Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro
Abstract
:1. Introduction
2. Materials and Methods
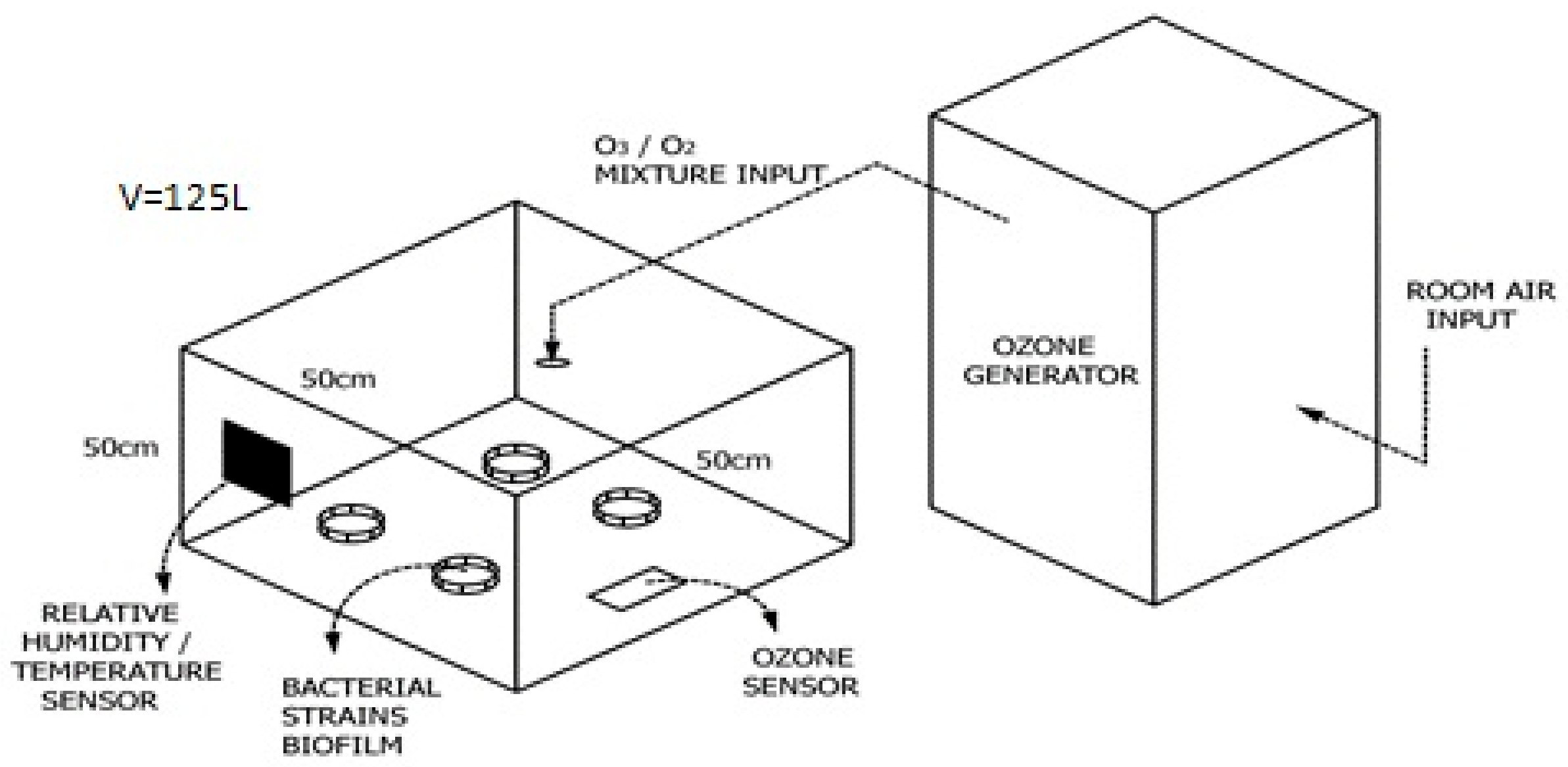
2.1. Equipment
2.2. Characterization of Ceramic Tiles
2.3. Bacterial Strains
2.4. Characterization of Bacterial Strains
2.4.1. Antimicrobial Resistance Profile
2.4.2. Characterization of Surface Physical Properties
2.5. Biofilm Formation on Ceramic Tiles
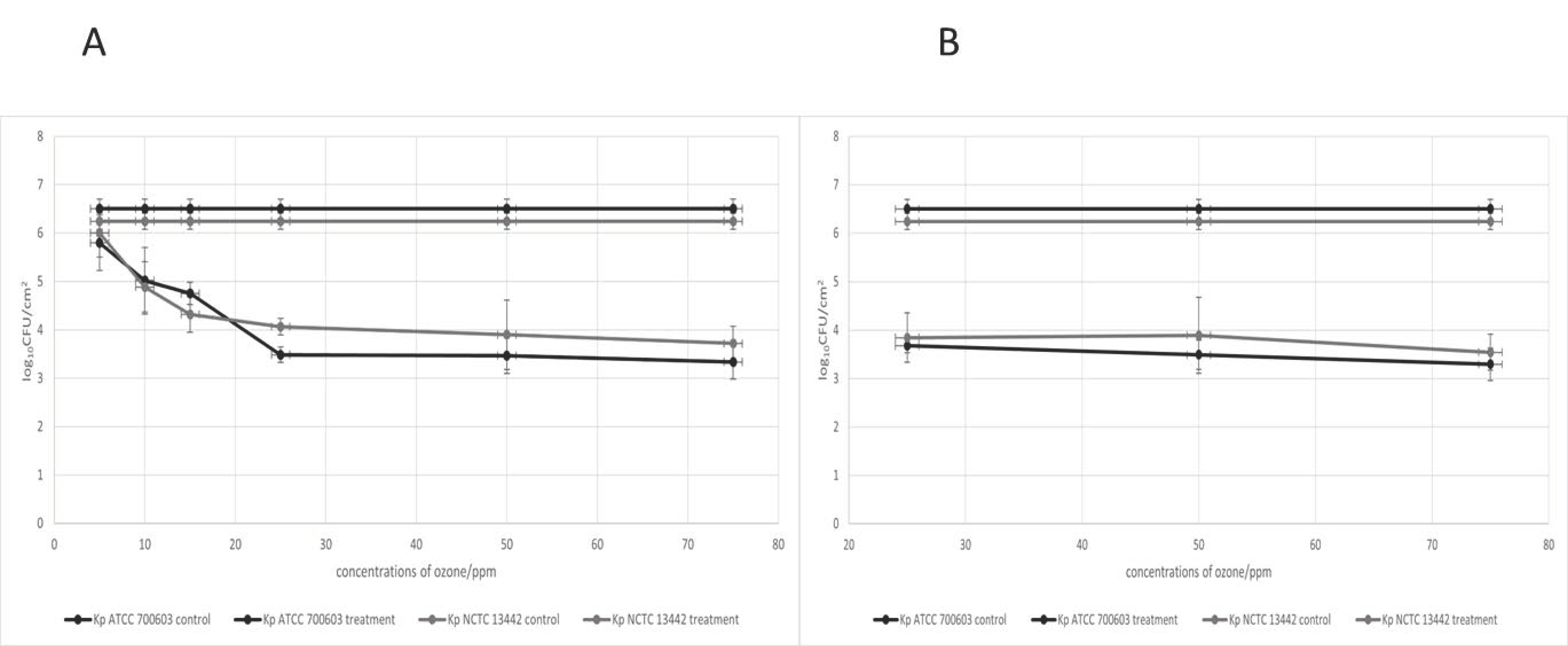
2.6. Determination of Optimal Gaseous Ozone Concentration
2.7. Determination of Total Bacterial Number
2.8. Determination of Cell Viability (Dead/Live Assay)
2.9. Biomass Determination by Crystal-Violet Staining
2.10. ATP Bioluminescence
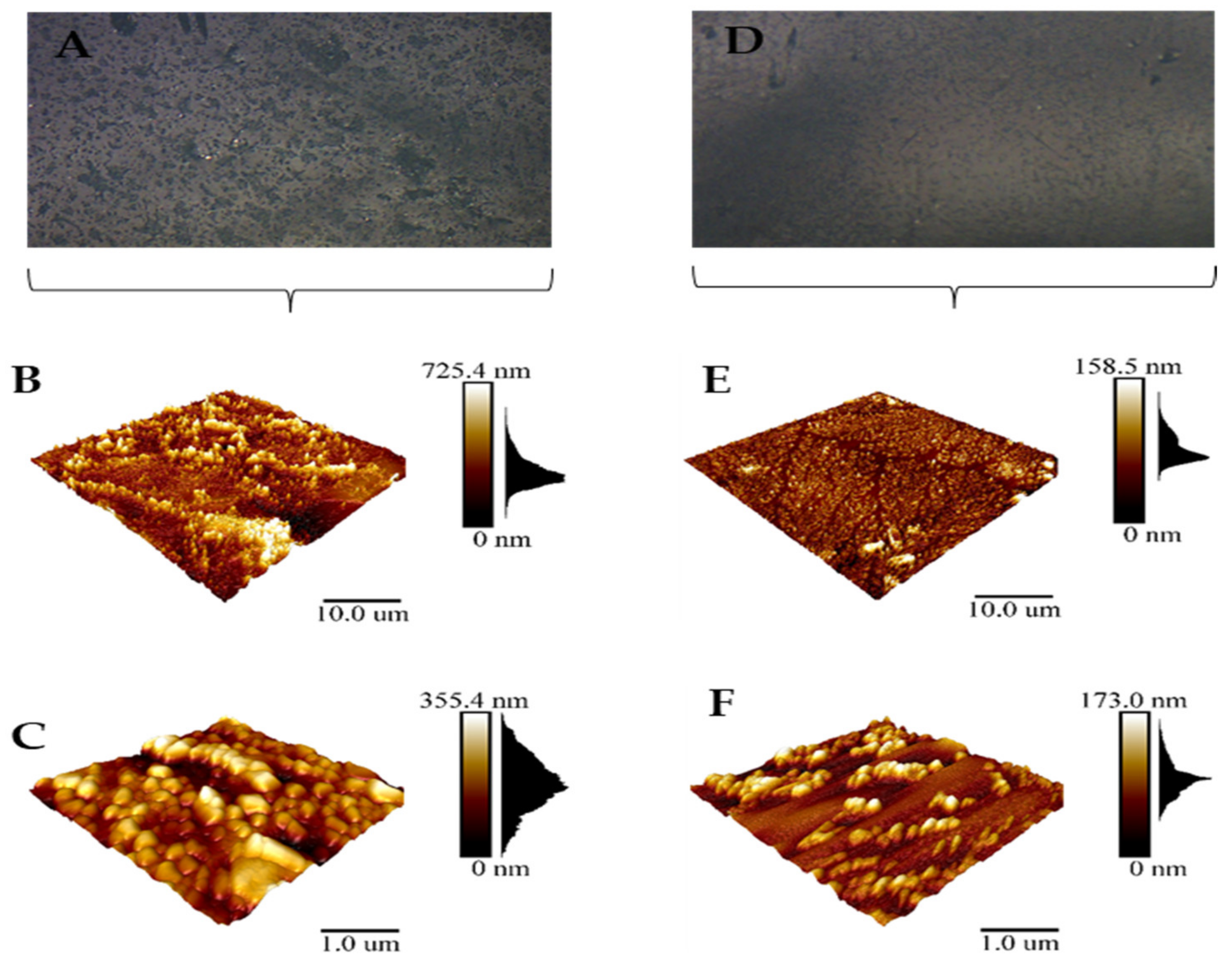
2.11. Atomic Force Microscopy
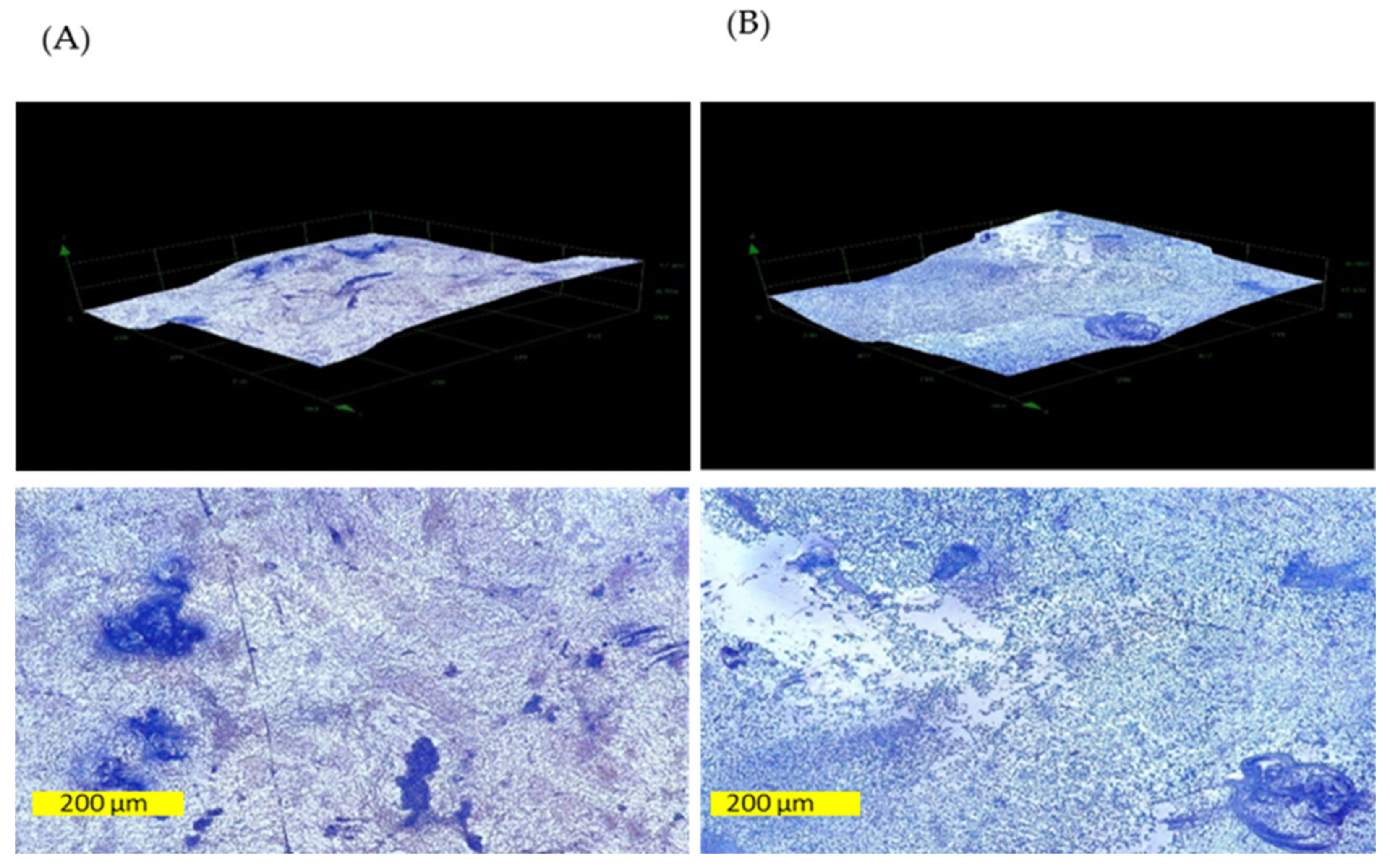
2.12. Digital Microscopy
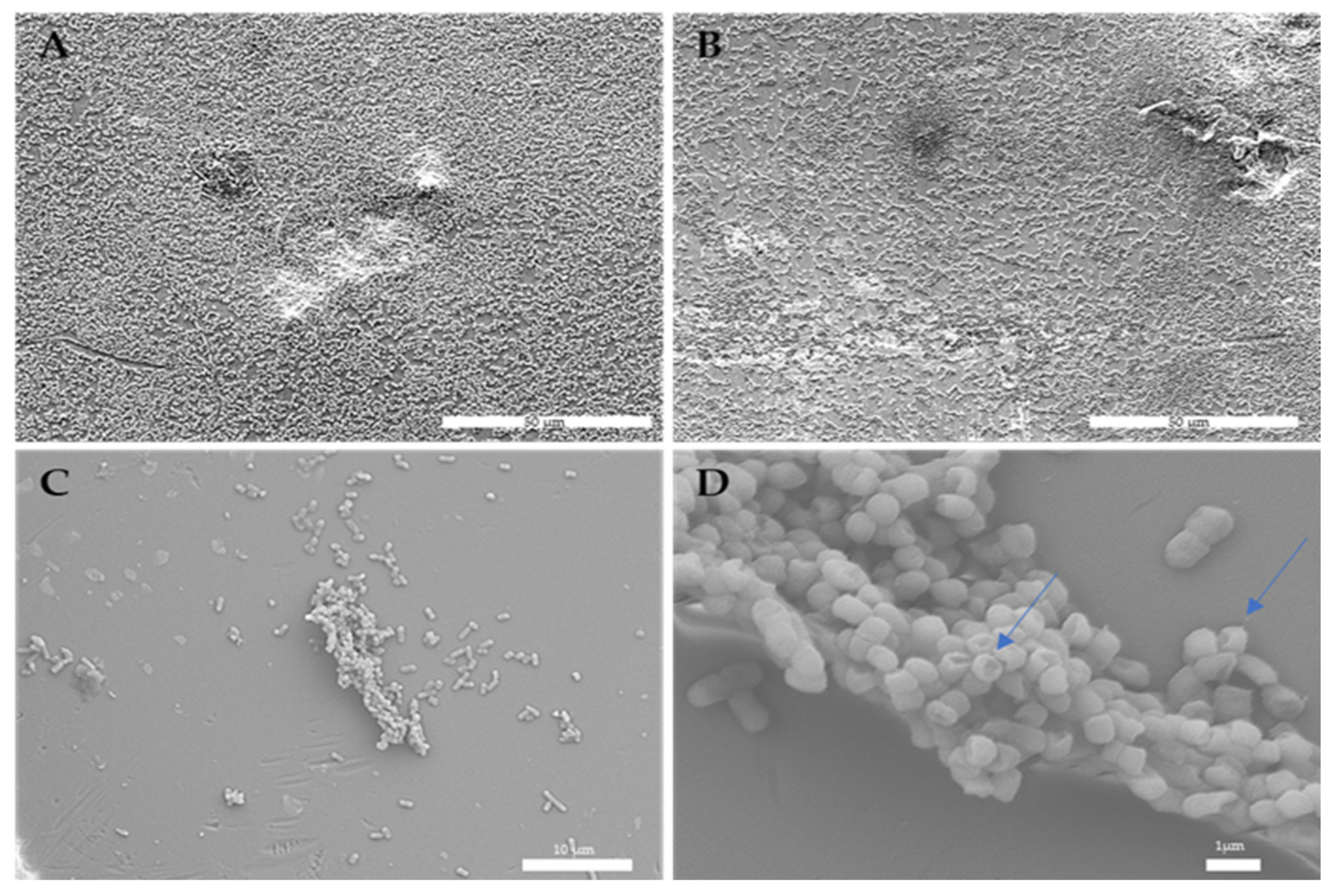
2.13. Scanning Electron Microscopy
2.14. Determination of Oxidative Stress
2.15. Statistical Analyses and Graphing
3. Results
3.1. Antimicrobial Resistance Profile of K. pneumoniae
3.2. Characterization of Ceramic Tiles
3.3. Cell Surface Characterization of Bacterial Strains
3.4. Optimal Concentration of Gaseous Ozone
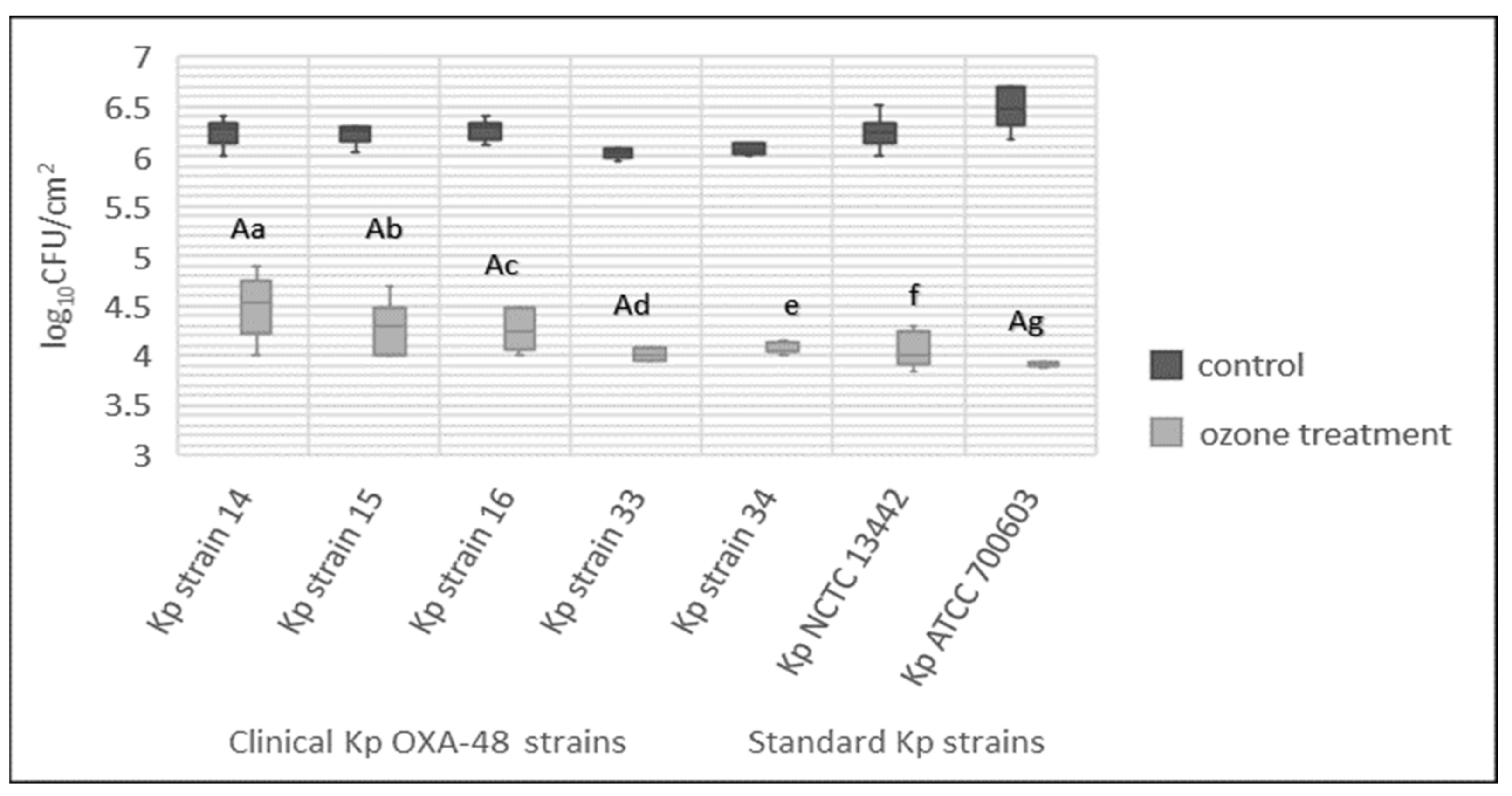
3.5. Total Bacteria Number
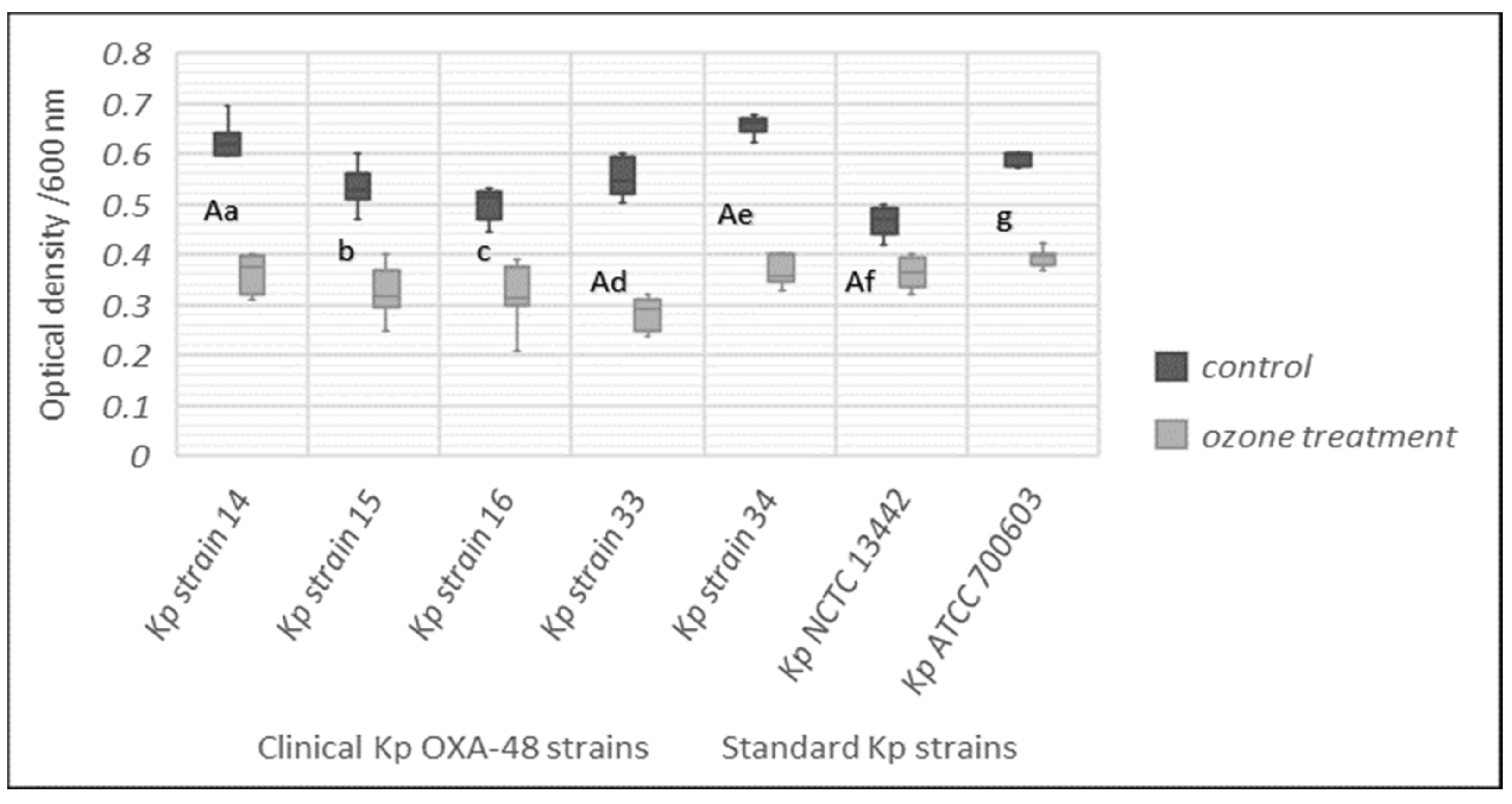
3.6. Effect of Gaseous Ozone on Biomass Production of K. pneumonia Strains
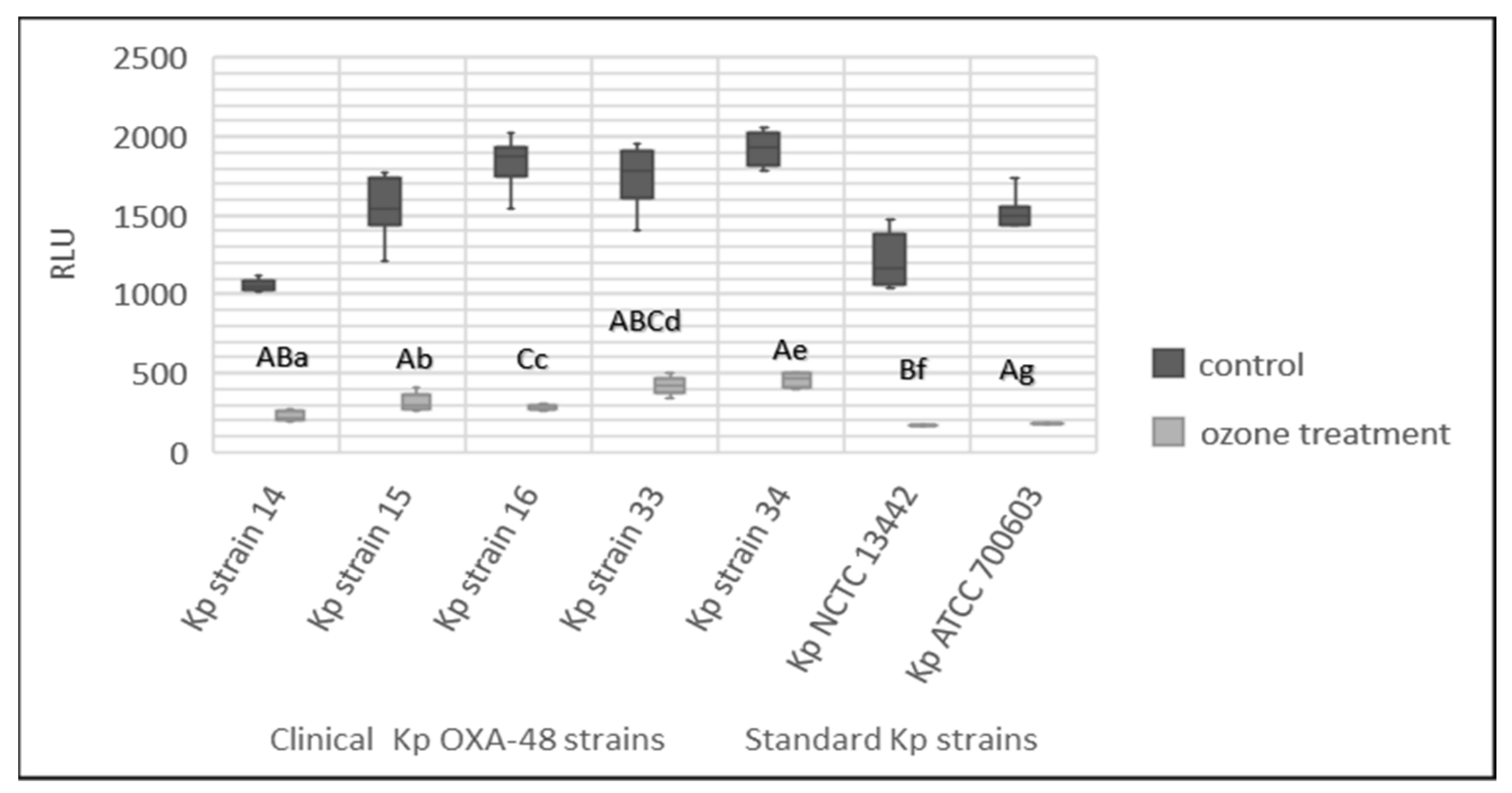
3.7. Effect of Gaseous Ozone on ATP Bioluminescence
3.8. Biofilm Inhibition
3.9. Effect of Gaseous Ozone on Topology of K. pneumoniae Biofilm
3.10. Effect of Gaseous Ozone on Bacterial Cells
3.11. Effect of Gaseous Ozone on K. pneumoniae Viability
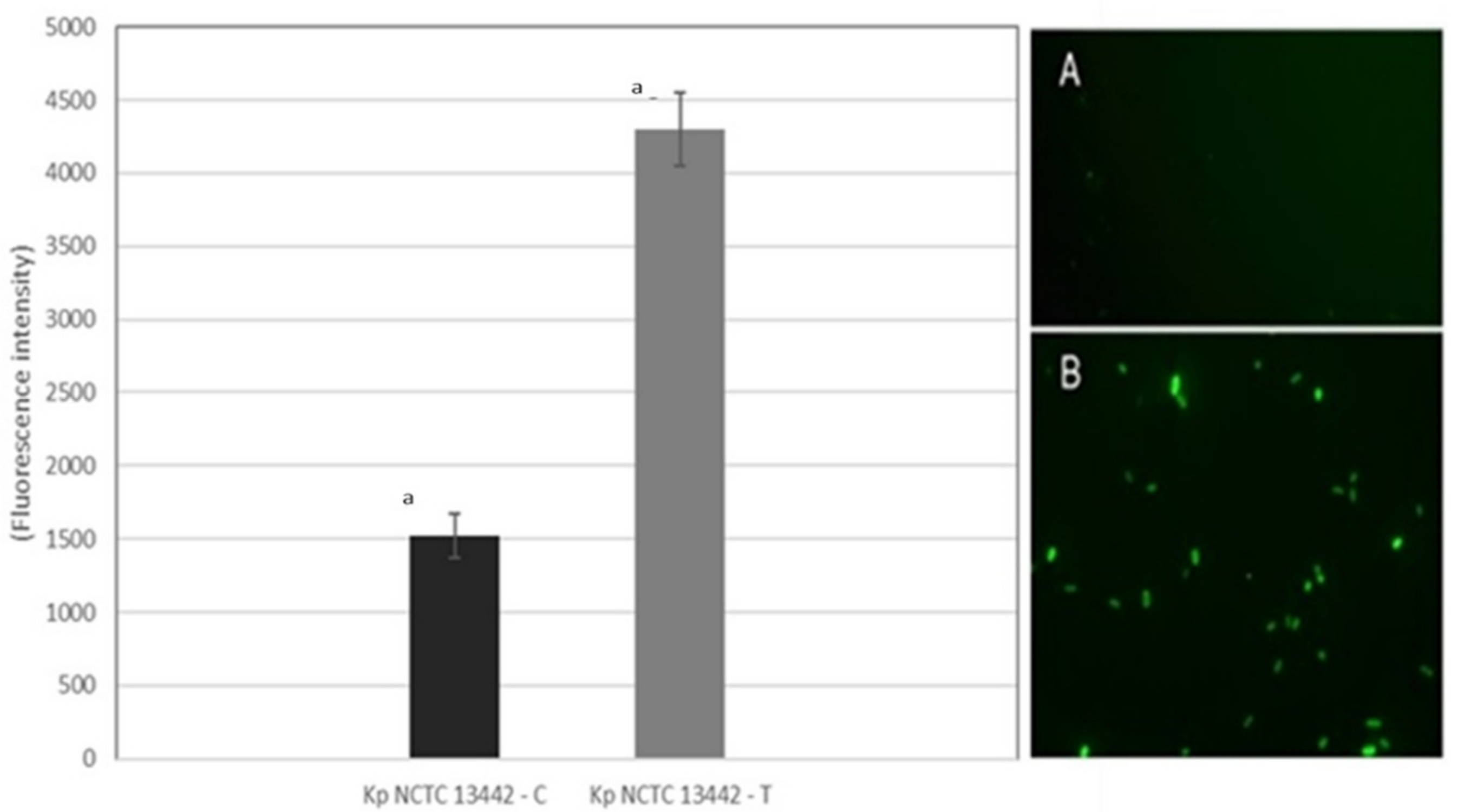
3.12. Effect of Gaseous Ozone on ROS Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Passaretti, C.L.; Otter, J.A.; Reich, N.G.; Myers, J.; Shepard, J.; Ross, T.; Carroll, K.C.; Lipsett, P.; Perl, T.M.; Costa, D.M.; et al. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: Implications for infection control. Am. J. Infect. Control 2013, 44, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Hudson, J.B. Ozone gas is an effective and practical antibacterial agent. Am. J. Infect. Control 2008, 36, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Škrobonja, I.; Ambrožić, D.; Repac-Antić, D.B.