One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom
Abstract
1. Introduction
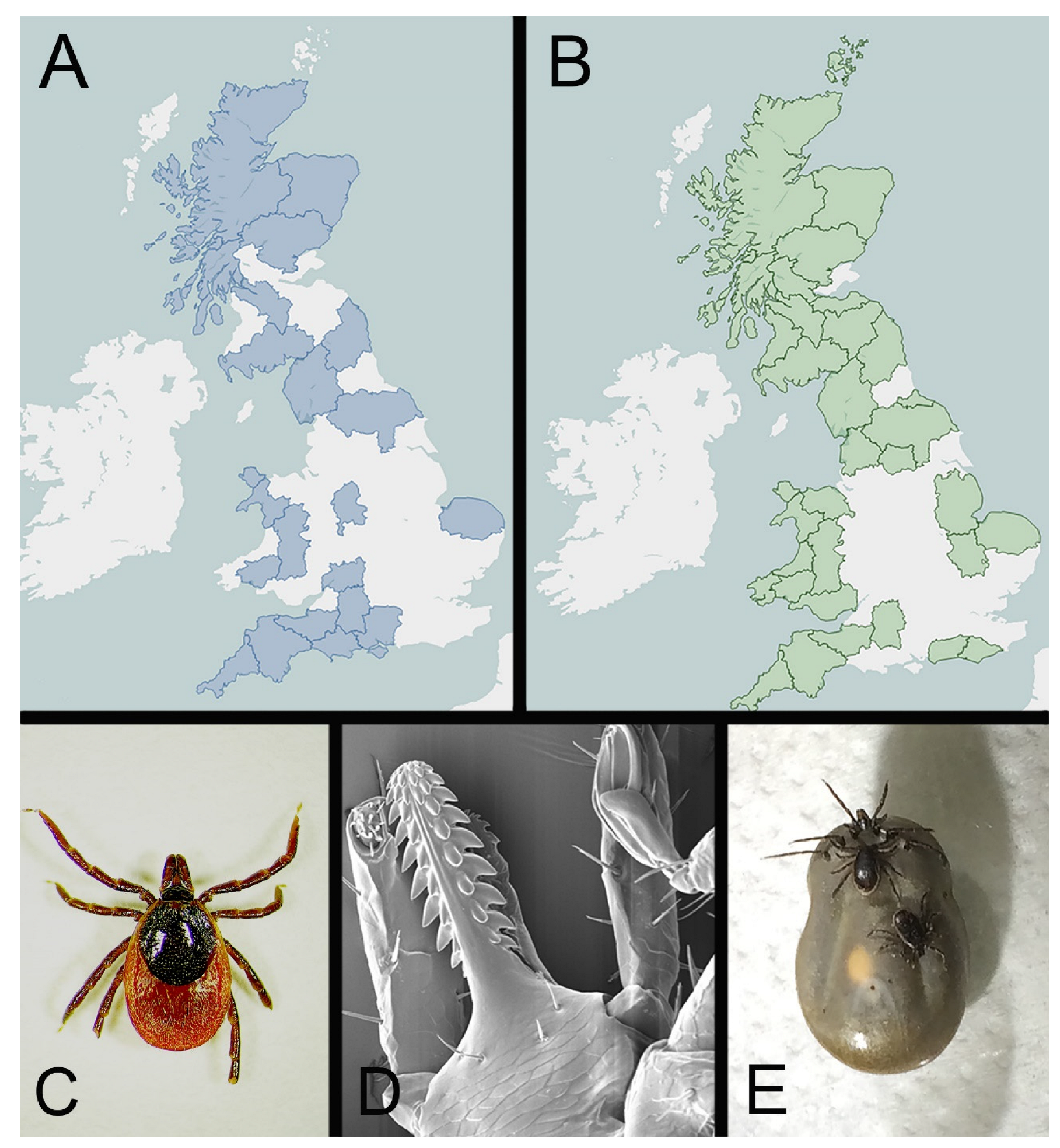
2. The Common Sheep Tick, Ixodes ricinus
3. The Challenge of Non-Native Tick Species
4. Surveillance for Ticks and Tick-Borne Disease
5. Detection of Tick-Borne Pathogens
6. A One Health Approach to Support Surveillance for Tick-Borne Diseases
7. Discussion
- Long-term surveys to characterize the distribution and abundance of I. ricinus on a country-wide scale, and to better understand seasonality of activity and biting.
