Blood Lead Monitoring in a Former Mining Area in Euskirchen, Germany—Volunteers across the Entire Population
Abstract
:1. Introduction
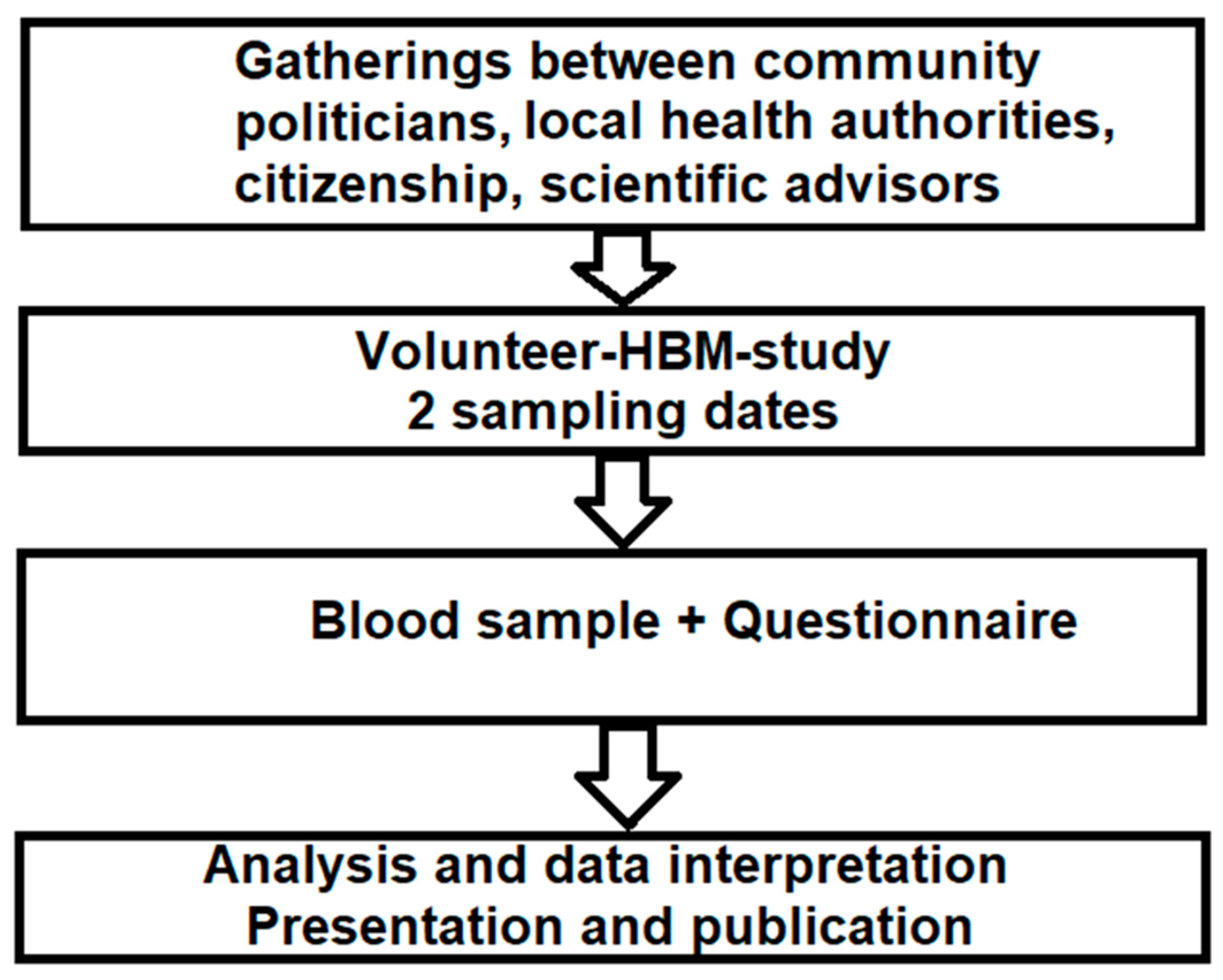
2. Materials and Methods
2.1. Sampling
2.2. Questionnaire
2.3. Analysis
2.4. Statistics
3. Results
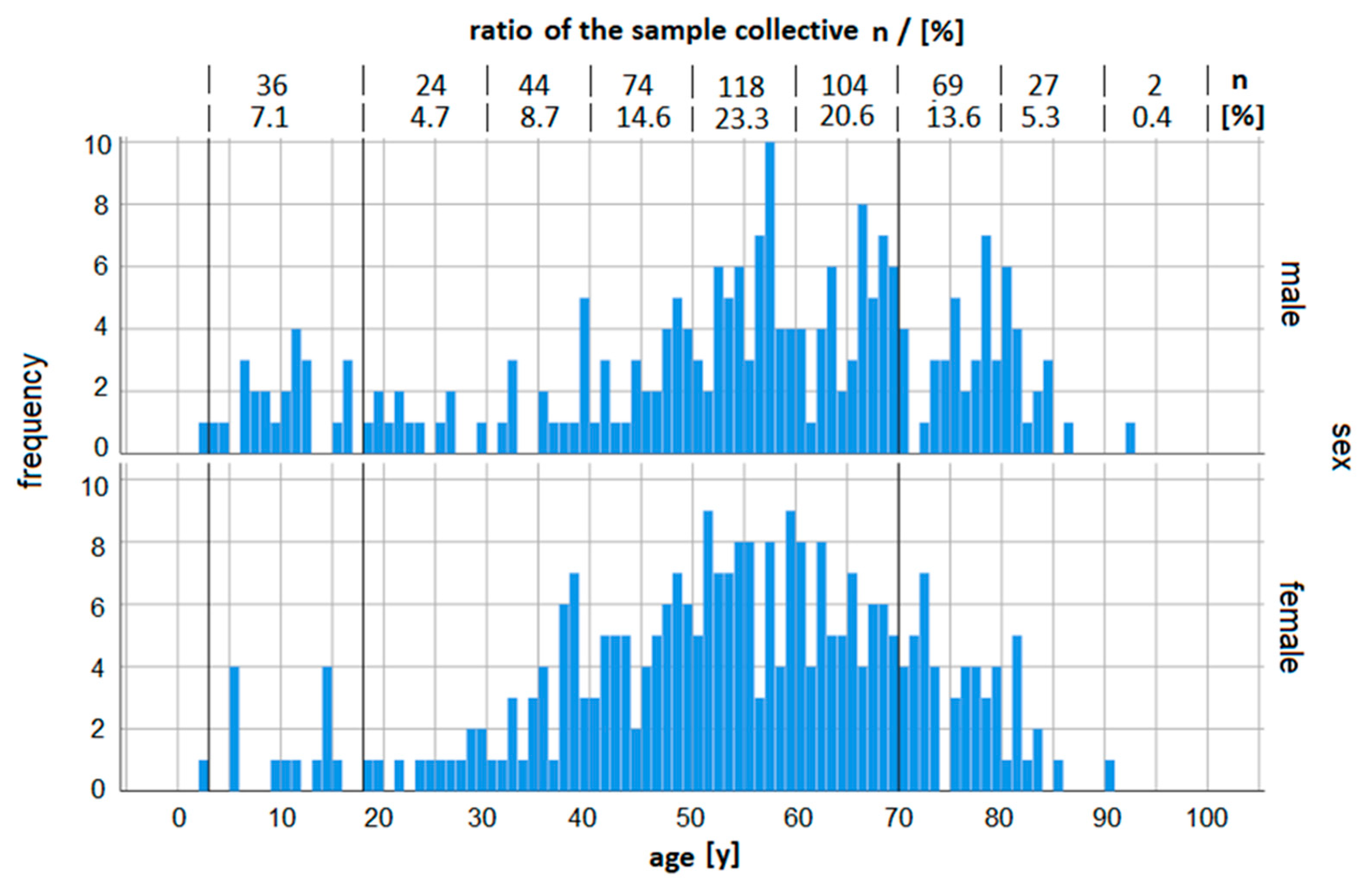