Š.M. ESKAPE—Bacteria that alert the world. Med. Flum. 2018, 54, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Hunter, I.S. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J. Med. Microbiol. 2008, 57, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Wang, Z.C.; Huang, H.Y.; Huang, H.D.; Peng, H.L. YjcC, a c-di-GMP Phosphodiesterase Protein, Regulates the Oxidative Stress Response and Virulence of Klebsiella pneumoniae CG43. PLoS ONE 2013, 8, e66740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Taoufik, L.; Amrani Hanchi, A.; Fatiha, B.; Nissrine, S.; Mrabih Rabou, M.F.; Nabila, S. Emergence of OXA-48 Carbapenemase Producing Klebsiella pneumoniae in a Neonatal Intensive Care Unit in Marrakech, Morocco. Clin. Med. Insights Pediatr. 2019, 13, 117955651983452. [Google Scholar] [CrossRef] [Green Version]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Montgomerie, J.Z. Epidemiology of klebsiella and hospital-associated infections. Rev. Infect. Dis. 1979, 1, 736. [Google Scholar] [CrossRef]
- Ostria-Hernandez, M.L.; Juárez-de la Rosa, K.C.; Arzate-Barbosa, P.; Lara-Hernández, A.; Sakai, F.; Ibarra, J.A.; Castro-Escarpulli, G.; Vidal, J.E. Nosocomial, Multidrug-Resistant Klebsiella pneumoniae Strains Isolated from Mexico City Produce Robust Biofilms on Abiotic Surfaces but Not on Human Lung Cells. Microb. Drug Resist. 2018, 24, 422–433. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Y.; Liu, C.; Chen, Z.; Zhou, D. Molecular pathogenesis of Klebsiella pneumoniae Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol. 2016, 9, 1071–1081. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Abram, M. Globalno širenje bakterija koje proizvode karbapenemaze. Med. Flum. 2014, 50, 128–149. [Google Scholar]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Epidemiología clínica de la expansión global de las carbapenemasas de Klebsiella pneumoniae. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef] [Green Version]
