The Developmental Origins of Health and Disease: Adolescence as a Critical Lifecourse Period to Break the Transgenerational Cycle of NCDs—A Narrative Review
Abstract
1. Introduction
2. Core Concept of the Developmental Origins of Health and Disease (DOHaD) Hypothesis: Focus on the Health of Mother and Offspring
3. Influences of Paternal Health Behaviours on Long-Term Health and NCDs
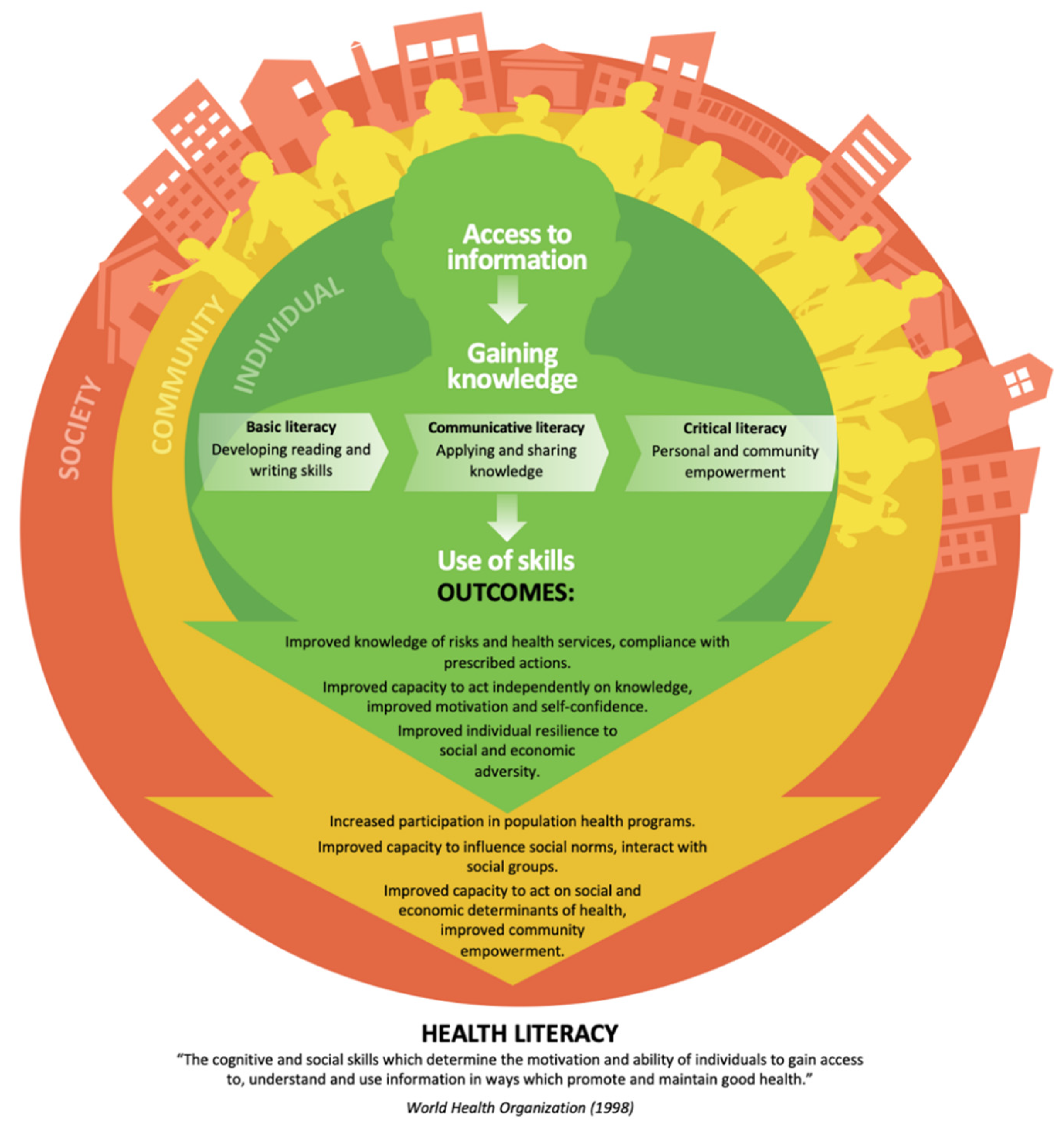
4. Knowledge Translation Approaches
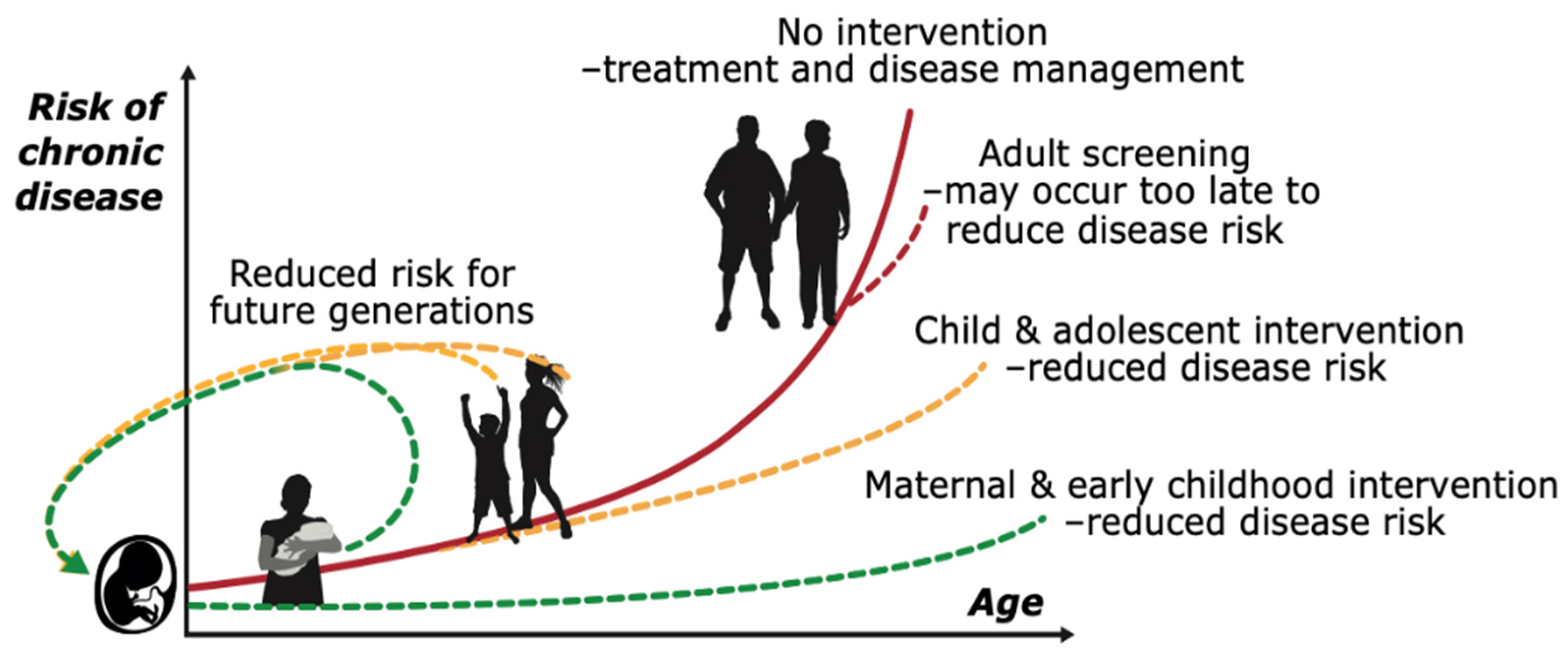
5. Adolescence: A Window of Opportunity for Better Health and Reduced NCD Risk
6. Systems Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases Country Profiles 2018. 2018, pp. 1–223. Available online: https://apps.who.int/iris/handle/10665/274512 (accessed on 12 April 2022).
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals. 2021, pp. 1–132. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/whs-2021_20may.pdf (accessed on 9 May 2022).
- Akseer, N.; Mehta, S.; Wigle, J.; Chera, R.; Brickman, Z.; Al-Gashm, S.; Sorichetti, B.; Vandermorris, A.; Hipgrave, D.; Schwalbe, N. Non-communicable diseases among adolescents: Current status, determinants, interventions and policies. BMC Public Health 2020, 20, 1908. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Reddy, K.S. Noncommunicable diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Ottawa Charter for Health Promotion, 1986; World Health Organization, Regional Office for Europe: Geneva, Switzerland, 1986.
