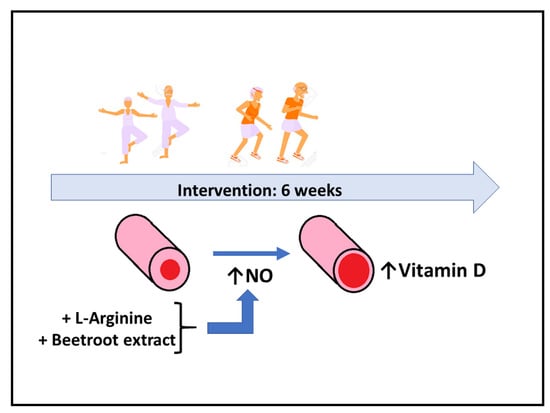
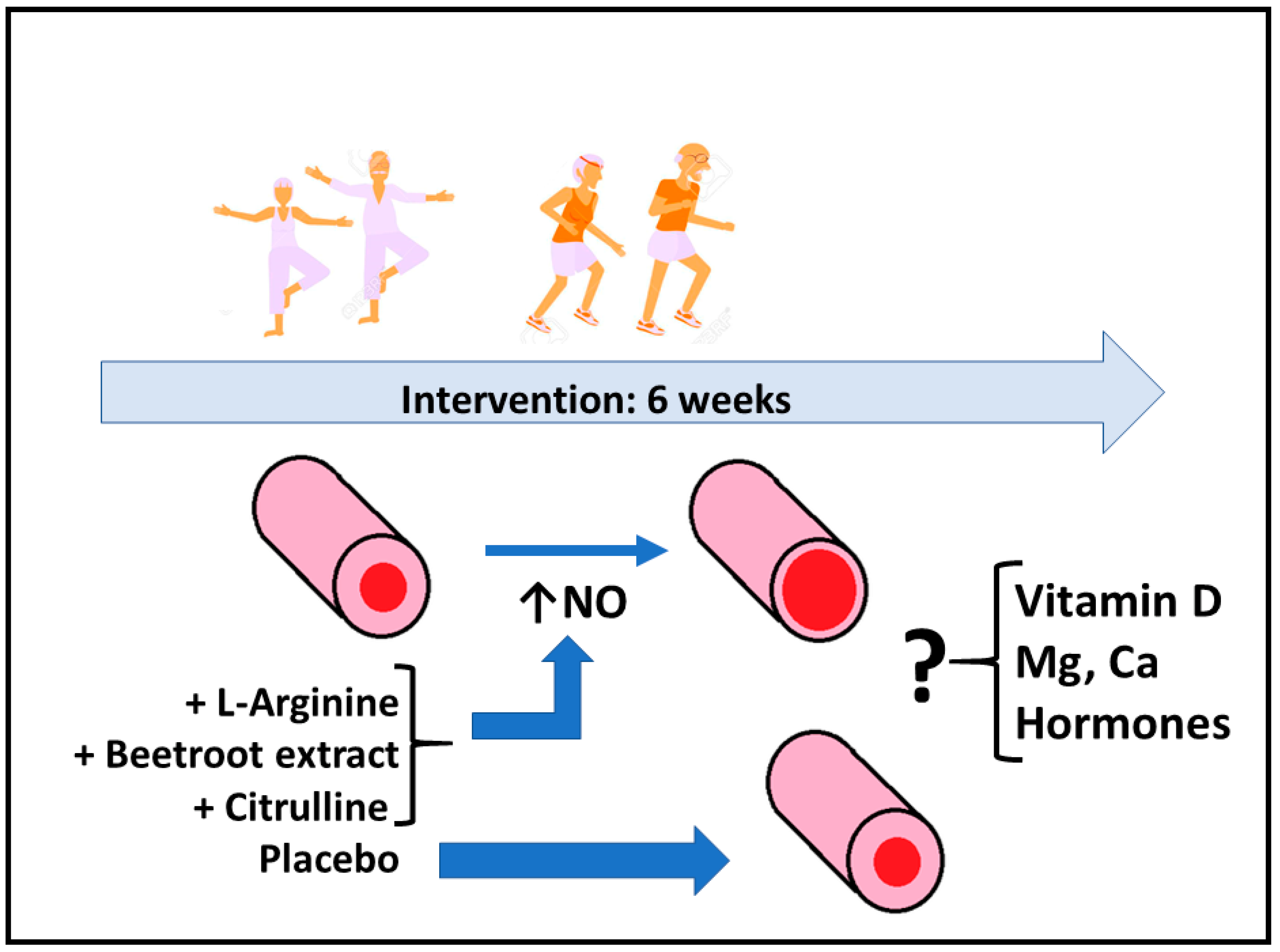
Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Supplementation
2.3. Physical Testing
2.4. Blood Analysis
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Wu, F.C.; Laurent, M.R.; Claessens, F.; Ward, K.A.; Boonen, S.; Bouillon, R.; et al. Endocrine determinants of incident sarcopenia in middle-aged and elderly European men. J. Cachexia Sarcopenia Muscle 2015, 6, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Aihie-Sayer, A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Fernández, J.; Fernández-Montero, A.; Córdova-Martínez, A.; Pastor, D.; Martínez-Rodríguez, A.; Roche, E. Sarcopenia: Molecular pathways and potential targets for intervention. Int. J. Mol. Sci. 2020, 21, 8844. [Google Scholar] [CrossRef]
- Szulc, P.; Duboeuf, F.; Marchand, F.; Delmas, P.D. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am. J. Clin. Nutr. 2004, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ren, B.; Chen, H.; Goltzman, D.; Yan, J.; Miao, D. 1,25-Dihydroxyvitamin D deficiency induces sarcopenia by inducing skeletal muscle cell senescence. Am. J. Transl. Res. 2021, 13, 12638–12649. [Google Scholar] [PubMed]
- Urena-Torres, P.; Souberbielle, J.C. Pharmacologic role of vitamin D natural products. Curr. Vasc. Pharmacol. 2014, 12, 278–285. [Google Scholar] [CrossRef]
- Seo, M.H.; Kim, M.K.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Lee, W.Y.; Baek, K.H.; Song, K.H.; Kang, M.I.; Oh, K.W. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: The fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr. J. 2013, 60, 679–686. [Google Scholar] [CrossRef]
- Ter Borg, S.; de Groot, L.C.; Mijnarends, D.M.; de Vries, J.H.; Verlaan, S.; Meijboom, S.; Luiking, Y.C.; Schols, J.M. Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults-results from the Maastricht Sarcopenia Study. J. Am. Med. Dir. Assoc. 2016, 17, 393–401. [Google Scholar] [CrossRef]
- van Dronkelaar, C.; van Velzen, A.; Abdelrazek, M.; van der Steen, A.; Weijs, P.J.M.; Tieland, M. Minerals and Sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: A systematic review. J. Am. Med. Dir. Assoc. 2018, 19, 6–11. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Michelucci, A.; Liang, C.; Protasi, F.; Dirksen, R.T. Altered Ca2+ handling and oxidative stress underlie mitochondrial damage and skeletal muscle dysfunction in aging and disease. Metabolites 2021, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Wallach, S. Effects of magnesium on skeletal metabolism. Magnes. Trace Elem. 1990, 9, 1–14. [Google Scholar] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium and aging. Curr. Pharmacol. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Berton, L.; Carraro, S.; Bolzetta, F.; De Rui, M.; Perissinotto, E.; Toffanello, E.D.; Bano, G.; Pizzato, S.; Miotto, F.; et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Menon, A.S.; Verma, V. Prevalence of testosterone deficiency in elderly male and its association with frailty and mobility at a tertiary care centre. Indian J. Endocrinol. Metab. 2021, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.T.; Ma, J.F.; Marco, S.D.; Gallouzi, I.E. Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging 2011, 3, 702–715. [Google Scholar] [CrossRef]
- Haynes, V.; Traaseth, N.J.; Elfering, S.; Fujisawa, Y.; Giulivi, C. Nitration of specific tyrosines in FoF1 ATP synthase and activity loss in aging. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E978–E987. [Google Scholar] [CrossRef]
- Beal, M.F. Oxidatively modified proteins in aging and disease. Free Radic. Biol. Med. 2002, 32, 797–803. [Google Scholar] [CrossRef]
- Braga, M.; Sinha Hikim, A.P.; Datta, S.; Ferrini, M.G.; Brown, D.; Kovacheva, E.L.; Gonzalez-Cadavid, N.F.; Sinha-Hikim, I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 2008, 13, 822–832. [Google Scholar] [CrossRef]
- Kanski, J.; Hong, S.J.; Schöneich, C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J. Biol. Chem. 2005, 280, 24261–24266. [Google Scholar] [CrossRef] [PubMed]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H. The pharmacodynamics of L-arginine. Altern. Health Med. 2014, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Caballero-García, A.; Pascual-Fernández, J.; Noriega-González, D.C.; Bello, H.J.; Pons-Biescas, A.; Roche, E.; Córdova-Martínez, A. L-Citrulline supplementation and exercise in the management of sarcopenia. Nutrients 2021, 13, 3133. [Google Scholar] [CrossRef]
- Sureda, A.; Pons, A. Arginine and citrulline supplementation in sports and exercise: Ergogenic nutrients? Med. Sport Sci. 2012, 59, 18–28. [Google Scholar] [CrossRef]
- Bescós, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef]
- Mielgo Ayuso, J.; Zourdos, M.C.; Urdampilleta, A.; Calleja-González, J.; Seco, J.; Córdova, A. Relationship of long-term macronutrients intake on anabolic-catabolic hormones in female elite volleyball players. Nutr. Hosp. 2017, 34, 1155–1162. [Google Scholar] [CrossRef]