- Determination of the presence and prevalence of tick-borne pathogens in at risk host populations and within indigenous tick instars that transmit pathogens.
- Provide evidence-based information for public and animal health policy to identify, mitigate and control tick-borne pathogen transmission and disease spread.
- Rapid detection of introduced tick species and timely responses to reduce potential impact and prevent establishment or spread to the wider environment.
- Collate robust data and develop climate-based assessments for future exotic tick establishment and disease risk.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Dedication
References
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020, 33, e00083-18. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic tick-borne pathogens in temperate and cold regions of Europe—A review on the prevalence in domestic animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef] [PubMed]
- Perveen, N.; Muzaffar, S.B.; Al-Deeb, M.A. Ticks and tick-borne diseases of livestock in the Middle East and North Africa: A review. Insects 2021, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.; Hiertqvist, M.; Bergström, T.; Lundkvist, A. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasites Vectors 2012, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Cayol, C.; Koskela, E.; Mappes, T.; Siukkola, A.; Kallio, E.R. Temporal dynamics of the tick Ixodes ricinus in northern Europe: Epidemiological implications. Parasites Vectors 2017, 10, 166. [Google Scholar] [CrossRef]
- Černý, J.; Lynn, G.; Hrnková, J.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. Management options for Ixodes ricinus-associated pathogens: A review of prevention strategies. Int. J. Environ. Res. Public Health 2020, 12, 1830. [Google Scholar] [CrossRef]
- Perret, J.-L.; Guigoz, E.; Rais, O.; Gern, L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 2000, 86, 554–557. [Google Scholar] [CrossRef]
- Johnson, N.; Phipps, L.P.; McFadzean, H.; Barlow, A.M. An outbreak of bovine babesiosis in February, 2019, triggered by above average winter temperature in southern England and co-infection with Babesia divergens and Anaplasma phagocytophilum. Parasites Vectors 2020, 13, 305. [Google Scholar] [CrossRef]
- Cull, B.; Hansford, K.M.; McGinley, L.; Gillingham, E.L.; Vaux, A.G.C.; Smith, R.; Medlock, J.M. A nationwide study on Borrelia burgdorferi s.l. infection rates in questing Ixodes ricinus: A six-year snapshot study in protected recreational areas in England and Wales. Med. Vet. Entomol. 2021, 35, 352–360. [Google Scholar] [CrossRef]
- Macrelli, M.; Phipps, P.; McGinley, L.; Medlock, J.; Johnson, N. First report of fatal tick pyaemia caused by heavy infestation with the rec sheep tick, Haemaphysalis punctata and co-infection with Babesia and Theileria species. Vet. Rec. Case Rep. 2020, 8, e001267. [Google Scholar] [CrossRef]
- Lv, J.; Fernández de Marco, M.D.M.; Goharriz, H.; Phipps, L.P.; McElhinney, L.M.; Hernández-Triana, L.M.; Wu, S.; Lin, X.; Fooks, A.R.; Johnson, N. Detection of tick-borne bacteria and babesia with zoonotic potential in Argas (Carios) vespertilionis (Latreille 1802) ticks from British bats. Sci. Rep. 2018, 8, 1865. [Google Scholar] [CrossRef] [PubMed]
- Labuda, M.; Nuttall, P.A. Tick-borne viruses. Parasitology 2004, 129, S221–S245. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; Fonville, M.; Docters van Leeuwen, A.; Stroo, A.; Duisterwinkel, M.; van Wieren, S.; Diuk-Wasser, M.; de Bruin, A.; Sprong, H. Pathogen communities of songbird-derived ticks in Europe’s low countries. Parasites Vectors 2017, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Vaux, A.G.C.; Cull, B.; Gillingham, E.; Leach, S. Assessment of the public health threats posed by vector-borne disease in the United Kingdom (UK). Int. J. Environ. Res. Public Health 2018, 15, 2145. [Google Scholar] [CrossRef] [PubMed]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Phipps, L.P.; Johnson, N. Emerging threats to animals in the United Kingdom by arthropod-borne diseases. Front. Vet. Sci. 2020, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Danas-Torres, F.; Santos-Silva, M.M. Ixodes ricinus (Lineaus, 1758) (Figs. 67–69). In Ticks of Europe and North Africa; Estrada-Peña, A., Mihalca, A.D., Petney, T.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 189–195. [Google Scholar]
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földovári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef]