- Bundy, D.A.; Horton, S. Impact of interventions on health and development during childhood and adolescence: A conceptual framework. In Child and Adolescent Health and Development, 3rd ed.; The International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2018; Chapter 6. [Google Scholar]
- World Health Organization. Preventing Noncommunicable Diseases in the Workplace through Diet and Physical Activity: WHO/World Economic Forum Report of a Joint Event. 2008, pp. 1–52. Available online: http://apps.who.int/iris/bitstream/handle/10665/43825/9789241596329_eng.pdf?sequence=1 (accessed on 12 April 2022).
- World Health Organization. Intercountry Consultation on Regional Strategy for Health Promotion for South-East Asia: Chiang Mai, Thailand, 26–29 June 2006; WHO Regional Office for South-East Asia: New Delhi, India, 2006. [Google Scholar]
- Pardell, H.; Roure, E.; Drygas, W.; Morava, E.; Nussel, E.; Puska, P.; Uhanov, M.; Laaksonen, M.; Tresserras, R.; Salto, E. East—West differences in reported preventive practices: A comparative study of six European areas of the WHO-CINDI programme. Eur. J. Public Health 2001, 11, 393–396. [Google Scholar] [CrossRef][Green Version]
- World Health Organization. Making every School a Health-Promoting School: Implementation Guidance. 2021, pp. 1–77. Available online: https://www.who.int/publications/i/item/9789240025073 (accessed on 12 April 2022).