- Martínez, A.C.; Seco-Calvo, J.; Tur-Marí, J.A.; Abecia-Inchaurregui, L.C.; Orella, E.E.; Pons-Biescas, A. Testosterone and cortisol changes in professional basketball players through a season competition. J. Strength Cond. Res. 2010, 24, 1102–1108. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Pons-Biescas, A.; Noriega, D.C.; Roche, E. L-Arginine and beetroot extract supplementation in the prevention of sarcopenia. Pharmaceuticals 2022, 15, 290. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Breen, L.; Phillips, S.M. Alterations in human muscle protein metabolism with aging: Protein and exercise as countermeasures to offset sarcopenia. Biofactors 2014, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Haynes, E.M.K.; Neubauer, N.A.; Cornett, K.M.D.; O’Connor, B.P.; Jones, G.R.; Jakobi, J.M. Age and sex-related decline of muscle strength across the adult lifespan: A scoping review of aggregated data. Appl. Physiol. Nutr. Metab. 2020, 45, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Kuh, D.; Cooper, C.; Sayer, A.A. Global variation in grip strength: A systematic review and meta-analysis of normative data. Age Ageing 2016, 45, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.E.; Walston, J.D.; Kim, M.; Won, C.W. Sex-specific differences in the effect of free testosterone on sarcopenia components in older adults. Front. Endocrinol. 2021, 12, 695614. [Google Scholar] [CrossRef]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in women–The Clinical Significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Carcaillon, L.; Blanco, C.; Alonso-Bouzon, C.; Alfaro-Acha, A.; Garcia-Garcia, F.J.; Rodriguez-Manas, L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: The Toledo Study for Healthy Aging. PLoS ONE 2012, 7, e32401. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J. Clin. Endocrinol. Metab. 2012, 97, 1153–1158. [Google Scholar] [CrossRef]
- Caballero-García, A.; Pérez-Valdecantos, D.; Guallar, P.; Caballero-Castillo, A.; Roche, E.; Noriega, D.C.; Córdova, A. Effect of vitamin D supplementation on muscle status in old patients recovering from COVID-19 infection. Medicina 2021, 57, 1079. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Haussler, C.A.; Bartik, L.; Whitfield, G.K.; Hsieh, J.C.; Slater, S.; Jurutka, P.W. Vitamin D receptor: Molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 2008, 66, S98–S112. [Google Scholar] [CrossRef] [PubMed]
