.030 | 1.318 |
| F11 | 2.940 (0.537) | 4.000 (0.704) | t(4) = −10.296, p = 0.001 | 0.230 |
| S | 11.340 (0.888) | 13.800 (1.005) | t(4) = −20.359, p = <0.001 | −9.105 |
| N95 | 12.480 (1.602) | 14.880 (2.153) | t(4) = −8.09, p = 0.001 | −3.618 |
| Mask Type | FE % (M(SD)) | Comparison | dP mm H2O (M(SD)) | Comparison | |
|---|---|---|---|---|---|
| GSM | <300 | 9.532 (5.768) | U(48) = 481, p < 0.001 | 4.582 (3.450) | U(48) = 307, p = 0.572 |
| >300 | 17.938 (5.163) | 4.000 (1.168) | |||
| Thickness | <1.0 mm | 9.477 (5.338) | U(48) = 458, p < 0.001 | 4.643 (3.408) | U(48) = 288, p = 0.992 |
| >1.0 mm | 16.307 (6.594) | 4.060 (1.953) | |||
| Fiber | Natural | 7.091 (2.969) | χ2(2, N = 48) = 16.676, p < 0.001 | 6.062 (3.843) | χ2(2, N = 48) = 18.800, p < 0.001 |
| Synthetic | 13.576 (7.088) | 2.545 (1.148) | |||
| Blend | 17.464 (5.407) | 5.240 (1.553) |
| Mask Type | FE % (M(SD)) | Comparison | dP mm H2O (M(SD)) | Comparison | |
|---|---|---|---|---|---|
| GSM | <300 | 8.963 (5.985) | U(48) = 478, p < 0.001 | 7.575 (5.494) | U(48) = 316, p = 0.451 |
| >300 | 16.736 (4.218) | 6.380 (1.908) | |||
| Thickness | <1.0 mm | 9.081 (5.711) | U(48) = 455, p < 0.001 | 6.787 (4.179) | U(48) = 306.5, p = 0.695 |
| >1.0 mm | 15.073 (5.995) | 7.344 (4.623) | |||
| Fiber | Natural | 6.672 (2.779) | χ2(2, N = 48) = 16.132, p < 0.001 | 8.377 (4.666) | χ2(2, N = 48) = 21.205, p < 0.001 |
| Synthetic | 12.903 (7.396) | 4.070 (1.799) | |||
| Blend | 16.060 (4.205) | 9.960 (4.231) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeman, C.; Burch, R.; Strawderman, L.; Black, C.; Saucier, D.; Rickert, J.; Wilson, J.; Seitz, H.; Stull, J. Do They Really Work? Quantifying Fabric Mask Effectiveness to Improve Public Health Messaging. Int. J. Environ. Res. Public Health 2022, 19, 6372. https://doi.org/10.3390/ijerph19116372
Freeman C, Burch R, Strawderman L, Black C, Saucier D, Rickert J, Wilson J, Seitz H, Stull J. Do They Really Work? Quantifying Fabric Mask Effectiveness to Improve Public Health Messaging. International Journal of Environmental Research and Public Health. 2022; 19(11):6372. https://doi.org/10.3390/ijerph19116372
Chicago/Turabian StyleFreeman, Charles, Reuben Burch, Lesley Strawderman, Catherine Black, David Saucier, Jaime Rickert, John Wilson, Holli Seitz, and Jeffrey Stull. 2022. "Do They Really Work? Quantifying Fabric Mask Effectiveness to Improve Public Health Messaging" International Journal of Environmental Research and Public Health 19, no. 11: 6372. https://doi.org/10.3390/ijerph19116372
APA StyleFreeman, C., Burch, R., Strawderman, L., Black, C., Saucier, D., Rickert, J., Wilson, J., Seitz, H., & Stull, J. (2022). Do They Really Work? Quantifying Fabric Mask Effectiveness to Improve Public Health Messaging. International Journal of Environmental Research and Public Health, 19(11), 6372. https://doi.org/10.3390/ijerph19116372







