Abstract
Background. Salivary metabolomics is garnering increasing attention in the health field because of easy, minimally invasive saliva sampling. Dihydrouracil (DHU) is a metabolite of pyrimidine metabolism present in urine, plasma, and saliva and of fluoropyrimidines-based chemotherapeutics. Its fast quantification would help in the identification of patients with higher risk of fluoropyrimidine-induced toxicity and inborn errors of pyrimidine metabolism. Few studies consider DHU as the main salivary metabolite, but reports of its concentration levels in saliva are scarce. We propose the direct determination of DHU in saliva by reversed-phase high-performance liquid chromatography (RP-HPLC-UV detector) as a simple, rapid procedure for non-invasive screening. Methods. The method used was validated and applied to 176 saliva samples collected from 21 nominally healthy volunteers and 4 saliva samples from metastatic colorectal cancer patients before and after receiving 5-fluorouracil chemotherapy. Results. DHU levels in all samples analyzed were in the μmol L−1 range or below proving that DHU is not the main metabolite in saliva and confirming the results found in the literature with LC-MS/MS instrumentation. Any increase of DHU due to metabolism dysfunctions can be suggestive of disease and easily monitored in saliva using common, low-cost instrumentation available also for population screening.
1. Introduction
Dihydrouracil (DHU) is a small metabolite of interest in clinical chemistry because, with dihydrothymine, it is the by-product of the pyrimidine metabolism [1]. Its increase in biological fluids is often a symptom of a disfunction of dihydropyrimidinase (DHP), the enzyme involved in the pyrimidine base degradation (uracil and thymine). DHP deficiency gives inborn errors of pyrimidine metabolism, with symptoms ranging from asymptomatic cases to epilepsy, intellectual disability, epilepsy, and autism [1,2,3]. 5-fluorouracil and its oral pre-prodrug capecitabine are the election treatment of colorectal, pancreatic, gastric, breast, and head and neck cancers [4]. The fluoropyrimidine toxicity (infections, septicemia, leucopenia, thrombocytopenia, neutropenia, angina pectoris, myocardial infarction, and gastrointestinal adverse effects) is due to an inherited deficiency of DHP in 15% of patients, which is responsible for 80% of the catabolism of fluoropyrimidines [5]. This limits or delays the administration of optimal or successive courses in chemotherapy treatments [6]. The detection of DPD activity is indeed recommended by the European Medicine Agency (https://www.ema.europa.eu/en/news/ema-recommendations-dpd-testing-prior-treatment-fluorouracil-capecitabine-tegafur-flucytosine) (accessed on 13 May 2022) before starting any treatment with fluoropyrimidine [7,8]. Thus, the quick and easy determination of DHU and of DHU-to-uracil (U) concentration ratio (DHU/U) in biological fluids (e.g., plasma, urine, and saliva) is a significant analytical target due to the potential relationship between these compounds and DHP deficiency.
Many analytical techniques have been developed for the determination of DHU in plasma based on UV [9,10,11,12,13] and mass spectrometry (MS) detection, including a fully automated LC-MS/MS method for the accurate and robust quantification of U and DHU in plasma [5,6]. They found that DHU in plasma ranges between 0.002–0.011 μmol L−1. Pan et al. recently reported an improved method for the detection of uracil and dihydrouracil in human plasma using reversed-phase high-performance liquid chromatography. Tsuchiya et al. determined DHU in urine by GC-MS and LC-MS reporting reference values in Fujita Health University Hospital in the range of 0–16 μmol mmol−1 creatine (corresponding to 0–0.416 μmol L−1 considering the reference interval of creatinine) [1]. Van Kuilenburg et al. quantified DHU in urine, plasma, and cerebrospinal fluid using reversed-phase LC combined with electrospray MS/MS [14], finding values in agreement with those reported above. Sparidans et al. reported a concentration of DHU in urine of 1.1 ± 0.9 μmol L−1 in healthy subjects determined by LC–MS/MS [15]. Analogous values were obtained by Sun et al. in urine by LC-MS [16]. Few data have been reported on the quantitation of DHU in saliva, which is an attractive matrix due to its non-invasive sampling. Venzon Antunes et al. validated a LC-MS/MS assay for the measurement of U and DHU concentrations in dried saliva spots of 77 individuals (38 healthy volunteers and 39 patients with gastrointestinal cancer scheduled to receive 5-fluorouracil chemotherapy) for the evaluation of DHP enzyme activity, finding a median of 0.926 μmol L−1 (0.673–1.798 μmol L−1 range) [17,18]. Galarza