- Spyridopoulou, K.; Psichogiou, M.; Sypsa, V.; Miriagou, V.; Karapanou, A.; Hadjihannas, L.; Tzouvelekis, L.; Daikos, G.L. Containing Carbapenemase-producing Klebsiella pneumoniae in an endemic setting. Antimicrob. Resist. Infect. Control 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Bolourchi, N.; Shahcheraghi, F.; Giske, C.G.; Nematzadeh, S.; Noori Goodarzi, N.; Solgi, H.; Badmasti, F. Comparative genome analysis of colistin-resistant OXA-48-producing Klebsiella pneumoniae clinical strains isolated from two Iranian hospitals. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 74. [Google Scholar] [CrossRef]
- Cuccaro, C.; Tarroni, M.; Tinturini, A.; Cresti, S.; Basagni, C.; Nante, N.; Messina, G.; De Marco, M.F. Carbapenem-resistant enterobacteriaceae: Don’t trust your neighbour. Eur. J. Public Health 2020, 30 (Suppl. 5), ckaa166.708. [Google Scholar] [CrossRef]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef]
- Vickery, K. Special Issue: Microbial biofilms in healthcare: Formation, prevention and treatment. Materials 2019, 12, 2001. [Google Scholar] [CrossRef] [Green Version]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Biofouling: The Journal of Bioadhesion and Biofilm Resistance of bacterial biofilms to disinfectants: A review. Biofouling J. Bioadhesion Biofilm Res. 2011, 27, 1017–1032. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Cholley, A.C.; Traoré, O.; Hennequin, C.; Aumeran, C. Klebsiella pneumoniae survival and regrowth in endoscope channel biofilm exposed to glutaraldehyde and desiccation. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1129–1136. [Google Scholar] [CrossRef]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, 57–68. [Google Scholar] [CrossRef]
- Marra, A.R.; Schweizer, M.L.; Edmond, M.B. No-touch disinfection methods to decrease multidrug-resistant organism infections: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018, 39, 20–31. [Google Scholar] [CrossRef]