3.1. Sample Collective
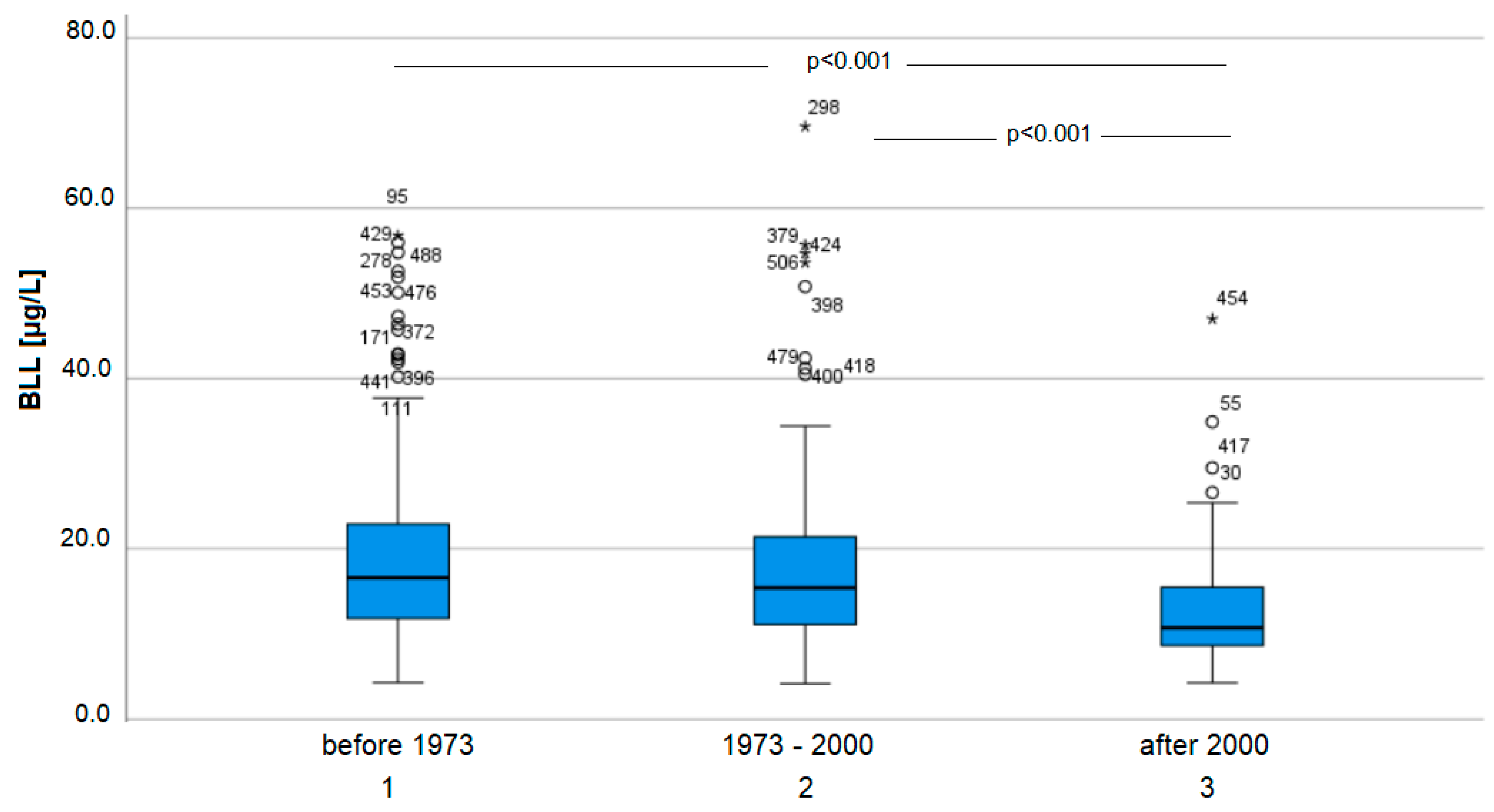
3.2. BLL
3.3. Statistics and Questionnaires
4. Discussion
4.1. Sample Collective
4.2. BLL
4.2.1. Minors
4.2.2. Adults
4.3. Hierarchical Regression Analysis
4.4. Comparison to Other Studies
4.4.1. Children Living in the Vicinity of Mining Sites
4.4.2. Adults Living in the Vicinity of Mining Sites
4.5. Policy Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead. U.S. Department of Health and Human Services. 2020. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp13.pdf (accessed on 29 October 2021).
