Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
- Cseq—carbon sequestration [g C m−2 a−1]
- TR—time since restoration [y]
- AL—lake area [m2]
- MOM—mass of organic matter in sediment after restoration [t]
- %OM—average organic matter content in sediment [%]
- MDS—mass of dry sediment [t]
- %DM—average percentage of dry matter in sediment [%]
- SpD—average specific density of sediment [g cm−3]
- SL—sediment layer thickness after restoration [m].
3. Results
4. Discussion
4.1. Carbon Accumulation in the Bottom Sediments of Water Bodies
4.2. Prospects for the Restoration of Formerly Drained Water Bodies
- Water bodies are dominant landscape features that enhance the local scenery;
- Water bodies are habitats for aquatic fauna and flora (this depends on their ecological state), which contribute to the biological diversity of rural areas;
- Measures aiming to improve water quality are difficult to implement in moderately to highly eutrophic water bodies. Restored lakes are initially characterised by clear-water conditions, but the clear state is much more difficult to stabilise than the turbid state with increased phytoplankton growth [38]. For this reason, the rationale behind many lake restoration projects is often questioned;
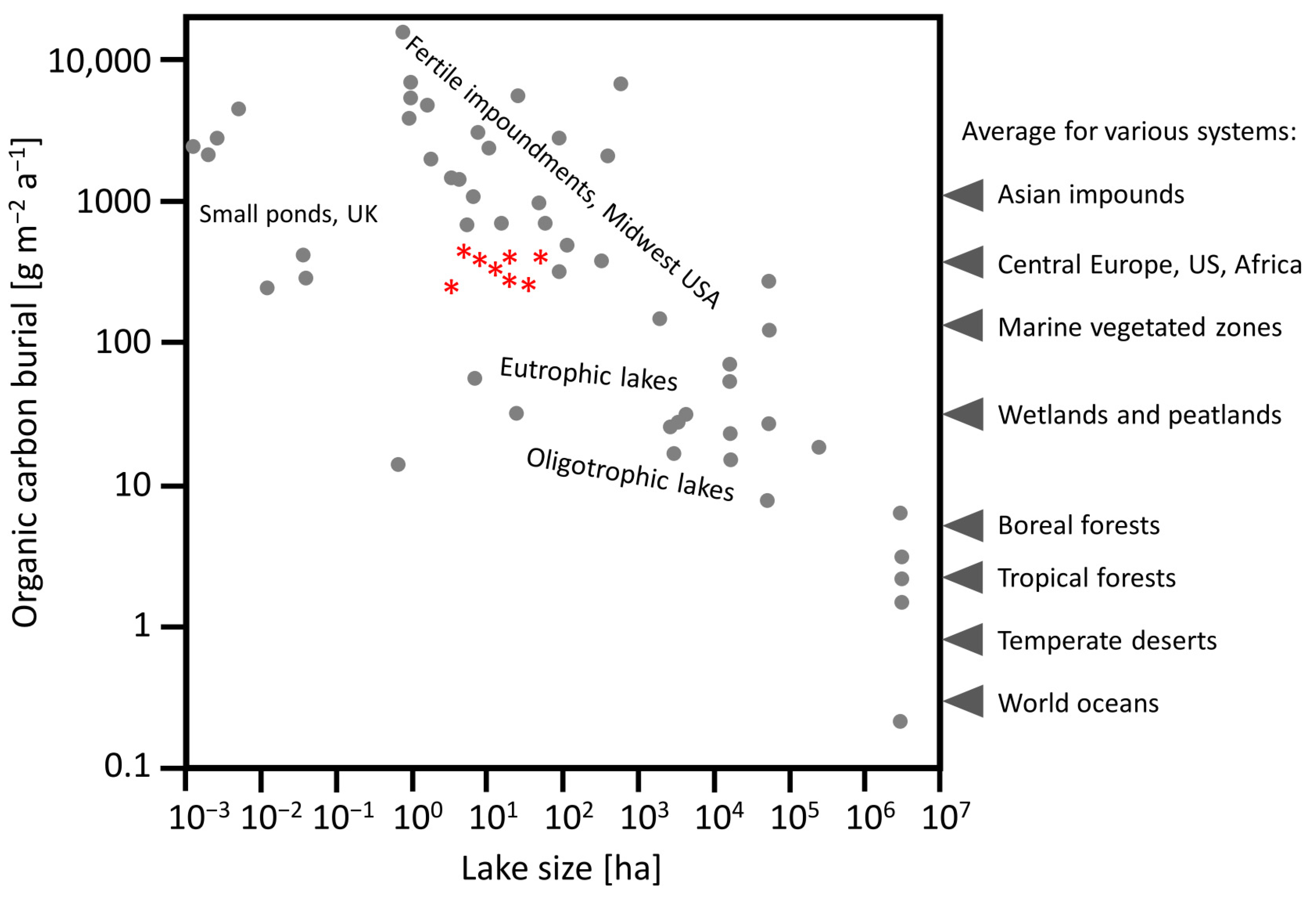
- Restored lakes can accumulate significant amounts of carbon in bottom sediments, and they can make some contribution to carbon sequestration possibilities, as a method that does not require large technical and financial outlays; also, carbon sequestration in restored lakes (Table 5) can be higher, even up to 20 times, than in meadows in the temperate climatic zone, according to the literature [39];
- Lakes can be used for economic activities (as water intakes), recreational purposes (depending on their trophic status), and fisheries;
- Lake restoration projects can activate local communities. The importance of water bodies that serve many functions is recognised by local residents. Deteriorating lakes deplete local resources, which increases the awareness of environmental issues.
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2021, 12, 3269–3340. [Google Scholar] [CrossRef]
- Einsele, G.; Yan, J.; Hinderer, M. Atmospheric carbon burial in modern lake basins and its significance for the global carbon budget. Glob. Planet. Chang. 2001, 30, 167–195. [Google Scholar] [CrossRef]