et al. measured by LC–MS/MS endogenous plasma and salivary DHU in 60 patients with gastrointestinal malignancies [19]. They found that in plasma DHU had a median of 0.799 μmol L−1 (range 0.537–1.023 μmol L−1); in saliva, DHU had a median of 2.168 μmol L−1 (range 1.139–5.013 μmol L−1) [19]. Al-Shehri et al. found DHU concentration level < 3 μmol L−1 in saliva of 10 healthy adults and 8.6 μmol L−1 in 10 neonates [3]. Carlsson et al. determined increasing levels of DHU in saliva of 73 colorectal cancer patients treated with 5-fluorouracil-based chemotherapy by HPLC method with UV detector at 220 nm using a first C18 reversed-phase column and by switching the C18 reversed-phase column to a second cation-exchange column for one minute to separate uracil from DHU [20]. Only after chemotherapy, they found DHU concentration of 0.043 ± 0.035 μmol L−1. In contrast with these values found in saliva, other fundamental papers on salivary metabolomics [21,22] reported DHU being the main metabolite of saliva itself, about 2000 μmol L−1, i.e., a concentration 1000 higher than that found in saliva, plasma, and urine by the previously mentioned works. DHU concentration has been reported to be 2168 ± 128 μmol L−1 by Dame et al. [22] and 2210 ± 353 μmol L−1 by Sugimoto et al. [21], measured by HPLC–UV and capillary electrophoresis-MS, respectively.
We recently approached the study of salivary metabolites with the aim of using saliva analysis as a minimally invasive, safe, and painless tool for the monitoring of the health status [23,24,25,26]. Here, we propose a fast bioanalytical procedure for the determination DHU in saliva based on saliva dilution and analysis reversed-phase liquid chromatography with diode array detection (RP-LC-DAD) in less than 6 min isocratic separation. No interferences from endogenous components were revealed. The method was validated and applied to 176 saliva samples collected from 21 nominally healthy volunteers and 4 saliva samples from metastatic colorectal cancer patients before and after receiving maintenance therapy with 5-fluorouracil chemotherapy.
2. Materials and Methods
2.1. Reagents, Materials, and Reference Standard Samples
Dihydrouracil (D-7628, CAS n. 504-07-4) was purchased from Sigma Aldrich (Milan, Italy). Methanol HPLC grade was purchased from Merck (Darmstadt, Germany). Purified water was obtained from an Elga Purelab Ultra system from Veolia Labwater (High Wycombe, United Kingdom). Additionally, 0.20 μm RC Mini-Uniprep filter units were obtained from (Agilent Tech., Milan, Italy). Saliva samples were collected using a Salivette device (Sarstedt, Nümbrecht, Germany).
2.2. Preparation of Solutions and Standards
DHU stock solution was prepared in water at the concentration of 10 mmol L−1. Working solutions (concentrations 1–2500 μmol L−1) were prepared by dilution of the stock solutions with the eluent phase (5 mM sulfuric acid).
2.3. Saliva Sample Preparation
Salivette® roll-shaped polyester swabs (Sarstedt, Nümbrecht, Germany) were used for the collection of non-stimulated saliva samples collected in different days from 21 nominally healthy volunteers and from 4 metastatic colorectal cancer patients receiving 5-fluorouracil at the University Hospital of Pisa (Pisa, Italy) within 1 h from the beginning of the 5-fluorouracil infusion and at the end of the infusion. 5-fluorouracil was administered as a continuous 48 h intravenous infusion at a dose of 2400 mg/mq (maintenance therapy). In total, N = 176 saliva samples were collected from healthy volunteers sampling in different days. This study was conducted in accordance with the principles set in the Helsinki Declaration. Ethical review and approval are not applicable because all subjects were volunteers. All subjects provided their written informed consent before any procedure. The healthy participant population included 11 men and 10 women, ranging in age from 26 to 62 y (mean age ± standard deviation, 40 ± 12 y); patients included 1 man and 3 women, ranging in age from 52 to 78 y (64 ± 14 y). For all participants, saliva samples were collected at the same time of the day (6:00 a.m. to 7:00 a.m.), to avoid fluctuation in the results due to the circadian saliva cycle, after at least 8 h of fasting or tooth brushing. Salivette® swabs (Sarstedt, Nümbrecht, Germany) were kept in the mouth for 5 min and, after collection were immediately stored at −20 °C. Prior to analysis, swabs were thawed at room temperature and then centrifuged at 4500× g for 10 min at 4 °C (Eppendorf™ 5804R Centrifuge) (Eppendorf, Milan, Italy). Saliva samples were diluted 5 times in 5 mM sulfuric acid, filtered using a 0.20 μm RC Mini-Uniprep filter, and then injected (Vinj = 5 μL) in the HPLC system.