- Pettersson, B. Some bitter-sweet reflections on the Ottawa Charter commemoration cake: A personal discourse from an Ottawa rocker. Health Promot. Int. 2011, 26 (Suppl. S2), ii173–ii179. [Google Scholar] [CrossRef]
- Jeet, G.; Thakur, J.S.; Prinja, S.; Singh, M.; Paika, R.; Kunjan, K.; Dhadwal, P. Effectiveness of targeting the health promotion settings for non-communicable disease control in low/middle-income countries: Systematic review protocol. BMJ Open 2018, 8, e014559. [Google Scholar] [CrossRef]
- Tu’akoi, S.; Bay, J.L.; Aung, Y.Y.M.; Tamarua-Herman, N.; Barrett-Watson, C.; Tairea, K.; Vickers, M.H. Birth Weight and Adolescent Health Indicators in Rarotonga, Cook Islands. Asia Pac. J. Public Health 2022, 34, 118–122. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Constância, M. Mechanisms of disease: The developmental origins of disease and the role of the epigenotype. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 539–546. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23 (Suppl. S6), 588S–595S. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Low, F.M. Evolutionary and developmental mismatches are consequences of adaptive developmental plasticity in humans and have implications for later disease risk. Philos. Trans. R. Soc. B 2019, 374, 20180109. [Google Scholar] [CrossRef]
- Bateson, P. Developmental plasticity and evolutionary biology. J. Nutr. 2007, 137, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J. The thrifty phenotype hypothesis: Type 2 diabetes. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D. The Fetal Matrix: Evolution, Development and Disease; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Godfrey, K.M.; Gluckman, P.D.; Hanson, M.A. Developmental origins of metabolic disease: Life course and intergenerational perspectives. Trends Endocrinol. Metab. 2010, 21, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Horton, R. Maternal and child undernutrition: An urgent opportunity. Lancet 2008, 371, 179. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Ahmed, T.; Black, R.E.; Cousens, S.; Dewey, K.; Giugliani, E.; Haider, B.A.; Kirkwood, B.; Morris, S.S.; Sachdev, H. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008, 371, 417–440. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Bateson, P.; Beedle, A.S.; Law, C.M.; Bhutta, Z.A.; Anokhin, K.V.; Bougnères, P.; Chandak, G.R.; Dasgupta, P. Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet 2009, 373, 1654–1657. [Google Scholar] [CrossRef]
- Newnham, J.P.; Ross, M.G. Early Life Origins of Human Health and Disease; Karger Medical and Scientific Publishers: Basel, Switzerland, 2009. [Google Scholar]
- Kassebaum, N.J.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, J.; Carter, A.; Casey, D.C.; Charlson, F.J.; Coates, M.M.; Coggeshall, M. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar] [CrossRef]
- Catalano, P.A.; Ehrenberg, H. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Li, M.; Sloboda, D.; Vickers, M. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef]
- Dontje, M.L.; Eastwood, P.; Straker, L. Western Australian pregnancy cohort (Raine) Study: Generation 1. BMJ Open 2019, 9, e026276. [Google Scholar] [CrossRef]
- Gaillard, R.; Welten, M.; Oddy, W.H.; Beilin, L.; Mori, T.; Jaddoe, V.W.; Huang, R.C. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, I.R.; Sly, P.D.; Schmidt, L.A.; van Lieshout, R.J.; Bienenstock, J.; Holt, P.G.; Arck, P.C. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J. Allergy Clin. Immunol. 2014, 134, 160–169.e7. [Google Scholar] [CrossRef] [PubMed]
- Ceesay, S.M.; Prentice, A.M.; Cole, T.J.; Foord, F.; Poskitt, E.M.; Weaver, L.T.; Whitehead, R.G. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ 1997, 315, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.; Watkinson, M.; Whitehead, R.; Lamb, W.; Cole, T. Prenatal dietary supplementation of African women and birth-weight. Lancet 1983, 321, 489–492. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Energy and protein intake in pregnancy. Cochrane Database Syst. Rev. 2003, CD000032. [Google Scholar] [CrossRef]
- Gebreselassie, S.G.; Gashe, F.E. A systematic review of effect of prenatal zinc supplementation on birthweight: Meta-analysis of 17 randomized controlled trials. J. Health Popul. Nutr. 2011, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Peña-Rosas, J.P.; Viteri, F.E. Effects and safety of preventive oral iron or iron+ folic acid supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2009, 7, CD004736. [Google Scholar]
- Fall, C.H.; Fisher, D.J.; Osmond, C.; Margetts, B.M. Multiple micronutrient supplementation during pregnancy in low-income countries: A meta-analysis of effects on birth size and length of gestation. Food Nutr. Bull. 2009, 30 (Suppl. S4), S533–S546. [Google Scholar] [CrossRef]
- Fricchione, G.L. The challenge of stress-related non-communicable diseases. Med. Sci. Monit. Basic Res. 2018, 24, 93. [Google Scholar] [CrossRef]