- Heathcote, A.J.; Anderson, N.J.; Prairie, Y.T.; Engstrom, D.R.; del Giorgio, P.A. Large increases in carbon burial in northern lakes during the Anthropocene. Nat. Commun. 2015, 6, 10016. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.E.; Gorham, E. Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 1998, 26, 535–538. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef]
- Alin, S.R.; Johnson, T.C. Carbon cycling in large lakes of the world: A synthesis of production, burial, and lake-atmosphere exchange estimates. Glob. Biogeochem. Cycles 2007, 21, GB3002. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.J.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and impoundments as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Mendonça, R.; Müller, R.A.; Clow, D.; Verpoorter, C.; Raymond, P.; Tranvik, L.J.; Sobek, S. Organic carbon burial in global lakes and reservoirs. Nat. Commun. 2017, 8, 1694. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valerac, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 13, 8049–8080. [Google Scholar] [CrossRef]
- Hinshaw, S.; Wohl, E. Quantitatively estimating carbon sequestration potential in soil and large wood in the context of river restoration. Front. Earth Sci. 2021, 9, 708895. [Google Scholar] [CrossRef]
- Bastin, J.-F.; Finegold, Y.; Garcia, C.; Mollicone, D.; Rezende, M.; Routh, D.; Zohner, C.M.; Crowther, T.W. The global tree restoration potential. Science 2019, 365, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Cole, J.J.; Middelburg, J.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Prairie, Y.T.; Laube, K.A. Sediment organic carbon burial in agriculturally eutrophic impoundments over the last century. Glob. Biogeochem. Cycles 2008, 22, GB1018. [Google Scholar] [CrossRef]
- Lossow, K. The role of lakes in young glacial lanscape of Mazurian Lakeland. Zesz. Probl. Postępów Nauk. Rol. 1996, 431, 47–59. (In Polish) [Google Scholar]
- Skwierawski, A. Past anthropogenic changes in the lake ecosystems of late glacial landscapes in north-eastern Poland. Landsc. Res. 2018, 43, 37–49. [Google Scholar] [CrossRef]
- Vollenweider, R.A.; Kerekes, J. Eutrophication of Waters. Monitoring, Assessment and Control; OECD Cooperative Programme on Monitoring of Inland Waters (Eutrophication Control), Environment Directorate; OECD: Paris, France, 1982; p. 154. [Google Scholar]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake Reserv. Manag. 1996, 12, 432–447. [Google Scholar] [CrossRef]