- Millins, C.; Gilbert, L.; Medlock, J.M.; Hansford, K.; Thomson, D.B.; Biek, R. Effects of conservation management of landscapes and vertebrate communities on Lyme borreliosis risk in the United Kingdom. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160123. [Google Scholar] [CrossRef]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis. 2016, 7, 992–1003. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. Species interactions in occurrence data for a community of tick-transmitted pathogens. Sci. Data 2016, 3, 160056. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. What do we still need to know about Ixodes ricinus? Tick Tick Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Léger, E.; Vourc’h, G.; Vial, L.; Chevillon, C.; McCoy, K.D. Changing distributions of ticks: Causes and consequences. Exp. Appl. Acarol. 2013, 59, 219–244. [Google Scholar] [CrossRef]
- De Marco, M.D.M.F.; Hernández-Triana, L.M.; Phipps, L.P.; Hansford, K.; Mitchell, E.S.; Cull, B.; Swainsbury, C.S.; Fooks, A.R.; Medlock, J.M.; Johnson, N. Emergence of Babesia canis in southern England. Parasites Vectors 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Pietzsch, M.; Quest, R.; Hillyard, P.D.; Medlock, J.M.; Leach, S. Importation of exotic ticks into the United Kingdom via the international trade in reptiles. Exp. Appl. Acarol. 2006, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, E.L.; Cull, B.; Pietzsch, M.E.; Phipps, L.P.; Medlock, J.M.; Hansford, K. The unexpected holiday souvenir: The public health risk to UK travellers from ticks acquired overseas. Int. J. Environ. Res. Public Health 2020, 17, 7957. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus into the United Kingdom by migratory birds. Ticks Tick. Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef] [PubMed]
- England, M.E.; Phipps, P.; Medlock, J.M.; Atkinson, P.M.; Atkinson, B.; Hewson, R.; Gale, P. Hyalomma ticks on northward migrating birds in southern Spain: Implications for the risk of entry of Crimean-Congo haemorrhagic fever virus to Great Britain. J. Vector. Ecol. 2016, 41, 128–134. [Google Scholar] [CrossRef][Green Version]
- Hansford, K.M.; Carter, D.; Gillingham, E.L.; Hernández-Triana, L.M.; Chamberlain, J.; Cull, B.; McGinley, L.; Phipps, L.M.; Medlock, J.M. Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Tick Tick Borne Dis. 2019, 10, 704–708. [Google Scholar] [CrossRef]
- Hansford, K.M.; Pietzsch, M.; Cull, B.; Medlock, J.M. Brown dog tick infestation of a home in England. Vet. Rec. 2015, 176, 129–130. [Google Scholar] [CrossRef]
- Allsopp, B.A. Heartwater—Ehrlichia ruminantium infection. Rev. Sci. Tech. 2015, 34, 557–568. [Google Scholar] [CrossRef]
- Watts, J.G.; Playford, M.C.; Hickey, K.L. Theileria orientalis: A review. N. Z. Vet. J. 2016, 64, 3–9. [Google Scholar] [CrossRef]
- Wang, H.H.; Grant, W.E.; Teel, P.D.; Lohmeyer, K.H.; Pérez, A.; de León, A. Enhanced biosurveillance of high-consequence invasive pests: Southern cattle fever ticks, Rhipicephalus (Boophilus) microplus on livestock and wildlife. Parasites Vectors 2020, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Barré, N.; Uilenberg, G. Spread of parasites transported with their hosts: Case study of two species of cattle tick. Rev. Sci. Tech. 2010, 29, 149–160. [Google Scholar] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Vaux, A.G.C.; Abdullah, S.; Pietzsch, M.E.; Wall, R.; Johnson, N.; Phipps, L.P. Distribution of the tick Dermacentor reticulatus in the United Kingdom. Med. Vet. Entomol. 2017, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Fooks, A.R.; Johnson, N. Jet set pets: Examining the zoonosis risk in animal import and travel across the European Union. Vet. Med. 2014, 6, 17–25. [Google Scholar]
- Phipps, L.P.; Hansford, K.; Hernández-Triana, L.M.; Johnson, N.; Medlock, J.M. Identification of the South African tortoise tick, Amblyomma marmoreum, imported in the United Kingdom on a leopard tortoise (Stigmochelys pardalis). Vet. Rec. 2021, 189, 208–209. [Google Scholar] [CrossRef]
- Mihalca, A.D. Ticks imported to Europe with exotic reptiles. Vet. Parasitol. 2015, 213, 67–71. [Google Scholar] [CrossRef]
- Tulloch, J.S.P.; Christley, R.M.; Radford, A.D.; Warner, J.C.; Beadsworth, M.B.J.; Beeching, N.J.; Vivancos, R. A descriptive epidemiological study of the incidence of newly diagnosed Lyme disease cases in a UK primary care cohort, 1998–2016. BMC Infect. Dis. 2020, 20, 285. [Google Scholar] [CrossRef]
- Zintl, A.; McGrath, G.; O’Grady, L.; Fanning, J.; Downing, K.; Roche, D.; Casey, M.; Gray, J.S. Changing incidence of bovine babesiosis in Ireland. Ir. Vet. J. 2014, 67, 19. [Google Scholar] [CrossRef]
- ECDC Technical Report: Field Sampling Methods for Mosquitoes, Sandflies, Biting Midges and Ticks. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Vector-sampling-field-protocol-2018.pdf?msclkid=a5c12082d05d11ec81c3f5dec1a02dab (accessed on 21 March 2022).