- Delgado, C.F.; Ullery, M.A.; Jordan, M.; Duclos, C.; Rajagopalan, S.; Scott, K. Lead Exposure and Developmental Disabilities in Preschool-Aged Children. J. Public Health Manag. Pract. 2018, 24, e10–e17. [Google Scholar] [CrossRef]
- Koshy, B.; Srinivasan, M.; Zachariah, S.M.; Karthikeyan, A.S.; Roshan, R.; Bose, A.; Mohan, V.R.; John, S.; Ramanujam, K.; Muliyil, J.; et al. Body iron and lead status in early childhood and its effects on development and cognition: A longitudinal study from urban Vellore. Public Health Nutr. 2020, 23, 1896–1906. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, M.A.; Filippelli, G.M.; Sadler, R.C.; Gonzales, C.R.; Ball, A.S.; Mielke, H.W. Children’s Blood Lead Seasonality in Flint, Michigan (USA), and Soil-Sourced Lead Hazard Risks. Int. J. Environ. Res. Public Health 2016, 13, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanphear, B.P.; Dietrich, K.; Auinger, P.; Cox, C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000, 115, 521–529. [Google Scholar] [CrossRef] [PubMed]
- McLaine, P.; Navas-Acien, A.; Lee, R.; Simon, P.; Diener-West, M.; Agnew, J. Elevated blood lead levels and reading readiness at the start of kindergarten. Pediatrics 2013, 131, 1081–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Human Biomonitoring Commission (HBM Commission) of the Federal Environmental Protection Agency; Umweltbundesamt—Kommission Human Biomonitoring des Umweltbundesamtes. 2. Addendum zur “Stoffmonographie Blei”—Referenz- und “Human-Biomonitoring”-Werte der Kommission “Human-Biomonitorng”. Bundesgesundheitsbl.-Gesundh.-Gesundh. 2009, 52, 983–986. [Google Scholar] [CrossRef] [PubMed]
- German Research Foundation (GRF)—Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK-Commission); Deutsche Forschungsgemeinschaft (DFG)—Ständige Senatskommission zur Prüfung gesundheitsschädlicher Arbeitsstoffe. MAK- and BAT-List Lead and Its Organic Compounds Apart from Lead Arsenate and Lead Chromate; GRF: Bonn, Germany, 2000; p. 17. [Google Scholar]
- Delile, H.; Keenan-Jones, D.; Blichert-Toft, J.; Goiran, J.P.; Arnaud-Godet, F.; Albarede, F. Rome’s urban history inferred from Pb-contaminated waters trapped in its ancient harbor basins. Proc. Natl. Acad. Sci. USA 2017, 114, 10059–10064. [Google Scholar] [CrossRef] [Green Version]
- Lermen, D.; Weber, T.; Goen, T.; Bartel-Steinbach, M.; Gwinner, F.; Mueller, S.C.; Conrad, A.; Rüther, M.; von Briesen, H.; Kolossa-Gehring, M. Long-term time trend of lead exposure in young German adults—Evaluation of more than 35 Years of data of the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health 2021, 231, 113665. [Google Scholar] [CrossRef] [PubMed]
- Mattisson, K.; Tekavec, E.; Lundh, T.; Stroh, E. Cadmium and Lead Levels in Blood and Arsenic Levels in Urine among Schoolchildren Living in Contaminated Glassworks Areas, Sweden. Int. J. Environ. Res. Public Health 2020, 17, 7382. [Google Scholar] [CrossRef]
- Pohl, H.R.; Ingber, S.Z.; Abadin, H.G. Historical View on Lead: Guidelines and Regulations. Met. Ions Life Sci. 2017, 17, 435–470. [Google Scholar] [CrossRef]
- Park, D.U.; Kim, D.S.; Yu, S.D.; Lee, K.M.; Ryu, S.H.; Kim, S.G.; Yang, W.H.; Park, D.Y.; Hong, Y.S.; Park, J.D.; et al. Blood levels of cadmium and lead in residents near abandoned metal mine areas in Korea. Environ. Monit. Assess. 2014, 186, 5209–5220. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Tohyama, H.; Fujita, W.; Nakayama, S.M.M.; Ishizuka, M.; Yabe, J.; Munyinda, N.S.; Sakala, D.; Choongo, K.; Yamasaki, S.; et al. The impact of elevated blood lead levels in children on maternal health-related quality of life. Chemosphere 2021, 279, 130490. [Google Scholar] [CrossRef] [PubMed]
- Schoof, R.A.; Johnson, D.L.; Handziuk, E.R.; Landingham, C.V.; Feldpausch, A.M.; Gallagher, A.E.; Dell, L.D.; Kephart, A. Assessment of blood lead level declines in an area of historical mining with a holistic remediation and abatement program. Environ Res. 2016, 150, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boreland, F.; Lesjak, M.S.; Lyle, D.M. Managing environmental lead in Broken Hill: A public health success. N. S. W. Public Health Bull. 2008, 19, 174–179. [Google Scholar] [CrossRef] [Green Version]