- Kincaid, D.W.; Lara, N.A.H.; Tiegs, S.D.; Hamilton, S.K. Decomposition in flocculent sediments of shallow freshwaters and its sensitivity to warming. Freshw. Sci. 2019, 38, 899–916. [Google Scholar] [CrossRef]
- Skwierawski, A. Water quality of restored shallow lake Nowe Włóki (Olsztyn Lake District, Poland). Ecol. Chem. Eng. 2006, 13, 345–354. (In Polish) [Google Scholar]
- Skwierawski, A. Nitrogen and phosphorus loads in the restored lake Sawag. Ecol. Chem. Eng. 2012, 19, 1029–1039. [Google Scholar] [CrossRef]
- Skwierawski, A. The use of the Integrated Trophic State Index in evaluation of the restored shallow water bodies. Ecol. Chem. Eng. 2013, 20, 1275–1283. [Google Scholar] [CrossRef]
- Cole, J.J.; Caraco, N.F. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar. Freshw. Res. 2001, 52, 101–110. [Google Scholar] [CrossRef]
- Kortelainen, P.; Pajunen, H.; Rantakari, M.; Saarnisto, M. A large carbon pool and small sink in boreal Holocene lake sediments. Glob. Chang. Biol. 2004, 10, 1648–1653. [Google Scholar] [CrossRef]
- Duarte, C.M.; Prairie, Y.T. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 2005, 8, 862–870. [Google Scholar] [CrossRef]
- Sobek, S.; Durisch-Kaiser, E.; Zurbrügg, R.; Wongfun, N.; Wessels, M.; Pasche, N.; Wehrli, B. Organic carbon burial efficiency in lake sediments controlled by oxygen exposure time and sediment source. Limnol. Oceanogr. 2009, 54, 2243–2254. [Google Scholar] [CrossRef]
- Waters, M.N.; Kenney, W.F.; Brenner, M.; Webster, B.C. Organic carbon sequestration in sediments of subtropical Florida lakes. PLoS ONE 2019, 14, e0226273. [Google Scholar] [CrossRef]
- Dong, X.; Anderson, N.J.; Yang, X.; Chen, X.; Shen, J. Carbon burial by shallow lakes on the Yangtze floodplain and its relevance to regional carbon sequestration. Glob. Chang. Biol. 2012, 18, 2205–2217. [Google Scholar] [CrossRef]
- Takamura, K.; Sugaya, Y.; Takamura, N.; Hanazato, T.; Yasuno, M.; Iwakuma, T. Primary production of phytoplankton and standing crops of zooplankton and zoobenthos in hypertrophic Lake Teganuma. Hydrobiologia 1989, 173, 173–184. [Google Scholar] [CrossRef]
- Hollinger, D.Y.; Aber, J.; Dail, B.; Davidson, E.A.; Goltz, S.M.; Hughes, H.; Leclerc, M.Y.; Lee, J.T.; Richardson, A.D.; Rodrigues, C.; et al. Spatial and temporal variability in forest-atmosphere CO2 exchange. Glob. Chang. Biol. 2004, 10, 1689–1706. [Google Scholar] [CrossRef]