- Newman, B.C.; Sutton, W.B.; Wang, Y.; Schweitzer, C.J.; Moncayo, A.C.; Miller, B.T. A standardized method for the construction of a tick drag/flag sampling approach and evaluation of sampling efficacy. Exp. Appl. Acarol. 2019, 79, 433–446. [Google Scholar] [CrossRef]
- Plantard, O.; Hoch, T.; Daveu, R.; Rispe, C.; Stachurski, F.; Boué, F.; Poux, V.; Cebe, N.; Verheyden, H.; René-Martellet, M.; et al. Where to find questing Ixodes frontalis ticks? Under bamboo bushes! Ticks Tick Borne Dis. 2021, 12, 101625. [Google Scholar] [CrossRef]
- Gondard, M.; Michelet, L.; Nisavanh, A.; Devillers, E.; Delannoy, S.; Fach, P.; Aspan, A.; Ullman, K.; Chirico, J.; Hoffmann, B.; et al. Prevalence of tick-borne viruses in Ixodes ricinus assessed by high-throughput real-time PCR. Pathog Dis. 2018, 76, fty083. [Google Scholar] [CrossRef] [PubMed]
- Bown, K.J.; Begon, M.; Bennett, M.; Birtles, R.J.; Burthe, S.; Lambin, X.; Telfer, S.; Woldehiwet, Z.; Ogden, N.H. Sympatric Ixodes trianguliceps and Ixodes ricinus ticks feeding on field voles (Microtus agrestis): Potential for increased risk of Anaplasma phagocytophilum in the United Kingdom? Vector Borne Zoonotic Dis. 2006, 6, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Sormunen, J.J.; Klemola, T.; Hänninen, J.; Mäkelä, S.; Vuorinen, I.; Penttinen, R.; Sääksiärvi, I.E.; Vesterinen, E.J. The importance of study duration and spatial scale in pathogen detection—Evidence from a tick-infested island. Emerg. Microbes Infect. 2018, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, P.; Lindblom, P.; Fryland, L.; Nyman, D.; Jaenson, T.G.; Forsberg, P.; Lindgren, P.E. Ixodes ricinus ticks removed from humans in Northern Europe: Seasonal pattern of infestation, attachment sites and duration of feeding. Parasites Vectors 2013, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, F.; González, J.; Tercero Jaime, J.M.; Olmeda, A.S. Long term study of Ixodid ticks feeding on red deer (Cervus elephus) in a meso-Mediterranean climate. Exp. Appl. Acarol. 2016, 69, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Scharlemann, J.P.; Johnson, P.J.; Smith, A.A.; Macdonald, D.W.; Randolph, S.E. Trends in Ixodid tick abundance and distribution in Great Britain. Med. Vet. Entomol. 2008, 22, 238–247. [Google Scholar] [CrossRef]
- Mowbray, F.; Amlôt, R.; Rubin, G.J. Predictors of protective behaviour against ticks in the UK: A mixed methods study. Ticks Tick Borne Dis. 2014, 5, 392–400. [Google Scholar] [CrossRef]
- Lihou, K.; Rose Vineer, H.; Wall, R. Distribution and prevalence of ticks and tick-borne disease on sheep and cattle farms in Great Britain. Parasites Vectors 2020, 13, 406. [Google Scholar] [CrossRef]
- Cull, B.; Pietzsch, M.E.; Hansford, K.M.; Gillingham, E.L.; Medlock, J.M. Surveillance of British ticks: An overview of species records, host associations, and new records of Ixodes ricinus distribution. Ticks Tick Borne Dis. 2018, 9, 605–614. [Google Scholar] [CrossRef]
- Cull, B.; Pietzsch, M.E.; Gillingham, E.L.; McGinley, L.; Medlock, J.M.; Cull, B. Seasonality and anatomical location of human tick bites in the United Kingdom. Zoonoses Public Health 2020, 67, 112–121. [Google Scholar] [CrossRef]
- Garcia-Marti, I.; Zurita-Milla, R.; Harms, M.G.; Swart, A. Using volunteered observations to map human exposure to ticks. Sci. Rep. 2019, 8, 15435. [Google Scholar] [CrossRef] [PubMed]