- German Research Foundation—Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK-Commission); Deutsche Forschungsgemeinschaft (DFG)—Ständige Senatskommission zur Prüfung gesundheitsschädlicher Arbeitsstoffe. MAK- und BAT-Werte-Liste 2007 Maximale Arbeitsplatzkonzentrationen und Biologische Arbeitsstofftoleranzwerte Mitteilung 43; Wiley: Weinheim, Germany, 2007. [Google Scholar]
- German Research Foundation—Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK-Commission); Deutsche Forschungsgemeinschaft (DFG)—Ständige Senatskommission zur Prüfung gesundheitsschädlicher Arbeitsstoffe. MAK- und BAT-Werte-Liste 2021 Maximale Arbeitsplatzkonzentrationen und Biologische Arbeitsstofftoleranzwerte Mitteilung 57 (Vol. Mitteilung 57); Wiley: Weinheim, Germany, 2021. [Google Scholar]
- Egan, K.B.; Cornwell, C.R.; Courtney, J.G.; Ettinger, A.S. Blood Lead Levels in U.S. Children Ages 1–11 Years, 1976–2016. Environ. Health Perspect. 2021, 129, 37003. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Egan, K.B.; Homa, D.M.; Brown, M.J. Blood Lead Levels in U.S. Women of Childbearing Age, 1976–2016. Environ. Health Perspect. 2020, 128, 17012. [Google Scholar] [CrossRef] [Green Version]
- Smolders, R.; Alimonti, A.; Cerna, M.; Den Hond, E.; Kristiansen, J.; Palkovicova, L.; Ranft, U.; Seldén, A.I.; Telišman, S.; Schoeters, G. Availability and comparability of human biomonitoring data across Europe: A case-study on blood-lead levels. Sci. Total Environ. 2010, 408, 1437–1445. [Google Scholar] [CrossRef]
- Von Storch, H.; Costa-Cabral, M.; Hagner, C.; Feser, F.; Pacyna, J.; Pacyna, E.; Kolb, S. Four decades of gasoline lead emissions and control policies in Europe: A retrospective assessment. Sci. Total Environ. 2003, 311, 151–176. [Google Scholar] [CrossRef]
- German Federal Environmental Agency [Umweltbundesamt]. Reference values (RV95) for Antimony, Arsenic and Metals (Pb, Cd, Ni, Hg, Pt, Tl, U) in Urine or in Blood. [Referenzwerte (RV95) für Antimon, Arsen und Metalle (Pb, Cd, Ni, Hg, Pt, Tl, U) im Urin Oder im Blut.]. 2019. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/4031/dokumente/tab._referenzwerte_-_metalle_30._september_2019_aktualisiert.pdf (accessed on 29 October 2021). (In German).
- Human Biomonitoring Commission (HBM Commission) of the Federal Environmental Protection Agency Umweltbundesamt—Kommission Human Biomonitoring des Umweltbundesamtes. Aktualisierung der Referenzwerte für Blei im Blut von Erwachsenen—Stellungnahme der Kommission Human-biomonitoring des Umweltbundesamtes. [Bekanntmachungen-Amtliche Mitteilungen]. Bundesgesundheitsblatt 2019, 62, 1280–1284. [Google Scholar] [CrossRef]
- Vogel, N.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Doyle, U.; Kampfe, A.; Hoera, C.; Hildebrand, J.; Schaefer, M.; Drexler, H.; et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany—Human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2021, 237, 113822. [Google Scholar] [CrossRef]
- Günther, P.; Krüger, G.; Barkowski, D. Investigation in the Region of Mechernich and Kall—House Construction Sites. [Detailuntersuchung im Mechernich-Kaller Bleibelastungsgebiet—Bebauungsplangebiet “Auf der Wäsche”]; Report; Institut für Umwelt-Analyse Projekt GmbH: Bielefeld, Germany, 2020. [Google Scholar]
- Haley, V.B.; Talbot, T.O. Seasonality and trend in blood lead levels of New York State children. BMC Pediatrics 2004, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, R.; Zlli Viera, C.L.; Mordarski, D.C.; Rosenbaum, M.H. Lead seasonality in humans, animal, and the natural environment. Environ. Res. 2020, 180, 108797. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Risk Management Strategy for Lead; Health Canada: Ottawa, ON, Canada, 2013; ISBN 978-1-100-21305-7.