- Caballero-García, A.; Córdova-Martínez, A.; Vicente-Salar, N.; Roche, E.; Pérez-Valdecantos, D. Vitamin D, Its Role in Recovery after Muscular Damage Following Exercise. Nutrients 2021, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Gruber, H.E.; Wei, L.Y.; Frausto, A. Immunolocalization of RANKL is increased and OPG decreased during dietary magnesium deficiency in the rat. Nutr. Metab. 2005, 2, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henkin, R.I.; Gouliouk, V.; Fordyce, A. Distinguishing patients with glossopyrosis from those with oropyrosis based upon clinical differences and differences in saliva and erythrocyte magnesium. Arch. Oral Biol. 2012, 57, 205–210. [Google Scholar] [CrossRef]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3636. [Google Scholar] [CrossRef]
- Sojka, J.E.; Weaver, C.M. Magnesium supplementation and osteoporosis. Nutr. Rev. 1995, 53, 71–74. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J.; Jiang, H. Association between serum level of magnesium and postmenopausal osteoporosis: A meta-analysis. Biol. Trace Elem. Res. 2014, 159, 8–14. [Google Scholar] [CrossRef]
- Begin, M.J.; Ste-Marie, L.G.; Coupal, L.; Ethier, J.; Rakel, A. Hypomagnesemia during teriparatide treatment in osteoporosis: Incidence and determinants. J. Bone Miner. Res. 2018, 33, 1444–1449. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.M.; Negru, A.G.; Căinap, S.S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and endothelial function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.; Weller, R.B. Investigating the potential for ultraviolet light to modulate morbidity and mortality from COVID-19: A narrative review and update. Front. Cardiovasc. Med. 2020, 7, 616527. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Men | Women | ANOVA p |
|---|---|---|---|
| n | 29 | 32 | |
| Anthropometric parameters | |||
| Height (cm) | 171.0 ± 4.3 | 158.1 ± 6.1 * | * 0.000 |
| Age (years) | 64.8 ± 3.6 | 65.4 ± 4.4 | 0.781 |
| Weight (kg) | 78.4 ± 9.0 | 63.9 ± 7.9 * | * 0.000 |
| BMI (kg/m2) | 26.6 ± 2.3 | 24.0 ± 2.9 * | * 0.032 |
| Fat (%) | 24.4 ± 2.7 | 34.9 ± 5.6 * | * 0.000 |
| Blood pressure values | |||
| Systolic (mmHg) | 133 ± 17 | 130 ± 21 | 0.070 |
| Diastolic (mmHg) | 79 ± 10 | 77 ± 11 | 0.338 |
| Circulating parameters | |||
| Calcium (mg/dL) | 9.5 ± 0.2 | 9.6 ± 0.3 | 0.097 |
| Magnesium (mg/dL) | 2.1 ± 0.1 | 2.2 ± 0.1 * | * 0.003 |
| Vitamin D (ng/mL) | 25.9 ± 8.0 | 26.1 ± 9.3 | 0.892 |
| Testosterone (ng/mL) | 4.2 ± 1.2 | 0.4 ± 0.2 * | 0.000 |