- Weber, D.J.; Kanamori, H.; Rutala, W.A. “No touch” technologies for environmental decontamination: Focus on ultraviolet devices and hydrogen peroxide systems. Curr. Opin. Infect. Dis. 2016, 29, 424–431. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Billah, M.M.; Sarker, A. COVID-19 pandemic and healthcare solid waste management strategy—A mini-review. Sci. Total Environ. 2021, 778, 146220. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Pottage, T.; Bennett, A.; Walker, J. Gaseous and air decontamination technologies for Clostridium difficile in the healthcare environment. J. Hosp. Infect. 2011, 77, 199–203. [Google Scholar] [CrossRef]
- Moat, J.; Cargill, J.; Shone, J.; Upton, M. Application of a novel decontamination process using gaseous ozone. Can. J. Microbiol. 2009, 55, 928–933. [Google Scholar] [CrossRef]
- De Boer, H.E.L.; van Elzelingen-Dekker, C.M.; van Rheenen-Verberg, C.M.F.; Spanjaard, L. Use of Gaseous Ozone for Eradication of Methicillin-Resistant Staphylococcus aureus From the Home Environment of a Colonized Hospital Employee. Infect. Control Hosp. Epidemiol. 2006, 27, 1120–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999, 62, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, G.; Ricevuti, G.; Galoforo, A.; Franzini, M. Microbiological aspects of ozone: Bactericidal activity and antibiotic/antimicrobial resistance in bacterial strains treated with ozone. Ozone Ther. 2018, 3, 1–4. [Google Scholar] [CrossRef]
- Megahed, A.; Aldridge, B.; Lowe, J. The microbial killing capacity of aqueous and gaseous ozone on different surfaces contaminated with dairy cattle manure. PLoS ONE 2018, 13, e0196555. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Valdramidis, V.P.; Karatzas, K.A.G.; Cullen, P.J.; Bourke, P. Assessing the microbial oxidative stress mechanism of ozone treatment through the responses of Escherichia coli mutants. J. Appl. Microbiol. 2011, 111, 136–144. [Google Scholar] [CrossRef]
- Li, C.S.; Wang, Y.C. Surface germicidal effects of ozone for microorganisms. Am. Ind. Hyg. Assoc. J. 2003, 64, 533–537. [Google Scholar] [CrossRef]
- Fontes, B.; Cattani Heimbecker, A.M.; de Souza Brito, G.; Costa, S.F.; van der Heijden, I.M.; Levin, A.S.; Rasslan, S. Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef] [Green Version]