- Kim, N.S.; Lee, B.K. National estimates of blood lead, cadmium, and mercury levels in the Korean general adult population. Int. Arch. Occup. Environ. Health 2011, 84, 53–63. [Google Scholar] [CrossRef]
- Einbrodt, H.J. Results of the Performance of a Medical Survey Regarding the Lead Burden in the General Public in the Region of Mechernich. [Ergebnisbericht über die Durchführung einer medizinischen Reihenuntersuchung zur Frage der Bleibelastung der Bevölkerung im Raum Mechernich]; Abteilung Hygiene und Arbeitsmedizin der RWTH Aachen: Aachen, Germany, 1982. [Google Scholar]
- Landes, F.C.; Inauen, J.; Ponce-Canchihuaman, J.; Markowski, K.; Ellis, T.K.; van Geen, A. Does Involving Parents in Soil Sampling Identify Causes of Child Exposure to Lead? A Case Study of Community Engagement in Mining-Impacted Towns in Peru. Geohealth 2019, 3, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Moonga, G.; Chisola, M.N.; Berger, U.; Nowak, D.; Yabe, J.; Nakata, H.; Nakayama, S.; Ishizuka, M.; Bose-O’Reilly, S. Geospatial approach to investigate spatial clustering and hotspots of blood lead levels in children within Kabwe, Zambia. Environ. Res. 2022, 207, 112646. [Google Scholar] [CrossRef] [PubMed]
- Surenbaatar, U.; Kim, B.G.; Seo, J.W.; Lim, H.J.; Kwon, J.Y.; Kang, M.K.; Altangerel, E.; Byambaa, T.; Batbaatar, S.; Myagmardorj, O.; et al. Environmental health survey for children residing near mining areas in South Gobi, Mongolia. Ann. Occup. Environ. Med. 2021, 33, e10. [Google Scholar] [CrossRef] [PubMed]
- Agyemang, V.; Acquaye, J.K.; Harrison, S.B.E.; Oppong, F.B.; Gyaase, S.; Asante, K.P.; Olayemi, E. Blood Lead Levels among Blood Donors and High-Risk Occupational Groups in a Mining Area in Ghana: Implications for Blood Transfusion among Vulnerable Populations. J. Trop. Med. 2020, 2020, 6718985. [Google Scholar] [CrossRef] [PubMed]
- Childebayeva, A.; Goodrich, J.M.; Chesterman, N.; Leon-Velarde, F.; Rivera-Ch, M.; Kiyamu, M.; Brutsaert, T.D.; Bigham, A.W.; Dolinoy, D.C. Blood lead levels in Peruvian adults are associated with proximity to mining and DNA methylation. Environ. Int. 2021, 155, 106587. [Google Scholar] [CrossRef]
- Malamba-Lez, D.; Tshala-Katumbay, D.; Bito, V.; Rigo, J.M.; Kipenge Kyandabike, R.; Ngoy Yolola, E.; Katchunga, P.; Koba-Bora, B.; Ngoy-Nkulu, D. Concurrent Heavy Metal Exposures and Idiopathic Dilated Cardiomyopathy: A Case-Control Study from the Katanga Mining Area of the Democratic Republic of Congo. Int. J. Environ. Res. Public Health 2021, 18, 4956. [Google Scholar] [CrossRef]


| Pb µg/L | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age [y] | Amount | Min | Max | Mean | RV | Number > RV | >RV [%] | |
| girls | <3 | 1 | – | – | 23.8 * | – | – | – |
| girls | 3–17 | 13 | 4.3 | 25.4 | 10.6 | 15 | 2 | 15.4 |
| boys | <3 | 1 | – | – | 12.4 * | – | – | – |
| boys | 3–10 | 12 | 9.3 | 23.4 | 14.6 | 20 | 2 | 16.7 |
| boys | 11–17 | 11 | 6.2 | 26.6 | 10.8 | 15 | 2 | 18.2 |
| women adults | 18–69 | 216 | 4.2 | 56 | 15.4 | 30 | 14 | 6.5 |
| men adults | 18–69 | 148 | 5 | 69.6 | 17.8 | 40 | 9 | 6.1 |
| women elderly | >70 | 49 | 4.5 | 54.8 | 22.2 | – | [8] | [16.3] |
| men elderly | >70 | 49 | 9.3 | 55.7 | 24.4 | – | [8] | [16.3] |
| unkown age & sex | – | 6 | 12.5 | 25.4 | 17.7 | – | – | – |
| total | – | 506 | 4.2 | 69.6 | 17.4 | – | – | – |
| Standardized Regression Coefficiant Beta | p-Value | Variabel Type | |