- Mishra, V.K.; Srivastava, S.; Muhammad, T.; Murthy, P. Relationship between tobacco use, alcohol consumption and non-communicable diseases among women in India: Evidence from National Family Health Survey-2015-16. BMC Public Health 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Ezzati, M.; Riboli, E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013, 369, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Khuwaja, A.K.; Kadir, M.M. Gender differences and clustering pattern of behavioural risk factors for chronic non-communicable diseases: Community-based study from a developing country. Chronic Illn. 2010, 6, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bista, B.; Dhungana, R.R.; Chalise, B.; Pandey, A.R. Prevalence and determinants of non-communicable diseases risk factors among reproductive aged women of Nepal: Results from Nepal Demographic Health Survey 2016. PLoS ONE 2020, 15, e0218840. [Google Scholar] [CrossRef] [PubMed]
- Rayfield, S.; Plugge, E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J. Epidemiol. Community Health 2017, 71, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Lawlor, D.A.; Alati, R.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M. Does maternal smoking during pregnancy have a direct effect on future offspring obesity? Evidence from a prospective birth cohort study. Am. J. Epidemiol. 2006, 164, 317–325. [Google Scholar] [CrossRef]
- Geerts, C.C.; Grobbee, D.E.; van der Ent, C.K.; de Jong, B.M.; van der Zalm, M.M.; van Putte-Katier, N.; Kimpen, J.L.; Uiterwaal, C.S. Tobacco smoke exposure of pregnant mothers and blood pressure in their newborns: Results from the wheezing illnesses study Leidsche Rijn birth cohort. Hypertension 2007, 50, 572–578. [Google Scholar] [CrossRef][Green Version]
- Charney, E.; Goodman, H.C.; McBride, M.; Lyon, B.; Pratt, R.; Breese, B.; Disney, F.; Marx, K. Childhood antecedents of adult obesity: Do chubby infants become obese adults? N. Engl. J. Med. 1976, 295, 6–9. [Google Scholar] [CrossRef]
- Leon, D.A.; Chenet, L.; Shkolnikov, V.M.; Zakharov, S.; Shapiro, J.; Rakhmanova, G.; Vassin, S.; McKee, M. Huge variation in Russian mortality rates 1984–94: Artefact, alcohol, or what? Lancet 1997, 350, 383–388. [Google Scholar] [CrossRef]
- Milliken-Smith, S.; Potter, C.M. Paternal origins of obesity: Emerging evidence for incorporating epigenetic pathways into the social determinants of health framework. Soc. Sci. Med. 2021, 271, 112066. [Google Scholar] [CrossRef]
- McPherson, N.O.; Fullston, T.; Aitken, R.J.; Lane, M. Paternal obesity, interventions, and mechanistic pathways to impaired health in offspring. Ann. Nutr. Metab. 2014, 64, 231–238. [Google Scholar] [CrossRef]
- Ng, S.-F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Messerlian, C.; Hauser, R. Fathers matter: Why it’s time to consider the impact of paternal environmental exposures on children’s health. Curr. Epidemiol. Rep. 2017, 4, 46–55. [Google Scholar] [CrossRef]
- Archer, E. In reply—Maternal, paternal, and societal efforts are needed to “cure” childhood obesity. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 555–557. [Google Scholar]
- Watkins, A.J.; Rubini, E.; Hosier, E.D.; Morgan, H.L. Paternal programming of offspring health. Early Hum. Dev. 2020, 150, 105185. [Google Scholar] [CrossRef] [PubMed]
- Rutter, H.; Savona, N.; Glonti, K.; Bibby, J.; Cummins, S.; Finegood, D.T.; Greaves, F.; Harper, L.; Hawe, P.; Moore, L. The need for a complex systems model of evidence for public health. Lancet 2017, 390, 2602–2604. [Google Scholar] [CrossRef]
- Mikkelsen, B.; Williams, J.; Rakovac, I.; Wickramasinghe, K.; Hennis, A.; Shin, H.-R.; Farmer, M.; Weber, M.; Berdzuli, N.; Borges, C. Life course approach to prevention and control of non-communicable diseases. BMJ 2019, 364, l257. [Google Scholar] [CrossRef] [PubMed]
- Rughani, A.; Friedman, J.E.; Tryggestad, J.B. Type 2 diabetes in youth: The role of early life exposures. Curr. Diabetes Rep. 2020, 20, 45. [Google Scholar] [CrossRef]
- Hanson, M.; Poston, L.; Gluckman, P. DOHaD–the challenge of translating the science to policy. J. Dev. Orig. Health Dis. 2019, 10, 263–267. [Google Scholar] [CrossRef]
- Straus, S.E.; Tetroe, J.; Graham, I.D. Introduction knowledge translation: What it is and what it isn’t. Knowl. Transl. Health Care 2013, 1–13. [Google Scholar] [CrossRef]
- World Health Organization. Prevention of Blindness from Diabetes Mellitus: Report of a WHO Consultation in Geneva, Switzerland, 9–11 November 2005; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. Oral Health Surveys: Basic Method; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Nurturing Care for Early Childhood Development: A Framework for Helping Children Survive and Thrive to Transform Health and Human Potential. 2018, pp. 1–64. Available online: https://apps.who.int/iris/bitstream/handle/10665/272603/9789241514064-eng.pdf (accessed on 12 April 2022).