- Bellon-Fontaine, M.N.; Rault, J.; Van Oss, C.J. Microbial adhesion to solvents: A novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces 1996, 7, 47–53. [Google Scholar] [CrossRef]
- Meylheuc, T.; Van Oss, C.J.; Bellon-Fontaine, M.N. Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [Google Scholar] [CrossRef] [Green Version]
- Ivanković, T.; Goić-Barišić, I.; Hrenović, J. Reduced susceptibility to disinfectants of Acinetobacter baumannii biofilms on glass and ceramic. Arh. Hig. Rada Toksikol. 2017, 68, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Rajneesh, J.P.; Chatterjee, A.; Singh, S.; Sinha, R. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2’,7’-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio-Protocol 2017, 7, e2545. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1897. [Google Scholar] [CrossRef] [Green Version]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012, 8, e1002440. [Google Scholar] [CrossRef] [Green Version]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Farniya, F.; Jamalli, A.; Dadgar, T. Physicochemical surface characteristics in different pathogenic bacteria. Cogent Biol. 2019, 5, 1638572. [Google Scholar] [CrossRef]
- Zeraik, A.E.; Nitschke, M. Influence of growth media and temperature on bacterial adhesion to polystyrene surfaces. Braz. Arch. Biol. Technol. 2012, 55, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.L.; Aiello, A.E.; Gomez-Duarte, C.; Lin, S.X.; Lee, L.; Della-Latta, P.; Lindhardt, C. Bioluminescence ATP monitoring as a surrogate marker for microbial load on hands and surfaces in the home. Food Microbiol. 2003, 20, 735–739. [Google Scholar] [CrossRef]
- Tebbutt, G.M. Comparison of traditional and rapid methods for assessing the risk of bacterial cross-contamination from cutting boards. Int. J. Environ. Health Res. 1999, 9, 67–74. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Bahnfleth, W.P.; Whittam, T.S. Bactericidal effects of high airborne ozone concentrations on Escherichia coli and Staphylococcus aureus. Ozone Sci. Eng. 1998, 20, 205–221. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Bahnfleth, W.P.; Striebig, B.A.; Whittam, T.S. Demonstration of a hermetic airborne ozone disinfection system: Studies on E. Coli. Am. Ind. Hyg. Assoc. J. 2003, 64, 222–227. [Google Scholar] [CrossRef]