- Verspagen, J.M.H.; Van de Waal, D.B.; Finke, J.F.; Visser, P.M.; Van Donk, E.; Huisman, J. Rising CO2 levels will intensify phytoplankton blooms in eutrophic and hypertrophic lakes. PLoS ONE 2014, 9, e104325. [Google Scholar] [CrossRef]
- Rose, N.L.; Morley, D.; Appleby, P.G.; Battarbee, R.W.; Alliksaar, T.; Guilizzoni, P.; Jeppesen, E.; Korhola, A.; Punning, J.-M. Sediment accumulation rates in European lakes since AD 1850: Trends, reference conditions and exceedence. J. Paleolimnol. 2011, 45, 447–468. [Google Scholar] [CrossRef]
- Dearing, J.A.; Jones, R.T. Coupling temporal and spatial dimensions of global sediment flux through lake and marine sediment record. Glob. Planet. Chang. 2003, 39, 147–168. [Google Scholar] [CrossRef]
- Marcé, R.; Obrador, B.; Gómez-Gener, L.; Catalán, N.; Koschorreck, M.; Arce, M.I.; Singer, G.; von Schiller, D. Emissions from dry inland waters are a blind spot in the global carbon cycle. Earth-Sci. Rev. 2019, 188, 240–248. [Google Scholar] [CrossRef]
- Brainard, A.S.; Fairchild, G.W. Sediment characteristics and accumulation rates in constructed ponds. J. Soil Water Conserv. 2012, 67, 425–432. [Google Scholar] [CrossRef]
- Kobus, S.; Glińska-Lewczuk, K.; Sidoruk, M.; Skwierawski, A.; Obolewski, K.; Timofte, C.M.; Sowiński, P. Effect of hydrological connectivity on physico-chemical properties of bottom sediments of floodplain lakes—A case study of the Łyna River, Northern Poland. Environ. Eng. Manag. J. 2016, 15, 1237–1246. [Google Scholar] [CrossRef]
- Bartout, P.; Touchart, L.; Terasmaa, J.; Choffel, Q.; Marzecova, A.; Koff, T.; Kapanen, G.; Qsair, Z.; Maleval, V.; Millot, C.; et al. A new approach to inventorying bodies of water, from local to global scale. Die Erde 2015, 146, 245–258. [Google Scholar] [CrossRef]
- Scheffer, M.; van Nes, E.H. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 2007, 584, 455–466. [Google Scholar] [CrossRef]
- Jones, M.B.; Donnelly, A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2. New Phytol. 2004, 164, 423–439. [Google Scholar] [CrossRef]
- De Vos, J.M.; Joppa, L.N.; Gittleman, J.L.; Stephens, P.R.; Pimm, S.L. Estimating the normal background rate of species extinction. Conserv. Biol. 2014, 29, 452–462. [Google Scholar] [CrossRef]