- Hanson, M.; Cooper, C.; Aihie Sayer, A.; Eendebak, R.; Clough, G.; Beard, J. Developmental aspects of a life course approach to healthy ageing. J. Physiol. 2016, 594, 2147–2160. [Google Scholar] [CrossRef]
- Kuruvilla, S.; Sadana, R.; Montesinos, E.V.; Beard, J.; Vasdeki, J.F.; de Carvalho, I.A.; Thomas, R.B.; Drisse, M.-N.B.; Daelmans, B.; Goodman, T. A life-course approach to health: Synergy with sustainable development goals. Bull. World Health Organ. 2018, 96, 42. [Google Scholar] [CrossRef]
- Poore, K.R.; Hanson, M.A.; Faustman, E.M.; Neira, M. Avoidable early life environmental exposures. Lancet Planet. Health 2017, 1, e172–e173. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Davies, P.; Funder, J.; Palmer, D.J.; Sinn, J.; Vickers, M.; Wall, C. Early life nutrition and the opportunity to influence long-term health: An Australasian perspective. J. Dev. Orig. Health Dis. 2016, 7, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, J.R.; Vickers, M.H.; Wall, C.R.; Bay, J.L. First 1000 days: New Zealand Mothers’ perceptions of early life nutrition resources. J. Dev. Orig. Health Dis. 2021, 12, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, M.L.; Evans, M.; Regnault, T.R.; de Vrijer, B. Translating developmental origins of health and disease in practice: Health care providers’ perspectives. J. Dev. Orig. Health Dis. 2021, 12, 404–410. [Google Scholar] [CrossRef]
- Fernandes, P.S.; Bernardo, C.D.O.; Campos, R.M.; de Vasconcelos, F.D.A. Evaluating the effect of nutritional education on the prevalence of overweight/obesity and on foods eaten at primary schools. J. De Pediatr. 2009, 85, 315–321. [Google Scholar] [CrossRef][Green Version]
- Ramírez-López, E.; Grijalva-Haro, M.I.; Valencia, M.E.; Ponce, J.A.; Artalejo, E. Effect of a School Breakfast Program on the prevalence of obesity and cardiovascular risk factors in children. Salud Publica De Mex. 2005, 47, 126–133. [Google Scholar]
- Bonhauser, M.; Fernandez, G.; Püschel, K.; Yañez, F.; Montero, J.; Thompson, B.; Coronado, G. Improving physical fitness and emotional well-being in adolescents of low socioeconomic status in Chile: Results of a school-based controlled trial. Health Promot. Int. 2005, 20, 113–122. [Google Scholar] [CrossRef]
- Draper, C.E.; De Kock, L.; Grimsrud, A.T.; Rudolph, M.; Nemutandani, S.; Kolbe-Alexander, T.; Lambert, E.V. Evaluation of a school-based physical activity intervention in Alexandra Township. South Afr. J. Sports Med. 2010, 22, 12–19. [Google Scholar] [CrossRef]
- Yan-Ping, L.; Xiao-Qi, H.; Schouten, E.G.; Ai-Ling, L.; Song-Ming, D.; Lin-Zhong, L.; Zhao-Hui, C.; Dong, W.; Frans, J.K.; Frank, B.H. Report on childhood obesity in China (8): Effects and sustainability of physical activity intervention on body composition of Chinese youth. Biomed. Environ. Sci. 2010, 23, 180–187. [Google Scholar]
- Mészáros, Z.; Kiss, K.; Szmodis, M.; Zsidegh, M.; Mavroudes, M.; Mészáros, J. Effects of attending elevated level school physical education in 7 to 11-year-old boys. Acta Physiol. Hung. 2009, 96, 349–357. [Google Scholar] [CrossRef]
- Taymoori, P.; Niknami, S.; Berry, T.; Lubans, D.; Ghofranipour, F.; Kazemnejad, A. A school-based randomized controlled trial to improve physical activity among Iranian high school girls. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, R.; Roberfroid, D.; Lachat, C.; Leroy, J.L.; Holdsworth, M.; Maes, L.; Kolsteren, P.W. Effectiveness of preventive school-based obesity interventions in low-and middle-income countries: A systematic review. Am. J. Clin. Nutr. 2012, 96, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.; de Silva-Sanigorski, A.; Burford, B.J.; Brown, T.; Campbell, K.J.; Gao, Y.; Armstrong, R.; Prosser, L.; Summerbell, C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2011, CD001871. [Google Scholar] [CrossRef]