2.4. HPLC Analytical Conditions
An Agilent 1260 Infinity HPLC system (G1311B quaternary pump) equipped with 1260 Infinity High-Performance Degasser, a TCC G1316A thermostat, 1260ALS autosampler (G1329B), and UV/vis diode array (1260 DAD G4212B) (Eppendorf, Milan, Italy) was employed. The identification of metabolites was based on the comparison of the retention time and UV spectra of standard compounds. The chromatographic separation was carried out by Zorbax Phenyl-Hexyl RP (Agilent Tech., Milan, Italy) 250 × 4.6 mm (silica particle size 5 μm) at 45 °C using an isocratic elution with 100% 5 mM sulfuric acid (pH 2.2) for 6 min. The column was rinsed with 100% methanol for 15 min, and then, a re-equilibration step was performed. All the solutions were filtered using a 0.22 μm regenerate cellulose filter (Millipore, Milan, Italy).
3. Results and Discussion
3.1. Method Validation
Figure 1A shows the absorbance chromatogram at 220 nm of 5, 10, and 45 μmol L−1 DHU eluting in our operating conditions at tR = 5.151. The method was validated in saliva by evaluating linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision. LOD and LOQ were determined by the analysis of samples with known concentrations of the standard analyte and by establishing the minimum level at which the analyte can be reliably detected and quantified with acceptable accuracy and precision, respectively. Linearity was calculated in the range 1–2500 μmol L−1 for the chemical standard DHU. Precision was recorded in standard solutions at three concentration levels in (QCLow = 10 μmol L−1, QCMedium = 200 μmol L−1, and QCHigh = 1000 μmol L−1) in three replicates to calculate the percentage of relative standard deviation (RSD), resulting as 3.4, 2.1, and 1.1%, respectively. In saliva at QCLow = 10 μmol L−1, QCMedium = 500 μmol L−1, and QCHigh = 2000 μmol L−1 RSD resulted as 6.1, 2.3, and 2.5%, respectively. DHU concentration was calculated based on the area of the peak at tR = 5.151 min and the corresponding calibration curve obtained in the matrix.
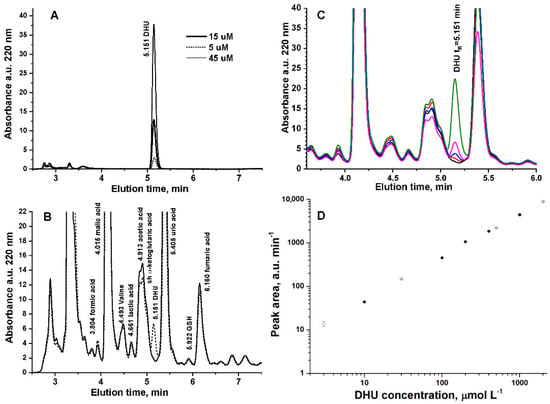
Figure 1.
(A) Absorbance chromatogram at 220 nm of 5, 10, and 45 μmol L−1 DHU. (B) Absorbance chromatogram at 220 nm of unspiked saliva (continuous line) and spiked with 10 μmol L−1 DHU (dotted line). (C) Absorbance chromatogram at 220 nm of unspiked saliva (continuous line) and spiked with 1 (red line), 3 (blue line), 10 (magenta line), or 30 μmol L−1 DHU (green line). (D) External calibration curve (full circle) and calibration curve of DHU analytical standard added in saliva (open circle) (N = 3 replicates).