- Strona, G.; Lafferty, K.D. Environmental change makes robust ecological networks fragile. Nat. Commun. 2016, 7, 12462. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]

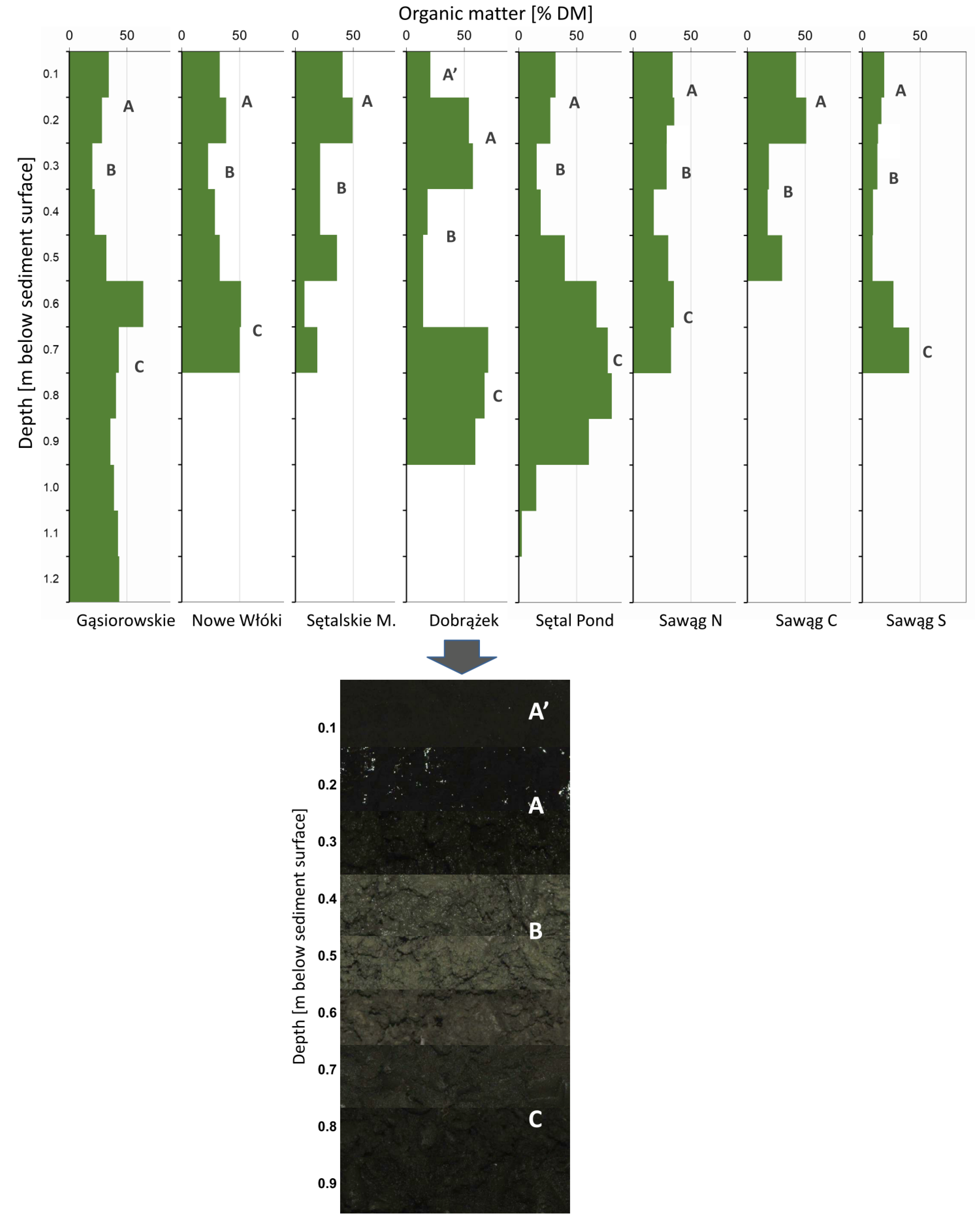

| Water Body | Location | Area | Agricultural Land in the Catchment | Maximum Depth | Time since Restoration | |
|---|---|---|---|---|---|---|
| Lake | Catchment (Including Direct Catchment) | |||||
| ha | % | m | Years | |||
| Gąsiorowskie | 53°43′13″ N 20°48′53″ E | 6.9 | 34 | 68 | 3.5 | 47 |
| Sętalskie Małe | 53°54′41″ N 20°28′49″ E | 12.5 | 813 (115) | 90 | 2.6 | 31 |
| Dobrążek | 53°49′50″ N 20°47′40″ E | 9.3 | 105 | 94 | 2.5 | 21 |
| Nowe Włóki | 53°53′58″ N 20°31′42″ E | 19.8 | 375 | 88 | 2.7 | 30 |
| Sętal Pond | 53°54′14″ N 20°28′57″ E | 3.7 | 103 | 96 | 1.6 | 29 |
| Sawąg N | 53°59′04″ N 20°19′44″ E | 53.2 | 187 | 93 | 4.0 | 13 |
| Sawąg C | 53°58′36″ N 20°18′51″ E | 15.4 | 338 (151) | 92 | 3.5 | 16 |
| Sawąg S | 53°58′15″ N 20°18′46″ E | 33.4 | 755 (417) | 94 | 3.5 | 16 |
| Water Body | EC [μS cm−1] | SD [m] | CHL [μg dm−3] | TP [μg dm−3] | SRP [μg dm−3] |
|---|---|---|---|---|---|
| Gąsiorowskie | 389 (±17) | 1.8 (±0.3) | 8.9 (±4.2) | 79 (±60) | 8 (±5) |
| Sętalskie Małe | 305 (±37) | 1.4 (±0.3) | 14.9 (±12.1) | 130 (±207) | 16 (±10) |
| Dobrążek | 347 (±37) | 1.0 (±0.4) | 22.0 (±10.3) | 138 (±90) | 20 (±13) |
| Nowe Włóki | 284 (±35) | 0.7 (±0.3) | 26.8 (±12.4) | 190 (±119) | 14 (±8) |
| Sętal Pond | 229 (±29) | 0.6 (±0.3) | 51.6 (±50.6) | 346 (±242) | 50 (±99) |
| Sawąg N | 390 (±48) | 1.0 (±0.3) | 21.6 (±11.3) | 233 (±126) | 54 (±50) |
| Sawąg C | 394 (±38) | 1.0 (±0.2) | 34.8 (±16.5) | 313 (±205) | 81 (±75) |
| Sawąg S | 372 (±61) | 0.9 (±0.3) | 25.6 (±11.6) | 247 (±164) | 72 (±61) |
| Water Body | Probability of Trophic State [%] | |||
|---|---|---|---|---|
| Oligotrophic | Mesotrophic | Eutrophic | Hypertrophic | |
| Gąsiorowskie | 1.8 | 31.4 | 49.0 | 17.8 |
| Sętalskie Małe | 1.0 | 23.5 | 49.2 | 26.4 |
| Dobrążek | 0.0 | 3.4 | 39.7 | 57.0 |
| Nowe Włóki | 0.0 | 2.0 | 30.8 | 67.2 |
| Sętal Pond | 0.0 | 0.2 | 19.4 | 80.5 |
| Sawąg N | 0.0 | 5.4 | 39.7 | 55.0 |
| Sawąg C | 0.0 | 0.0 | 23.2 | 76.9 |
| Sawąg S | 0.0 | 2.5 | 32.2 | 65.4 |
| Water Body | Specific Density g cm−3 | Water Content [%] | Organic Matter [% DM] | Calcium Content [% DM] | ||||
|---|---|---|---|---|---|---|---|---|