- Bay, J.L.; Hipkins, R.; Siddiqi, K.; Huque, R.; Dixon, R.; Shirley, D.; Tairea, K.; Yaqona, D.; Mason-Jones, A.; Vickers, M.H. School-based primary NCD risk reduction: Education and public health perspectives. Health Promot. Int. 2017, 32, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, J.-A.; Walker, D.; Fox-Rushby, J. Economic evaluations of non-communicable disease interventions in developing countries: A critical review of the evidence base. Cost Eff. Resour. Alloc. 2006, 4, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hingle, M.D.; O’Connor, T.M.; Dave, J.M.; Baranowski, T. Parental involvement in interventions to improve child dietary intake: A systematic review. Prev. Med. 2010, 51, 103–111. [Google Scholar] [CrossRef]
- Van Lippevelde, W.; Verloigne, M.; De Bourdeaudhuij, I.; Bjelland, M.; Lien, N.; Fernández-Alvira, J.M.; Moreno, L.A.; Kovacs, E.; Brug, J.; Maes, L. What do parents think about parental participation in school-based interventions on energy balance-related behaviours? A qualitative study in 4 countries. BMC Public Health 2011, 11, 881. [Google Scholar] [CrossRef]
- Dooris, M.; Poland, B.; Kolbe, L.; Leeuw, E.D.; McCall, D.S.; Wharf-Higgins, J. Healthy settings. In Global Perspectives on Health Promotion Effectiveness; Springer: Berlin/Heidelberg, Germany, 2007; pp. 327–352. [Google Scholar]
- Bay, J.; Yaqona, D.; Oyamada, M. DOHaD interventions: Opportunities during adolescence and the periconceptional period. In Pre-Emptive Medicine: Public Health Aspects of Developmental Origins of Health and Disease; Springer: Berlin/Heidelberg, Germany, 2019; pp. 37–51. [Google Scholar]
- Lee, J.M.; Pilli, S.; Gebremariam, A.; Keirns, C.C.; Davis, M.M.; Vijan, S.; Freed, G.L.; Herman, W.H.; Gurney, J.G. Getting heavier, younger: Trajectories of obesity over the life course. Int. J. Obes. 2010, 34, 614–623. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Guo, G.; Harris, K.M. Trends in body mass index in adolescence and young adulthood in the United States: 1959–2002. J. Adolesc. Health 2011, 49, 601–608. [Google Scholar] [CrossRef][Green Version]
- Bonnie, R.J.; Stroud, C.; Breiner, H.; Committee on Improving the Health, Safety, and Well-Being of Young Adults; National Research Council. Young adults in the 21st century. In Investing in the Health and Well-Being of Young Adults; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Epstein, L.H.; Wrotniak, B.H. Future directions for pediatric obesity treatment. Obesity 2010, 18 (Suppl. S1), S8. [Google Scholar] [CrossRef]
- Bay, J.; Vickers, M. Adolescent education: An opportunity to create a Developmental Origins of Health and Disease (DOHaD) circuit breaker. J. Dev. Orig. Health Dis. 2016, 7, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Kluge, H.H.P.; Wickramasinghe, K.; Rippin, H.L.; Mendes, R.; Peters, D.H.; Kontsevaya, A.; Breda, J. Prevention and control of non-communicable diseases in the COVID-19 response. Lancet 2020, 395, 1678–1680. [Google Scholar] [CrossRef]
- Morton, S.; Grant, C.; Berry, S.D.; Walker, C.G.; Corkin, M.; Ly, K.; De Castro, T.G.; Atatoa Carr, P.E.; Bandara, D.K.; Mohal, J. Growing Up in New Zealand: A Longitudinal Study of New Zealand Children and Their Families. Now We Are Four: Describing the Preschool Years. 2017, pp. 1–76. Available online: https://cdn.auckland.ac.nz/assets/growingup/research-findings-impact/GUiNZ_Now%20we%20are%20four%20report.pdf (accessed on 12 April 2022).
- Braine, T. Adolescent pregnancy: A culturally complex issue. World Health Organ. Bull. World Health Organ. 2009, 87, 410. [Google Scholar]
- Wodon, Q.; Male, C.; Nayihouba, A.; Onagoruwa, A.; Savadogo, A.; Yedan, A.; Edmeades, J.; Kes, A.; John, N.; Murithi, L. Economic Impacts of Child Marriage: Global Synthesis Report. 2017, pp. 1–99. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/eicm_global_conference_edition_june_27_final.pdf (accessed on 12 April 2022).