The accuracy of the method was evaluated by spiking a pooled saliva sample with the DHU standard solution, diluting five times in 5 mM sulfuric acid, filtering using a 0.20 μm RC Mini-Uniprep filter, and then injecting (Vinj = 5 μL) in the HPLC system (N = 3 replicates). Figure 1B shows the absorbance chromatogram at 220 nm of unspiked saliva (continuous line) and spiked with 10 μmol L−1 DHU. The spike of DHU to saliva sample showed a peak eluting at tR = 5.151 min, which was not detected in unspiked saliva. Figure 1C shows a detail of the absorbance chromatogram at 220 nm of unspiked saliva and saliva and spiked with 1, 3, 10, or 30 μmol L−1 DHU. Figure 1D shows the comparison of the external calibration curve (open circle) and the calibration curve of the analytical standards added in saliva (full circle). We also verified that DHU is quantitatively recovered (98 ± 3%) from the swab by analyzing 50 μmol L−1 DHU standard solution before and after adsorption/centrifugation of Salivette®. Table 1 reports the fitting parameters of the calibration curves (Figure 1D) and the quantitation limits (LOQs). Although the nominal LOQ of DHU in standard solutions is 0.103 μmol L−1, in saliva, LOQ spans from 0.103 to 3 μmol L−1 depending on the concentration level of α-ketoglutaric acid (shoulder at 5.022 min in Figure 1B), which has a variable concentration in saliva [26]. Figure 1B shows the worst situation, in which α-ketoglutaric acid has high concentration and coelutes with acetic acid.

Table 1.
Calibration curves and limit of quantitation LOQ for the determination of dihydrouracil DHU by HPLC-DAD (N = 3 replicates).
3.2. Application
In all saliva samples of 18 out of 21 nominally healthy volunteers (total 161 saliva sample analyses), DHU was below the LOQ of this method. In three subjects, DHU concentration was found 4.01 ± 1.15 (nominally S19, N = 8 sampling in different days), 0.66 ± 0.67 (nominally S20, N = 3 sampling in different days), and 0.19 ± 0.14 μmol L−1 (nominally S21, N = 4 sampling in different days). Figure 2 shows representative absorbance chromatograms at 220 nm of saliva samples of S20 and S21 compared with the chromatogram of a nominally healthy volunteer, with DHU < LOQ (S18).
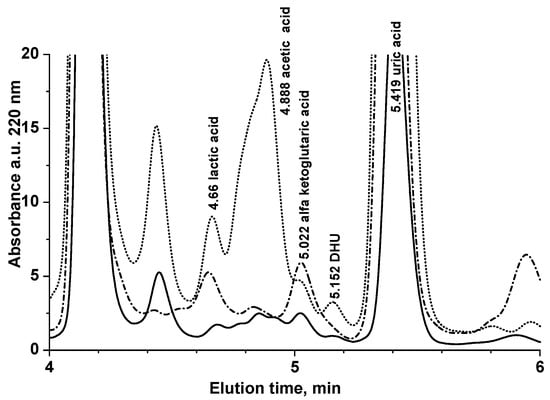
Figure 2.
Representative absorbance chromatogram at 220 nm of saliva samples of S20 (dotted line, 0.66 ± 0.67 μmol L−1 DHU, N = 3 sampling in different days) and S21 (continuous line, 0.19 ± 0.14 μmol L−1 DHU, N = 4 sampling in different days) compared with the chromatogram of a nominally healthy volunteer with DHU < LOQ (S18, dash-dotted line).
DHU concentration in saliva sample from four metastatic colorectal cancer patients receiving 5-fluorouracil sampled before and after the infusion was always below the LOQ. These results obtained agree with the DHU concentration value found in saliva by other authors [17,18,19,20], clarifying that DHU is not the main metabolite in saliva [21,22].
Table 2 summarizes the scarce data reported in the literature on DHU concentration in saliva, including the results found in this work.

Table 2.
Summary of data on DHU quantitation in saliva.
Despite the limited number of cases examined, the finding of a slightly higher concentration of DHU in 3 out of 21 nominally healthy volunteers arouses interest in the role of DHU in metabolism. DHU monitoring is indeed commonly related to DPD dysfunctions during chemotherapy treatments. However, DHU is an oxidation product of the nucleotide uracil and may provide a stable marker of altered nucleotide metabolism in several diseases [14,28,29,30,31,32]. In inflammatory colon tissue (ulcerative colitis or Crohn’s disease), DPD activity was significantly higher than in normal tissue (p = 0.006) [33]. Wikoff et al. found distinct metabolic perturbations associated with early stage lung adenocarcinoma [34]. DHU was significantly elevated by 2.4-fold in cancer compared with control tissue and constituted the single best multivariate predictor for cancer [34]. Thus, the wide screening of DHU in an “easy” matrix such as saliva and also in not-highly equipped laboratories as well as eventually the definition of its reference range within a population is of the utmost importance for metabolic studies. This topic is beyond the methodological goal of this work, but in perspective, the screening of DHU in saliva could address the clinician to more informative, detailed investigations, which is the goal of personalized medicine.