|---|---|---|---|
| age | 0.218 | 0.000 | numeric |
| occupancy | 0.156 | 0.008 | numeric |
| smoking | 0.024 | 0.009 | categorial |
| sex | −0.107 | 0.024 | categorial |
| intense soil contact | 0.104 | 0.035 | categorial |
| age real estate | −0.102 | 0.043 | numeric |
| garden time | 0.095 | 0.058 | numeric |
| region | −0.080 | 0.095 | categorial |
| pregnancy | −0.042 | 0.374 | categorial |
| bowels | −0.038 | 0.443 | categorial |
| Mean | |||||||
|---|---|---|---|---|---|---|---|
| Country | Region/Name | Sampling | Lead Source | Age [a] | BLL [µg/L] | n | Reference |
| Australia | Broken Hill | 1991 | Lead mine before remediation | 1–4 | 163 | n.d. | [16] |
| Australia | Broken Hill | 2007 | Lead mine after remediation | 1–4 | 83 | n.d. | [16] |
| Peru | Corcona, Tornamesa | 2015 | Several mining activities including lead; Past/Present | 0–6 | 72 | 200 | [32] |
| Zambia | Kabwe | 2017 | Lead-zinc mining town | 6–12 | 197 | 208 | [14] |
| USA | Butte | 2003 | Copper mining site | 1–5 | 34.8 | 351 | [15] |
| USA | Butte | 2010 | Copper mining site | 1–5 | 15.3 | 461 | [15] |
| Mean | ||||||||
|---|---|---|---|---|---|---|---|---|
| Country | Region/Name | Sampling | Lead Source | Age [a] | BLL [µg/L] | n | Reference | Country |
| Ghana | Kenyasi | 2017 | Gold mining area | 18–57 | 50 | 40 | [33] | Ghana |
| Peru | Cerro de Pasco | 2012/2013 | Mostly lead and zinc mining | 18–35 | 47.5 | 157 | [32] | Peru |
| Congo | Katanga | 2017/2019 | Copper and cobalt mining area | 34–62 | 56.5 | 29 | [35] | Congo |
| Zambia | Kabwe | 2017 | Lead-zinc mining town | adult mothers | 106 | 404 | [14] | Zambia |
| Zambia | Kabwe | 2017 | Lead-zinc mining town | adult fathers | 116 | 125 | [14] | Zambia |
| Korea | various | 2008–2011 | 38 abandoned gold, silver, copper metal mine areas | 18–39 | 26.9 | 164 | [13] | Korea |
| Korea | various | 2008–2011 | 38 abandoned gold, silver, copper metal mine areas | 40–64 | 32.3 | 2077 | [13] | Korea |
| Korea | various | 2008–2011 | 38 abandoned gold, silver, copper metal mine areas | >65 | 30.6 | 3441 | [13] | Korea |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertram, J.; Ramolla, C.; Esser, A.; Schettgen, T.; Fohn, N.; Kraus, T. Blood Lead Monitoring in a Former Mining Area in Euskirchen, Germany—Volunteers across the Entire Population. Int. J. Environ. Res. Public Health 2022, 19, 6083. https://doi.org/10.3390/ijerph19106083
Bertram J, Ramolla C, Esser A, Schettgen T, Fohn N, Kraus T. Blood Lead Monitoring in a Former Mining Area in Euskirchen, Germany—Volunteers across the Entire Population. International Journal of Environmental Research and Public Health. 2022; 19(10):6083. https://doi.org/10.3390/ijerph19106083
Chicago/Turabian StyleBertram, Jens, Christian Ramolla, André Esser, Thomas Schettgen, Nina Fohn, and Thomas Kraus. 2022. "Blood Lead Monitoring in a Former Mining Area in Euskirchen, Germany—Volunteers across the Entire Population" International Journal of Environmental Research and Public Health 19, no. 10: 6083. https://doi.org/10.3390/ijerph19106083
APA StyleBertram, J., Ramolla, C., Esser, A., Schettgen, T., Fohn, N., & Kraus, T. (2022). Blood Lead Monitoring in a Former Mining Area in Euskirchen, Germany—Volunteers across the Entire Population. International Journal of Environmental Research and Public Health, 19(10), 6083. https://doi.org/10.3390/ijerph19106083