- Lernout, T.; De Regge, N.; Tersago, K.; Fonville, M.; Suin, V.; Sprong, H. Prevalence of pathogens in ticks collected from humans through citizen science in Belgium. Parasites Vectors 2019, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Laakesonen, M.; Klemola, T.; Feuth, E.; Sormunen, J.J.; Puisto, A.; Mäkelä, S.; Penttinen, R.; Ruohomäki, K.; Hänningen, J.; Sääksiärvi, I.E.; et al. Tick-borne pathogens in Finland: Comparison of Ixodes ricinus and I. persulcatus in sympatric and parapatric areas. Parasites Vectors 2018, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Drehman, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—Evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 578220. [Google Scholar] [CrossRef]
- Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Ticks infesting domestic dogs in the UK: A large-scale surveillance programme. Parasites Vectors 2016, 9, 391. [Google Scholar] [CrossRef]
- Davies, S.; Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Prevalence of ticks and tick-borne pathogens: Babesia and Borrelia species in ticks infesting cats of Great Britain. Vet. Parasitol. 2017, 244, 129–135. [Google Scholar] [CrossRef]
- Tulloch, J.S.P.; McGinley, L.; Sánchez-Vizcaíno, F.; Medlock, J.M.; Radford, A.D. The passive surveillance of ticks using companion animal electronic health records. Epidemiol. Infect. 2017, 145, 2020–2029. [Google Scholar] [CrossRef]
- Eisen, L.; Eisen, R.J. Benefits and drawbacks of citizen science to complement traditional data gathering approaches for medically important hard ticks (Acari: Ixodidae) in the United States. J. Entomol. 2021, 58, 1–9. [Google Scholar] [CrossRef]
- Johnson, N.; Voller, K.; Phipps, L.P.; Fooks, A.R. Rapid molecular detection methods for arboviruses of livestock of importance to northern Europe. J. Biomed. Biotechnol. 2012, 2012, 719402. [Google Scholar] [CrossRef]
- Liu, Q.; Ne, B.; Huang, S.Y.; Wei, F.; Zhu, Z.O. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Shi, M.; Bohlin, J.; Eidholm, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, Å.; Holmes, E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.; Sameroff, S.; Tagliafierro, T.; Jain, K.; Williams, S.H.; Cucura, D.M.; Rochlin, I.; Monzon, J.; Carpi, G.; Tufts, D.; et al. Identification of novel viruses in Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks. mSphere 2018, 3, e00614-17. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial nature of tick-borne diseases. mBio 2019, 10, e02055-19. [Google Scholar] [CrossRef]
- Choi, B.C.; Pak, A.W. Multidisciplinary, interdisciplinary and transdisciplinarity in health research, services, education and policy: 1 Definitions, objectives, and evidence of effectiveness. Clin. Investig. Med. 2006, 29, 351–364. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Bonfoh, B.; Fooks, A.R.; Kasymbekov, J.; Waltner-Toews, D.; Tanner, M. Towards a “One Health” research and application toolbox. Vet. Ital. 2009, 45, 119–131. [Google Scholar]
- Mathison, B.A.; Pritt, B.S. Laboratory identification of arthropod ectoparasites. Clin. Microbiol. Rev. 2014, 27, 48–67. [Google Scholar] [CrossRef]
- Mediannikov, O.; Fenollar, F. Looking in ticks for human bacterial pathogens. Microb. Pathog. 2014, 77, 142–148. [Google Scholar] [CrossRef]
- Pfister, K.; Armstrong, R. Systematically and cutaneously distributed ectoparasiticides: A review of the efficacy against ticks and fleas on dogs. Parasites Vectors 2016, 9, 436. [Google Scholar] [CrossRef]
- Kosmider, R.; Gibbens, J.; Avigad, R. Identification, assessment and management of new and re-emerging animal-related risks: UK perspective. Vet. Rec. 2017, 181, 67. [Google Scholar] [CrossRef]
- Human Animal Infection and Risk Surveillance (HAIRS) Group. Available online: https://www.gov.uk/government/collections/human-animal-infections-and-risk-surveillance-group-hairs (accessed on 8 February 2022).