| Cortisol (μg/mL) | 14.6 ± 3.2 | 17.0 ± 3.6 * | * 0.001 |
| Testosterone/Cortisol Index | 29.4 ± 9.2 | 2.3 ± 0.9 * | * 0.000 |
| Physical condition tests | |||
| Strength Dynamometry (kg) | 42.1 ± 8.6 | 28.5 ± 7.8 * | * 0.000 |
| Endurance 6 min (m) | 1055 ± 220 | 820 ± 143 * | * 0.000 |
| 4 m walking speed (s) | 2.2 ± 0.3 | 2.3 ± 0.3 | 0.122 |
| 5 Squats (s) | 10.2 ± 2.0 | 11.3 ± 1.9 * | * 0.011 |
| Group: | PL | CM | ARG | BEET | ||||
|---|---|---|---|---|---|---|---|---|
| Parameter | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 |
| Glucose (mg/dL) | 89.7 ± 6.2 | 90.0 ± 6.0 | 90.0 ± 8.0 | 83.7 ± 6.2 | 88.2 ± 7.7 | 81.5 ± 11.5 | 90.2 ± 2.7 | 86.5 ± 4.5 |
| Urea (mg/dL) | 42.2 ± 9.2 | 40.5 ± 6.5 | 40.2 ± 2.7 | 40.5 ± 3.5 | 43.5 ± 11.5 | 38.0 ± 7.0 | 36.7 ± 5.2 | 37.0 ± 6.0 |
| Uric acid (mg/dL) | 5.15 ± 0.9 | 5.0 ± 0.7 | 4.8 ± 0.7 | 4.8 ± 0.7 | 4.7 ± 0.7 | 4.7 ± 0.6 | 4.4 ± 0.7 | 4.8 ± 1.0 |
| Cholesterol (mg/dL) | 212 ± 18.5 | 236 ± 22.5 | 220 ± 17.5 | 212 ± 18.5 | 242 ± 17.0 | 239 ± 30.0 | 211 ± 8.5 | 214 ± 10.5 |
| HDL (mg/dL) | 66.0 ± 7.0 | 62.0 ± 6.0 | 61.0 ± 3.5 | 59.0 ± 5.0 | 64.0 ± 8.0 | 59.0 ± 6.0 | 58.7 ± 5.2 | 59.5 ± 6.5 |
| Triglycerides (mg/dL) | 81.2 ± 7.2 | 89.2 ± 19.3 | 78.7 ± 15.7 | 79.2 ± 16.7 | 80.5 ± 17.5 | 73.5 ± 13.5 | 90.0 ± 24.0 | 77.5 ± 14.5 |
| Total proteins (g/dL) | 7.2 ± 0.3 | 7.3 ± 0.2 | 6.9 ± 0.1 | 7.1 ± 0.1 | 7.1 ± 0.2 | 7.0 ± 0.2 | 7.3 ± 0.1 | 7.0 ± 0.3 |
| WOMEN | MEN | |
|---|---|---|
| Calcium (Ca) | Mg (0.269) p = 0.05 * T/C index (−0.278) p = 0.046 * | |
| Magnesium (Mg) | Ca (0.269) p = 0.05 * Endurance (m) (0.271) p = 0.052 * | Vitamin D (−0.330) p = 0.038 * |
| Vitamin D | Mg (−0.330) p = 0.038 * Strength (kg) (−0.364) p = 0.024 * | |
| Testosterone (T) | C (0.440) p = 0.001 ** T/C index (0.717) p = 0.000 ** Endurance (m) (−0.289) p = 0.038 * | C (0.396) p = 0.011 * T/C index (0.766) p = 0.000 ** |
| Cortisol © | T (0.440) p = 0.001 ** | T (0.396) p = 0.011 * Strength (kg) (0.532) p = 0.001 ** |
| T/C index | T (0.717) p = 0.000 ** Ca (−0.278) p = 0.046 * | T (0.766) p = 0.000 ** Speed (s) (0.379) p = 0.019 * |
| Strength (kg) | C (0.532) p = 0.001 ** Vitamin D (−0.369) p = 0.024 * Endurance (m) (0.332) p = 0.036 * | |
| Endurance (m) | Mg (0.271) p = 0.05 * Speed (s) (−0.634) p = 0.000 ** Squats (s) (−0.457) p = 0.001 ** | Strength (kg) (0.437) p = 0.006 ** Speed (s) (−0.371) p = 0.022 * |
| Speed (s) | Endurance (m) (−0.634) p = 0.000 ** Squats (s) (0.544) p = 0.000 ** | Index T/C (0.379) p = 0.019 * Endurance (m) (−0.371) p = 0.022 * Squats (s) (0.486) p = 0.002 ** |