| After Refilling | Before Drainage | After Refilling | Before Drainage | After Refilling | Before Drainage | After Refilling | Before Drainage | |
| Gąsiorowskie | 1.07 | 1.18 | 86.6 | 71.2 | 31.2 | 21.0 | 11.43 | 2.02 |
| Sętalskie Małe | 1.10 | 1.29 | 88.5 | 65.6 | 45.4 | 21.5 | 1.15 | 0.10 |
| Dobrążek | 1.06 | 1.29 | 89.6 | 54.9 | 44.3 | 15.8 | 1.02 | 0.01 |
| Nowe Włóki | 1.13 | 1.27 | 87.5 | 67.1 | 35.7 | 24.7 | 5.72 | 0.23 |
| Sętal Pond | 1.15 | 1.37 | 82.4 | 56.5 | 29.6 | 17.4 | 0.30 | 0.09 |
| Sawąg N | 1.25 | 1.57 | 74.7 | 41.9 | 17.8 | 10.4 | 3.04 | 0.09 |
| Sawąg C | 1.10 | 1.36 | 87.7 | 63.1 | 46.7 | 18.3 | 0.34 | 0.08 |
| Sawąg S | 1.05 | 1.30 | 88.7 | 61.0 | 35.1 | 23.5 | 2.15 | 0.13 |
| Average | 1.11 | 1.33 | 85.7 | 60.2 | 35.7 | 19.1 | 3.14 | 0.34 |
| Water Body | Accumulation Rate | Mass of Carbon Buried in the Top Sediment Layer | Carbon Burial Rate | CO2 Equivalent | |||
|---|---|---|---|---|---|---|---|
| in Organic Matter | in CaCO3 | in Organic Matter | in CaCO3 | in Organic Matter | in CaCO3 | ||
| mm a−1 | Mg | g C m−2 a−1 | Mg ha−1 a−1 | ||||
| Gąsiorowskie | 4.3 | 358 | 68 | 111 | 21 | 4.1 | 0.8 |
| Sętalskie Małe | 6.5 | 833 | 11 | 215 | 3 | 7.9 | 0.1 |
| Dobrążek | 14.3 | 791 | 9 | 405 | 5 | 14.8 | 0.2 |
| Nowe Włóki | 6.7 | 1163 | 96 | 196 | 16 | 7.2 | 0.6 |
| Sętal Pond | 6.9 | 257 | 1 | 239 | 1 | 8.8 | 0.1 |
| Sawąg N | 11.5 | 2619 | 231 | 379 | 33 | 13.9 | 1.2 |
| Sawąg C | 9.4 | 1842 | 7 | 345 | 1 | 12.6 | 0.1 |
| Sawąg S | 9.4 | 557 | 18 | 226 | 7 | 8.3 | 0.3 |
| Average | 8.6 | 264.5 | 11.0 | 9.7 | 0.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwierawski, A. Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes. Int. J. Environ. Res. Public Health 2022, 19, 6308. https://doi.org/10.3390/ijerph19106308
Skwierawski A. Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes. International Journal of Environmental Research and Public Health. 2022; 19(10):6308. https://doi.org/10.3390/ijerph19106308
Chicago/Turabian StyleSkwierawski, Andrzej. 2022. "Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes" International Journal of Environmental Research and Public Health 19, no. 10: 6308. https://doi.org/10.3390/ijerph19106308
APA StyleSkwierawski, A. (2022). Carbon Sequestration Potential in the Restoration of Highly Eutrophic Shallow Lakes. International Journal of Environmental Research and Public Health, 19(10), 6308. https://doi.org/10.3390/ijerph19106308