- Eisen, R.J.; Paddock, C.D. Tick and tickborne pathogen surveillance as a public health tool in the United States. J. Med. Entomol. 2021, 58, 1490–1502. [Google Scholar] [CrossRef]
| Tick Species | Habitat in the United Kingdom | Main Hosts | Pathogen | Disease |
|---|---|---|---|---|
| Ixodes ricinus | Deciduous and mixed forest, upland grazing areas, permanent lowland grazing areas | Immature forms feed on a variety of mammals, reptiles and birds. Adults favour large mammals including humans, livestock, domestic pets and deer | Babesia divergens | Bovine and human babesiosis |
| Babesia venatorum | Human babesiosis | |||
| Louping ill virus | Ovine encephalomyelitis | |||
| Borrelia burgdorferi s.l. | Lyme disease | |||
| Borrelia miyamotoi | Relapsing fever | |||
| Anaplasma phagocytophilum | Tick-borne fever in ruminants | |||
| Tick-borne encephalitis virus | Human encephalitis | |||
| Rickettsia spp. | Disease not reported in UK | |||
| Dermacentor reticulatus | Coastal locations including sand dunes and grazing land | Adults feed on humans, livestock and domestic pets | Babesia canis | Canine babesiosis |
| Rickettsia spp. | Disease not reported in UK | |||
| Haemaphysalis punctata | Chalk grassland and grazing marsh | Adults feed mainly on cattle and sheep. Occasional reports from dogs and humans | Theileria luwenshuni | Theileriosis in ruminants |
| Babesia motasi | Ovine babesiosis | |||
| Babesia major | Bovine babesiosis | |||
| Theileria orientalis | Bovine theileriosis | |||
| Rickettsia massiliae | Disease not reported in UK |
| Family | Species |
|---|---|
| Argasidae (Soft ticks) | Argas reflexus |
| Argas vespertilionis Ornithodoros maritimus | |
| Ixodidae (Hard ticks) | Dermacentor reticulatus |
| Haemaphysalis punctata | |
| Ixodes acuminatus | |
| Ixodes apronophorus | |
| Ixodes arboricola | |
| Ixodes caledonicus | |
| Ixodes canisuga | |
| Ixodes frontalis | |
| Ixodes hexagonus | |
| Ixodes lividus | |
| Ixodes ricinus | |
| Ixodes rothschildi | |
| Ixodes trianguliceps Ixodes univacatus | |
| Ixodes uriae Ixodes ventalloi Ixodes vespertilionis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, N.; Phipps, L.P.; Hansford, K.M.; Folly, A.J.; Fooks, A.R.; Medlock, J.M.; Mansfield, K.L. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. Int. J. Environ. Res. Public Health 2022, 19, 5833. https://doi.org/10.3390/ijerph19105833
Johnson N, Phipps LP, Hansford KM, Folly AJ, Fooks AR, Medlock JM, Mansfield KL. One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. International Journal of Environmental Research and Public Health. 2022; 19(10):5833. https://doi.org/10.3390/ijerph19105833
Chicago/Turabian StyleJohnson, Nicholas, Lawrence Paul Phipps, Kayleigh M. Hansford, Arran J. Folly, Anthony R. Fooks, Jolyon M. Medlock, and Karen L. Mansfield. 2022. "One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom" International Journal of Environmental Research and Public Health 19, no. 10: 5833. https://doi.org/10.3390/ijerph19105833
APA StyleJohnson, N., Phipps, L. P., Hansford, K. M., Folly, A. J., Fooks, A. R., Medlock, J. M., & Mansfield, K. L. (2022). One Health Approach to Tick and Tick-Borne Disease Surveillance in the United Kingdom. International Journal of Environmental Research and Public Health, 19(10), 5833. https://doi.org/10.3390/ijerph19105833