| Parameter | Groups | T1 | T2 | ANOVA |
|---|---|---|---|---|
| Ca (mg/dL) | PL | 9.6 ± 0.3 | 9.6 ± 0.3 | S (0.719) T (0.309) SxT (0.246) |
| CM | 9.5 ± 0.2 | 9.6 ± 0.3 | ||
| ARG | 9.6 ± 0.2 | 9.5 ± 0.2 | ||
| BEET | 9.7 ± 0.4 | 9.5 ± 0.3 | ||
| Mg (mg/dL) | PL | 2.2 ± 0.1 | 2.1 ± 0.1 | S (0.496) T (0.211) SxT (0.604) |
| CM | 2.2 ± 0.2 | 2.1 ± 0.1 | ||
| ARG | 2.2 ± 0.1 | 2.2 ± 0.2 | ||
| BEET | 2.1 ± 0.1 | 2.1 ± 0.2 | ||
| Vitamin D (ng/mL) | PL | 22.6 ± 7.3 | 22.7 ± 6.4 | S (0.0.032) T (0.05) SxT (0.553) |
| CM | 24.7 ± 10.3 | 26.5 ± 9.1 | ||
| ARG | 26.6 ± 6.6 | 32.7 ± 9.5 a,b | ||
| BEET | 26.0 ± 7.4 | 32.5 ± 7.0 a,b |
| Parameter | Group | T1 | T2 | ANOVA |
|---|---|---|---|---|
| Testosterone (T) (ng/mL) | PL | 1.7 ± 1.9 | 1.6 ± 1.9 | S (0.031) T (0.671) SxT (0.998) |
| CM | 1.9 ± 1.9 | 1.7 ± 1.8 | ||
| ARG | 2.1 ± 2.3 | 1.9 ± 2.3 | ||
| BEET | 3.7 ± 2.5 * | 3.4 ± 2.2 | ||
| Cortis © (C) (µg/mL) | PL | 16.6 ± 4.5 | 16.8 ± 3.9 | S (0.561) T (0.123) SxT (0.387) |
| CM | 15.9 ± 4.1 | 15.9 ± 2.8 | ||
| ARG | 17.6 ± 3.4 | 14.5 ± 2.7 | ||
| BEET | 16.1 ± 3.5 | 13.8 ± 3.1 | ||
| T/C index | PL | 11.1 ± 13.5 | 9.66 ± 12.4 | S (0.007) T (0.955) SxT (0.972) |
| CM | 13.1 ± 13.4 | 11.2 ± 12.0 | ||
| ARG | 12.7 ± 14.3 | 14.0 ± 16.9 | ||
| BEET | 25.7 ± 18.6 * | 27.0 ± 18.1 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova, A.; Caballero-García, A.; Noriega-González, D.; Bello, H.J.; Pons, A.; Roche, E. Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 5938. https://doi.org/10.3390/ijerph19105938
Córdova A, Caballero-García A, Noriega-González D, Bello HJ, Pons A, Roche E. Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia. International Journal of Environmental Research and Public Health. 2022; 19(10):5938. https://doi.org/10.3390/ijerph19105938
Chicago/Turabian StyleCórdova, Alfredo, Alberto Caballero-García, David Noriega-González, Hugo J. Bello, Antoni Pons, and Enrique Roche. 2022. "Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia" International Journal of Environmental Research and Public Health 19, no. 10: 5938. https://doi.org/10.3390/ijerph19105938
APA StyleCórdova, A., Caballero-García, A., Noriega-González, D., Bello, H. J., Pons, A., & Roche, E. (2022). Nitric-Oxide-Inducing Factors on Vitamin D Changes in Older People Susceptible to Suffer from Sarcopenia. International Journal of Environmental Research and Public Health, 19(10), 5938. https://doi.org/10.3390/ijerph19105938