- Hanson, M.A.; Gluckman, P.D.; Ma, R.C.; Matzen, P.; Biesma, R.G. Early life opportunities for prevention of diabetes in low and middle income countries. BMC Public Health 2012, 12, 1025. [Google Scholar] [CrossRef]
- Day, R.E.; Sahota, P.; Christian, M.S. Effective implementation of primary school-based healthy lifestyle programmes: A qualitative study of views of school staff. BMC Public Health 2019, 19, 1239. [Google Scholar] [CrossRef]
- Fleary, S.A.; Joseph, P.; Pappagianopoulos, J.E. Adolescent health literacy and health behaviors: A systematic review. J. Adolesc. 2018, 62, 116–127. [Google Scholar] [CrossRef]
- Bay, J.; Mora, H.; Sloboda, D.; Morton, S.; Vickers, M.; Gluckman, P. Adolescent understanding of DOHaD concepts: A school-based intervention to support knowledge translation and behaviour change. J. Dev. Orig. Health Dis. 2012, 3, 469–482. [Google Scholar] [CrossRef]
- Oyamada, M.; Lim, A.; Dixon, R.; Wall, C.; Bay, J. Development of understanding of DOHaD concepts in students during undergraduate health professional programs in Japan and New Zealand. J. Dev. Orig. Health Dis. 2018, 9, 253–259. [Google Scholar] [CrossRef]
- Bay, J.L.; Fohoko, F.; La’Akulu, M.; Leota, O.; Pulotu, L.; Tu’Ipuloto, S.; Tutoe, S.; Tovo, O.; Vekoso, A.; Pouvalu, E.H. Questioning in Tongan science classrooms: A pilot study to identify current practice, barriers and facilitators. In Asia-Pacific Forum on Science Learning and Teaching; The Education University of Hong Kong, Department of Science and Evironmental Studies: Hong Kong, China, 2016; pp. 1–25. [Google Scholar]
- Macnab, A.J.; Mukisa, R. Priorities for African youth for engaging in DOHaD. J. Dev. Orig. Health Dis. 2018, 9, 15–19. [Google Scholar] [CrossRef]
- Grace, M.; Woods-Townsend, K.; Griffiths, J.; Godfrey, K.; Hanson, M.; Galloway, I.; Azaola, M.C.; Harman, K.; Byrne, J.; Inskip, H. Developing teenagers’ views on their health and the health of their future children. Health Educ. 2012, 112, 543–559. [Google Scholar] [CrossRef]
- Bay, J.; Yaqona, D.; Barrett-Watson, C.; Tairea, K.; Herrmann, U.; Vickers, M. We learnt and now we are teaching our family. J. Dev. Orig. Health Dis. 2017, 8 (Suppl. S1), s152. [Google Scholar]
- Grace, M.; Bay, J. Developing a pedagogy to support science for health literacy. In Asia-Pacific Forum on Science Learning and Teaching; The Education University of Hong Kong, Department of Science and Evironmental Studies: Hong Kong, China, 2011. [Google Scholar]
- Bay, J.L.; Sloboda, D.M.; Vickers, M.H.; Mora, H.A. Multi-Dimentional Connections: The Liggins Education Network for Science: Developing Partnerships to Enhance Science Education. In Bringing Communities Together; Brill Sense: Leiden, The Netherlands, 2012; pp. 161–173. [Google Scholar]
- Bay, J.L.; Vickers, M.H.; Mora, H.A.; Sloboda, D.M.; Morton, S.M. Adolescents as agents of healthful change through scientific literacy development: A school-university partnership program in New Zealand. Int. J. STEM Educ. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.; Low, F.; Bay, J. The value of evidence-based life skills education. Improv. Transit. 2011, 87–96. Available online: https://cpb-ap-se2.wpmucdn.com/blogs.auckland.ac.nz/dist/f/688/files/2020/02/Improving-the-Transition-report.pdf#page=97 (accessed on 12 April 2022).
- Samdal, O.; Rowling, L. The Implementation of Health Promoting Schools; Routledge: London, UK, 2012. [Google Scholar]
- Macnab, A.; Daar, A.; Norris, S.; Pauw, J. Advancing the DOHaD agenda in Africa. J. Dev. Orig. Health Dis. 2018, 9, 2–4. [Google Scholar] [CrossRef]
- Macnab, A.; Mukisa, R. Celebrity endorsed music videos: Innovation to foster youth health promotion. Health Promot. Int. 2019, 34, 716–725. [Google Scholar] [CrossRef]
- Altares, A.; Sobel, D.; Hobbs, S.; Nelson, T.; Serpa, M.; Bellows, L. P54 Youth CAN: Cultivating Community Change Through Youth-Driven Health Initiatives. J. Nutr. Educ. Behav. 2020, 52, S41. [Google Scholar] [CrossRef]
- Löhr, K.; Weinhardt, M.; Sieber, S. The “World Café” as a participatory method for collecting qualitative data. Int. J. Qual. Methods 2020, 19, 1609406920916976. [Google Scholar] [CrossRef]
- Zambon, A.; Morgan, A.; Vereecken, C.; Colombini, S.; Boyce, W.; Mazur, J.; Lemma, P.; Cavallo, F. The contribution of club participation to adolescent health: Evidence from six countries. J. Epidemiol. Community Health 2010, 64, 89–95. [Google Scholar] [CrossRef]
- Johnson, S.M.; Trejo, G.; Beck, K.L.; Worsley, C.; Tranberg, H.; Plax, K.L.; Linton, J.M. Building Community Support Using a Modified World Café Method for Pregnant and Parenting Teenagers in Forsyth County, North Carolina. J. Pediatric Adolesc. Gynecol. 2018, 31, 614–619. [Google Scholar] [CrossRef]