4. Conclusions
The direct RP-HPLC-DAD method proposed allows the analyst to determine DHU in saliva in less than 6 min after an easy, fast dilution and filtration of the saliva sample. The DHU concentration levels found in saliva in this work as well as the values found by other authors [17,18,19,20] confirm that DHU is not the main metabolite in human saliva, and its concentration in nominally healthy volunteers is in the micromolar range or below. Despite many of these data have been obtained using LC-MS/MS approaches, we found that HPLC with UV detector can be a straightforward approach suitable for a fast, low-cost screening of DHU in saliva whenever the operating conditions allow the control of potential compounds interfering with the DHU determination. In HPLC, methods with UV detection interferences must indeed be accurately checked because of the scarce selectivity of UV spectra. The LOQ of DHU found using this methodology is indeed comparable with the LOD found in saliva by other authors using LC-MS/MS [3]. Due to the role of DHU as a marker of pyrimidine metabolism, the wide screening of DHU in a nominally healthy population may provide a personalized diagnostic to identify cohorts of patients with alterations in DPD activity.
Author Contributions
Conceptualization, E.B. (Emilia Bramanti), B.C. and E.B. (Edoardo Benedetti); methodology, E.B. (Emilia Bramanti), B.C., M.O. and R.N.; validation, E.B. (Emilia Bramanti), T.L., M.O., R.N., F.M. and C.C.; resources, E.B. (Emilia Bramanti); writing—original draft preparation, E.B. (Emilia Bramanti); writing—review and editing, E.B. (Emilia Bramanti), E.B. (Edoardo Benedetti), B.C. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki (https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/) (last accessed on 13 May 2022). Ethical review and approval are not applicable because all subjects were volunteers.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from volunteers to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsuchiya, H.; Akiyama, T.; Kuhara, T.; Nakajima, Y.; Ohse, M.; Kurahashi, H.; Kato, T.; Maeda, Y.; Yoshinaga, H.; Kobayashi, K. A case of dihydropyrimidinase deficiency incidentally detected by urine metabolome analysis. Brain Dev. 2019, 41, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. Urine Pyrimidine Metabolite Determination by HPLC Tandem Mass Spectrometry. In Clinical Applications of Mass Spectrometry in Biomolecular Analysis: Methods and Protocols; Garg, U., Ed.; Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1378, pp. 237–242. ISBN1 978-1-4939-3182-8. ISBN2 978-1-4939-3181-1. [Google Scholar]
- Al-Shehri, S.; Henman, M.; Charles, B.G.; Cowley, D.; Shaw, P.N.; Liley, H.; Tomarchio, A.; Punyadeera, C.; Duley, J.A. Collection and determination of nucleotide metabolites in neonatal and adult saliva by high performance liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 931, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chionh, F.; Lau, D.; Yeung, Y.; Price, T.; Tebbutt, N. Oral versus intravenous fluoropyrimidines for colorectal cancer. Cochrane Database Syst. Rev. 2017, 7, CD008398. [Google Scholar] [CrossRef] [PubMed]
- Robin, T.; Saint-Marcoux, F.; Toinon, D.; Tafzi, N.; Marquet, P.; El Balkhi, S. Automatic quantification of uracil and dihydrouracil in plasma. J. Chromatogr. B 2020, 1142, 122038. [Google Scholar] [CrossRef] [PubMed]
- Tafzi, N.; Woillard, J.-B.; Fleytoux, A.; Picard, N.; Marquet, P. Phenotyping of uracil and 5-fluorouracil metabolism using LC-MS/MS for prevention of toxicity and dose adjustment of fluoropyrimidines. Ther. Drug Monit. 2020, 42, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Chamorey, E.; Francois, E.; Etienne, M.-C.; Ferrero, J.-M.; Peyrade, F.; Barranger, E.; Bozec, A.; Largillier, R.; Cassuto, O.; Viotti, J.; et al. DPD status and fluoropyrimidines-based treatment: High activity matters too. BMC Cancer 2020, 20, 436. [Google Scholar] [CrossRef]