- Aydogan, A.; Gurol, M.D. Application of Gaseous Ozone for Inactivation of Bacillus subtilis Spores. J. Air Waste Manag. Assoc. 2006, 56, 179–185. [Google Scholar] [CrossRef]
- Nicholas, R.; Dunton, P.; Tatham, A.; Fielding, L. The effect of ozone and open air factor on surface-attached and biofilm environmental Listeria monocytogenes. J. Appl. Microbiol. 2013, 115, 555–564. [Google Scholar] [CrossRef]
- Arana, I.; Santorum, P.; Muela, A.; Barcina, I. Chlorination and ozonation of waste-water: Comparative analysis of efficacy through the effect on Escherichia coli membranes. J. Appl. Microbiol. 1999, 86, 883. [Google Scholar] [CrossRef]
- Nagayoshi, M.; Fukuizumi, T.; Kitamura, C.; Yano, J.; Terashita, M.; Nishihara, T. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol. Immunol. 2004, 19, 240–246. [Google Scholar] [CrossRef]
- Mao, Y.; Doyle, M.P.; Chen, J. Role of colanic acid exopolysaccharide in the survival of enterohaemorrhagic Escherichia coli O157:H7 in simulated gastrointestinal fluids. Lett. Appl. Microbiol. 2006, 42, 642–647. [Google Scholar] [CrossRef]
- Ionescu, M.; Belkin, S. Overproduction of Exopolysaccharides by an Escherichia coli K-12 rpoS Mutant in Response to Osmotic Stress. Appl. Environ. Microbiol. 2009, 75, 483. [Google Scholar] [CrossRef] [Green Version]
- Panebianco, F.; Rubiola, S.; Chiesa, F.; Civera, T.; Di Ciccio, P.A. Effect of gaseous ozone on listeria monocytogenes planktonic cells and biofilm: An in vitro study. Foods 2021, 10, 1484. [Google Scholar] [CrossRef]
- Dyas, A.; Boughton, B.J.; Das, B.C. Ozone killing action against bacterial and fungal species; microbiological testing of a domestic ozone generator. J. Clin. Pathol. 1983, 36, 1102–1104. [Google Scholar] [CrossRef] [Green Version]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalh, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental effect of ozone on pathogenic bacteria. Microorganisms 2022, 10, 40. [Google Scholar] [CrossRef]