- Patton, G.C.; Olsson, C.A.; Skirbekk, V.; Saffery, R.; Wlodek, M.E.; Azzopardi, P.S.; Stonawski, M.; Rasmussen, B.; Spry, E.; Francis, K. Adolescence and the next generation. Nature 2018, 554, 458–466. [Google Scholar] [CrossRef]
- Sawyer, S.M.; Azzopardi, P.S.; Wickremarathne, D.; Patton, G.C. The age of adolescence. Lancet Child Adolesc. Health 2018, 2, 223–228. [Google Scholar] [CrossRef]
- Groth, M.V.; Fagt, S.; Brøndsted, L. Social determinants of dietary habits in Denmark. Eur. J. Clin. Nutr. 2001, 55, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Crozier, S.; Borland, S.; Hammond, J.; Barker, D.; Inskip, H. Impact of educational attainment on the quality of young women’s diets. Eur. J. Clin. Nutr. 2004, 58, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Lawrence, W.T.; Skinner, T.C.; Haslam, C.O.; Robinson, S.M.; Inskip, H.M.; Margetts, B.M.; Jackson, A.A.; Barker, D.J.; Cooper, C. Constraints on food choices of women in the UK with lower educational attainment. Public Health Nutr. 2008, 11, 1229–1237. [Google Scholar] [CrossRef]
- Borzekowski, D. Considering children and health literacy: A theoretical approach. Pediatrics 2009, 124, S282–S288. [Google Scholar] [CrossRef]
- Woods-Townsend, K.; Leat, H.; Bay, J.; Bagust, L.; Davey, H.; Lovelock, D.; Christodoulou, A.; Griffiths, J.; Grace, M.; Godfrey, K. LifeLab Southampton: A programme to engage adolescents with DOHaD concepts as a tool for increasing health literacy in teenagers—A pilot cluster-randomized control trial. J. Dev. Orig. Health Dis. 2018, 9, 475–480. [Google Scholar] [CrossRef]
- Nutbeam, D. Health literacy as a public health goal: A challenge for contemporary health education and communication strategies into the 21st century. Health Promot. Int. 2000, 15, 259–267. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.; Zimmet, P.; Forrester, T. Losing the war against obesity: The need for a developmental perspective. Sci. Transl. Med. 2011, 3, 93cm19. [Google Scholar] [CrossRef]
- Chisolm, D.J.; Manganello, J.A.; Kelleher, K.J.; Marshal, M.P. Health literacy, alcohol expectancies, and alcohol use behaviors in teens. Patient Educ. Couns. 2014, 97, 291–296. [Google Scholar] [CrossRef]
- Salgado, M.V.; Pérez-Stable, E.J.; Primack, B.A.; Kaplan, C.P.; Mejia, R.M.; Gregorich, S.E.; Alderete, E. Association of media literacy with cigarette smoking among youth in Jujuy, Argentina. Nicotine Tob. Res. 2012, 14, 516–521. [Google Scholar] [CrossRef][Green Version]
- Hoffman, S.; Marsiglia, F.F.; Nevarez, L.; Porta, M. Health literacy among youth in Guatemala City. Soc. Work. Public Health 2017, 32, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; Lategan, F.; Erasmus, M.; Lombaard, C.M.; Mc Carthy, N.; Olivier, J.; van Niekerk, M.; Hanekom, S. Health education interventions to promote health literacy in adults with selected non-communicable diseases living in low-to-middle income countries: A systematic review and meta-analysis. J. Eval. Clin. Pract. 2021, 27, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Tu’akoi, S.; Tamarua-Herman, N.; Tairea, K.; Vickers, M.H.; Aung, Y.Y.M.; Bay, J.L. Supporting Cook Island communities to access DOHaD evidence. J. Dev. Orig. Health Dis. 2020, 11, 564–572. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohi, M.; Bay, J.L.; Tu’akoi, S.; Vickers, M.H. The Developmental Origins of Health and Disease: Adolescence as a Critical Lifecourse Period to Break the Transgenerational Cycle of NCDs—A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 6024. https://doi.org/10.3390/ijerph19106024
Tohi M, Bay JL, Tu’akoi S, Vickers MH. The Developmental Origins of Health and Disease: Adolescence as a Critical Lifecourse Period to Break the Transgenerational Cycle of NCDs—A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(10):6024. https://doi.org/10.3390/ijerph19106024
Chicago/Turabian StyleTohi, Melenaite, Jacquie Lindsay Bay, Siobhan Tu’akoi, and Mark Hedley Vickers. 2022. "The Developmental Origins of Health and Disease: Adolescence as a Critical Lifecourse Period to Break the Transgenerational Cycle of NCDs—A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 10: 6024. https://doi.org/10.3390/ijerph19106024
APA StyleTohi, M., Bay, J. L., Tu’akoi, S., & Vickers, M. H. (2022). The Developmental Origins of Health and Disease: Adolescence as a Critical Lifecourse Period to Break the Transgenerational Cycle of NCDs—A Narrative Review. International Journal of Environmental Research and Public Health, 19(10), 6024. https://doi.org/10.3390/ijerph19106024