- Martens, F.K.; Huntjens, D.W.; Rigter, T.; Bartels, M.; Bet, P.M.; Cornel, M.C. DPD Testing Before Treatment With Fluoropyrimidines in the Amsterdam UMCs: An Evaluation of Current Pharmacogenetic Practice. Front. Pharmacol. 2020, 10, 1069. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Li, Y.; Feng, Y.; Yang, F.; Liu, H. A new sample preparation and separation combination for the precise, accurate, and simultaneous determination of uracil and dihydrouracil in human plasma by reversed-phase HPLC. J. Sep. Sci. 2017, 40, 3763–3770. [Google Scholar] [CrossRef]
- Déporte, R.; Amiand, M.; Moreau, A.; Charbonnel, C.; Campion, L. High-performance liquid chromatographic assay with UV detection for measurement of dihydrouracil/uracil ratio in plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 834, 170–177. [Google Scholar] [CrossRef]
- Garg, M.B.; Sevester, J.C.; Sakoff, J.A.; Ackland, S.P. Simple liquid chromatographic method for the determination of uracil and dihydrouracil plasma levels: A potential pretreatment predictor of 5-fluorouracil toxicity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 774, 223–230. [Google Scholar] [CrossRef]
- Remaud, G.; Boisdron-Celle, M.; Hameline, C.; Morel, A.; Gamelin, E. An accurate dihydrouracil/uracil determination using improved high performance liquid chromatography method for preventing fluoropyrimidines- related toxicity in clinical practice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 823, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Svobaite, R.; Solassol, I.; Pinguet, F.; Ivanauskas, L.; Brès, J.; Bressolle, F.M.M. HPLC with UV or mass spectrometric detection for quantifying endogenous uracil and dihydrouracil in human plasma. Clin. Chem. 2008, 54, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- van Kuilenburg, A.B.P.; Dobritzsch, D.; Meijer, J.; Meinsma, R.; Benoist, J.-F.; Assmann, B.; Schubert, S.; Hoffmann, G.F.; Duran, M.; de Vries, M.C.; et al. Dihydropyrimidinase deficiency: Phenotype, genotype and structural consequences in 17 patients. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparidans, R.W.; Bosch, T.M.; Jorger, M.; Schellens, J.H.M.; Beijnen, J.H. Liquid chromatography-tandem mass spectrometric assay for the analysis of uracil breakdown in urine for the measurement of the activities and phenotyping of the corresponding pyrimidine catabolic enzymes. J. Chromatogr. B 2006, 839, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Garg, U. Clinical Applications of Mass Spectrometry in Drug Analysis: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 1493932519. [Google Scholar]
- Antunes, M.V.; Raymundo, S.; Cezimbra da Silva, A.C.; Muller, V.V.; Vicente Neto, O.J.; Schwartsmann, G.; Linden, R. Determination of Endogenous Concentrations of Uracil and Dihydrouracil in Dried Saliva Spots by LC-MS/MS. Ther. Drug Monit. 2019, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Neto, O.V.; Raymundo, S.; Franzoi, M.A.; do Carmo Artmann, A.; Tegner, M.; Müller, V.V.; Hahn, R.Z.; Alves, G.V.; Schwartsmann, G.; Linden, R.; et al. DPD functional tests in plasma, fresh saliva and dried saliva samples as predictors of 5-fluorouracil exposure and occurrence of drug-related severe toxicity. Clin. Biochem. 2018, 56, 18–25. [Google Scholar] [CrossRef]
- Andrade Galarza, A.F.; Linden, R.; Antunes, M.V.; Hahn, R.Z.; Raymundo, S.; da Silva, A.C.; Staggemeier, R.; Spilki, F.R.; Schwartsmann, G. Endogenous plasma and salivary uracil to dihydrouracil ratios and DPYD genotyping as predictors of severe fluoropyrimidine toxicity in patients with gastrointestinal malignancies. Clin. Biochem. 2016, 49, 1221–1226. [Google Scholar] [CrossRef]
- Carlsson, G.; Odin, E.; Gustavsson, B.; Wettergren, Y. Pretherapeutic uracil and dihydrouracil levels in saliva of colorectal cancer patients are associated with toxicity during adjuvant 5-fluorouracil-based chemotherapy. CANCER Chemother. Pharmacol. 2014, 74, 757–763. [Google Scholar] [CrossRef]