- Moccia, G.; Motta, O.; Pironti, C.; Proto, A.; Capunzo, M.; De Caro, F. An alternative approach for the decontamination of hospital settings. J. Infect. Public Health 2020, 13, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Zoutman, D.; Shannon, M.; Mandel, A. Effectiveness of a novel ozone-based system for the rapid high-level disinfection of health care spaces and surfaces. Am. J. Infect. Control 2011, 39, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.; Griffith, C.; Peters, A. Bactericidal properties of ozone and its potential application as a terminal disinfectant. J. Food Prot. 2000, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Berrington, A.W.; Pedler, S.J. Investigation of gaseous ozone for MRSA decontamination of hospital side-rooms. J. Hosp. Infect. 1998, 40, 61–65. [Google Scholar] [CrossRef]
- Marino, M.; Maifreni, M.; Baggio, A.; Innocente, N. Inactivation of foodborne bacteria biofilms by aqueous and gaseous ozone. Front. Microbiol. 2018, 9, 2024. [Google Scholar] [CrossRef] [Green Version]
- Boch, T.; Tennert, C.; Vach, K.; Al-Ahmad, A.; Hellwig, E.; Polydorou, O. Effect of gaseous ozone on Enterococcus faecalis biofilm–an in vitro study. Clin. Oral Investig. 2016, 20, 1733–1739. [Google Scholar] [CrossRef]
- Bialoszewski, D.; Pietruczuk-Padzik, A.; Kalicinska, A.; Bocian, E.; Czajkowska, M.; Bukowska, B.; Tyski, S. Activity of ozonated water and ozone against staphylococcus aureus and pseudomonas aeruginosa bioflms. Med. Sci. Monit. 2011, 17, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Hassett, D.J.; Elkins, J.G.; Ma, J.F.; Mcdermott, T.R. Pseudomonas aeruginosa biofilm sensitivity to biocides: Use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol. 1999, 310, 599–608. [Google Scholar] [CrossRef]
- Costa, D.M.; Johani, K.; Melo, D.S.; Lopes, L.K.O.; Lopes Lima, L.K.O.; Tipple, A.F.V.; Hu, H.; Vickery, K. Biofilm contamination of high-touched surfaces in intensive care units: Epidemiology and potential impacts. Lett. Appl. Microbiol. 2019, 68, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Bentivegna, E.; Alessio, G.; Spuntarelli, V.; Luciani, M.; Santino, I.; Simmaco, M.; Martelletti, P. Impact of COVID-19 prevention measures on risk of health care-associated Clostridium difficile infection. Am. J. Infect. Control 2021, 49, 640–642. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of multidrug-resistant (Mdr) bacterial infections during the COVID-19 pandemic: A retrospective study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef]
- Doan, L.; Forrest, H.; Fakis, A.; Craig, J.; Claxton, L.; Khare, M. Clinical and cost effectiveness of eight disinfection methods for terminal disinfection of hospital isolation rooms contaminated with Clostridium difficile 027. J. Hosp. Infect. 2012, 82, 114–121. [Google Scholar] [CrossRef]
- Conto, A. The EU chemical strategy for sustainability towards a toxic-free environment. Chim. Oggi/Chem. Today 2021, 39, 40–41. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piletić, K.; Kovač, B.; Perčić, M.; Žigon, J.; Broznić, D.; Karleuša, L.; Lučić Blagojević, S.; Oder, M.; Gobin, I. Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro. Int. J. Environ. Res. Public Health 2022, 19, 6177. https://doi.org/10.3390/ijerph19106177
Piletić K, Kovač B, Perčić M, Žigon J, Broznić D, Karleuša L, Lučić Blagojević S, Oder M, Gobin I. Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro. International Journal of Environmental Research and Public Health. 2022; 19(10):6177. https://doi.org/10.3390/ijerph19106177
Chicago/Turabian StylePiletić, Kaća, Bruno Kovač, Marko Perčić, Jure Žigon, Dalibor Broznić, Ljerka Karleuša, Sanja Lučić Blagojević, Martina Oder, and Ivana Gobin. 2022. "Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro" International Journal of Environmental Research and Public Health 19, no. 10: 6177. https://doi.org/10.3390/ijerph19106177
APA StylePiletić, K., Kovač, B., Perčić, M., Žigon, J., Broznić, D., Karleuša, L., Lučić Blagojević, S., Oder, M., & Gobin, I. (2022). Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro. International Journal of Environmental Research and Public Health, 19(10), 6177. https://doi.org/10.3390/ijerph19106177