- Sugimoto, M.; Saruta, J.; Matsuki, C.; To, M.; Onuma, H.; Kaneko, M.; Soga, T.; Tomita, M.; Tsukinoki, K. Physiological and environmental parameters associated with mass spectrometry-based salivary metabolomic profiles. Metabolomics 2013, 9, 454–463. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Biagini, D.; Lomonaco, T.; Ghimenti, S.; Fusi, J.; Cerri, E.; De Angelis, F.; Bellagambi, F.G.; Oger, C.; Galano, J.M.; Bramanti, E.; et al. Saliva as a non-invasive tool for monitoring oxidative stress in swimmers athletes performing a VO2max cycle ergometer test. Talanta 2020, 216, 120979. [Google Scholar] [CrossRef] [PubMed]
- Campanella, B.; Onor, M.; Lomonaco, T.; Benedetti, E.; Bramanti, E. HS-SPME-GC-MS approach for the analysis of volatile salivary metabolites and application in a case study for the indirect assessment of gut microbiota. Anal. Bioanal. Chem. 2019, 411, 7551–7562. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, T.; Ghimenti, S.; Biagini, D.; Bramanti, E.; Onor, M.; Bellagambi, F.G.; Fuoco, R.; Di Francesco, F. The effect of sampling procedures on the urate and lactate concentration in oral fluid. Microchem. J. 2018, 136, 255–262. [Google Scholar] [CrossRef]
- Campanella, B.; Lomonaco, T.; Benedetti, E.; Onor, M.; Nieri, R.; Bramanti, E. Validation and Application of a Derivatization-Free RP-HPLC-DAD Method for the Determination of Low Molecular Weight Salivary Metabolites. Int. J. Environ. Res. Public Health 2020, 17, 6158. [Google Scholar] [CrossRef] [PubMed]
- Bhavyasri, K.; Vishnumurthy, K.M.; Rambabu, D.; Sumakanth, M. ICH guidelines—Q series (quality guidelines). A review. GSC Biol. Pharm. Sci. 2019, 6, 89–106. [Google Scholar]
- Sumi, S.; Kidouchi, K.; Ohba, S.; Wada, Y. Automated screening system for purine and pyrimidine metabolism disorders using high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1995, 672, 233–239. [Google Scholar] [CrossRef]
- Hubert, L.; Sutton, V.R. Chapter 12—Disorders of Purine and Pyrimidine Metabolism. In Clinical Aspects and Laboratory Determination; Garg, U., Smith, L., Eds.; Elsevier: San Diego, CA, USA, 2017; pp. 283–299. ISBN 978-0-12-802896-4. [Google Scholar]
- Schmidt, C.; Hofmann, U.; Kohlmuller, D.; Murdter, T.; Zanger, U.M.; Schwab, M.; Hoffmann, G.F. Comprehensive analysis of pyrimidine metabolism in 450 children with unspecific neurological symptoms using high-pressure liquid chromatography-electrospray ionization tandem mass spectrometry. J. Inherit. Metab. Dis. 2005, 28, 1109–1122. [Google Scholar] [CrossRef]
- Xia, J.-F.; Liang, Q.-L.; Liang, X.-P.; Wang, Y.-M.; Hu, P.; Li, P.; Luo, G.-A. Ultraviolet and tandem mass spectrometry for simultaneous quantification of 21 pivotal metabolites in plasma from patients with diabetic nephropathy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1930–1936. [Google Scholar] [CrossRef]
- Marin, C.; Krache, A.; Palmaro, C.; Lucas, M.; Hilaire, V.; Ugdonne, R.; De Victor, B.; Quaranta, S.; Solas, C.; Lacarelle, B.; et al. A Simple and Rapid UPLC-UV Method for Detecting DPD Deficiency in Patients with Cancer. CTS Clin. Transl. Sci. 2020, 13, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Guimbaud, R.; Guichard, S.; Dusseau, C.; Bertrand, V.; Aparicio, T.; Lochon, I.; Chatelut, E.; Couturier, D.; Bugat, R.; Chaussade, S.; et al. Dihydropyrimidine dehydrogenase activity in normal, inflammatory and tumour tissues of colon and liver in humans. Cancer Chemother. Pharmacol. 2000, 45, 477–482. [Google Scholar] [CrossRef]
- Wikoff, W.R.; Grapov, D.; Fahrmann, J.F.; DeFelice, B.; Rom, W.N.; Pass, H.I.; Kim, K.; Nguyen, U.; Taylor, S.L.; Gandara, D.R.; et al. Metabolomic Markers of Altered Nucleotide Metabolism in Early Stage Adenocarcinoma. Cancer Prev. Res. 2015, 8, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).