Full-Scale Application of One-Stage Simultaneous Nitrification and Denitrification Coupled with Anammox Process for Treating Collagen Casing Wastewater
Abstract
:1. Introduction
2. Materials and Methods
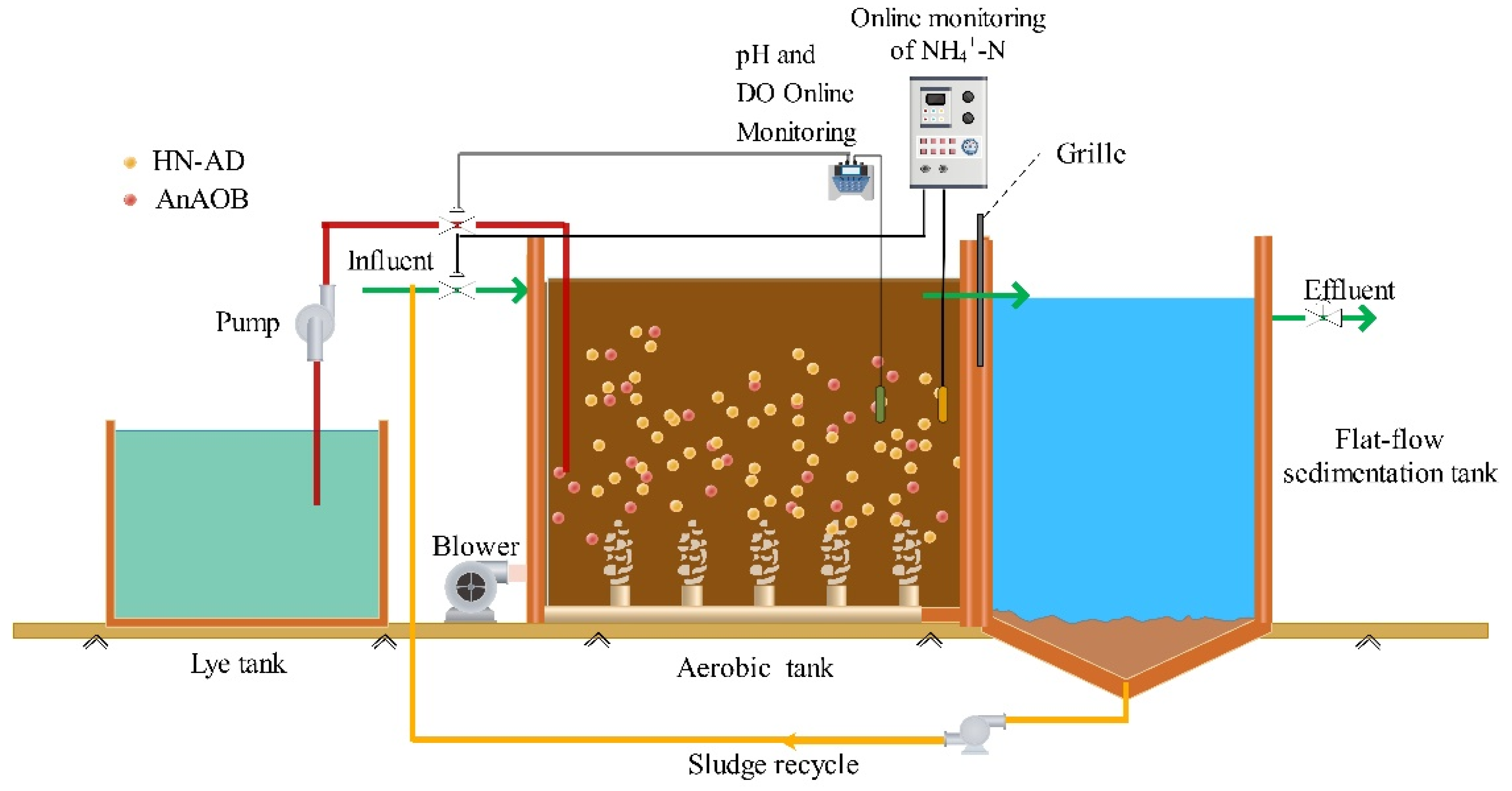
2.1. Project Introduction
2.2. Inoculated Sludge and Wastewater
2.3. Analytical Method
2.4. Microbial Diversity Analysis
3. Results and Discussion
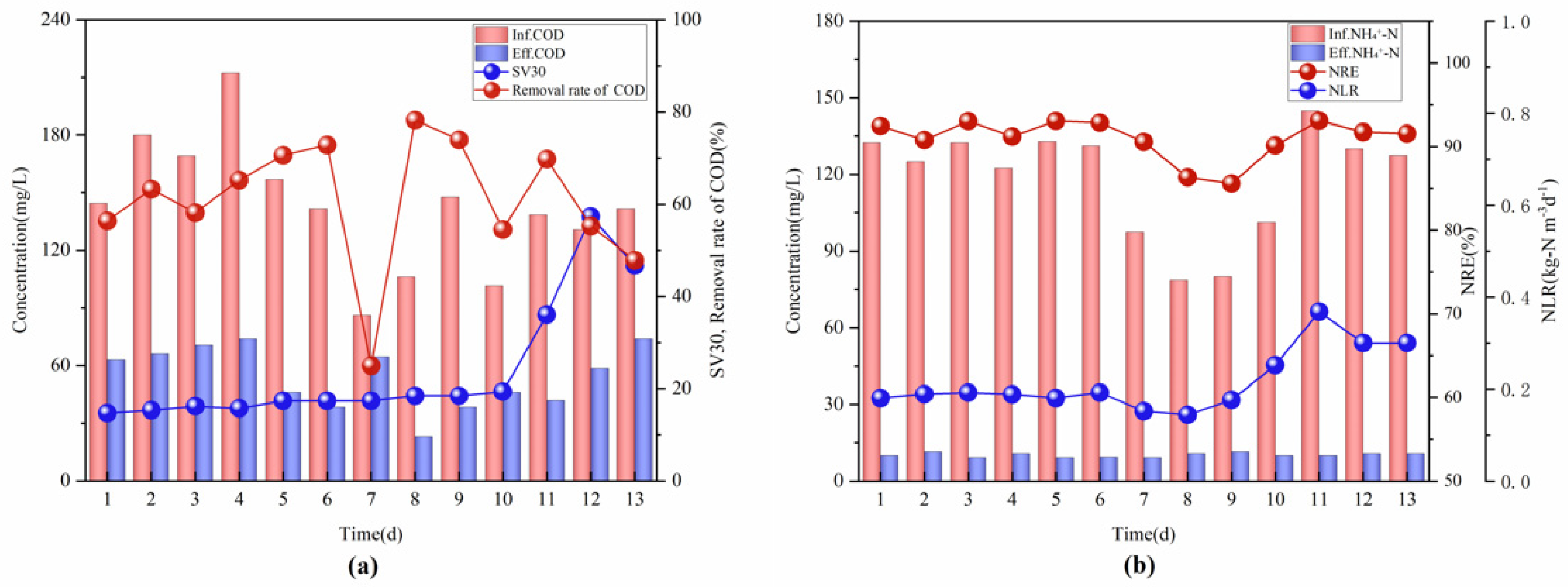
3.1. Start-Up Period
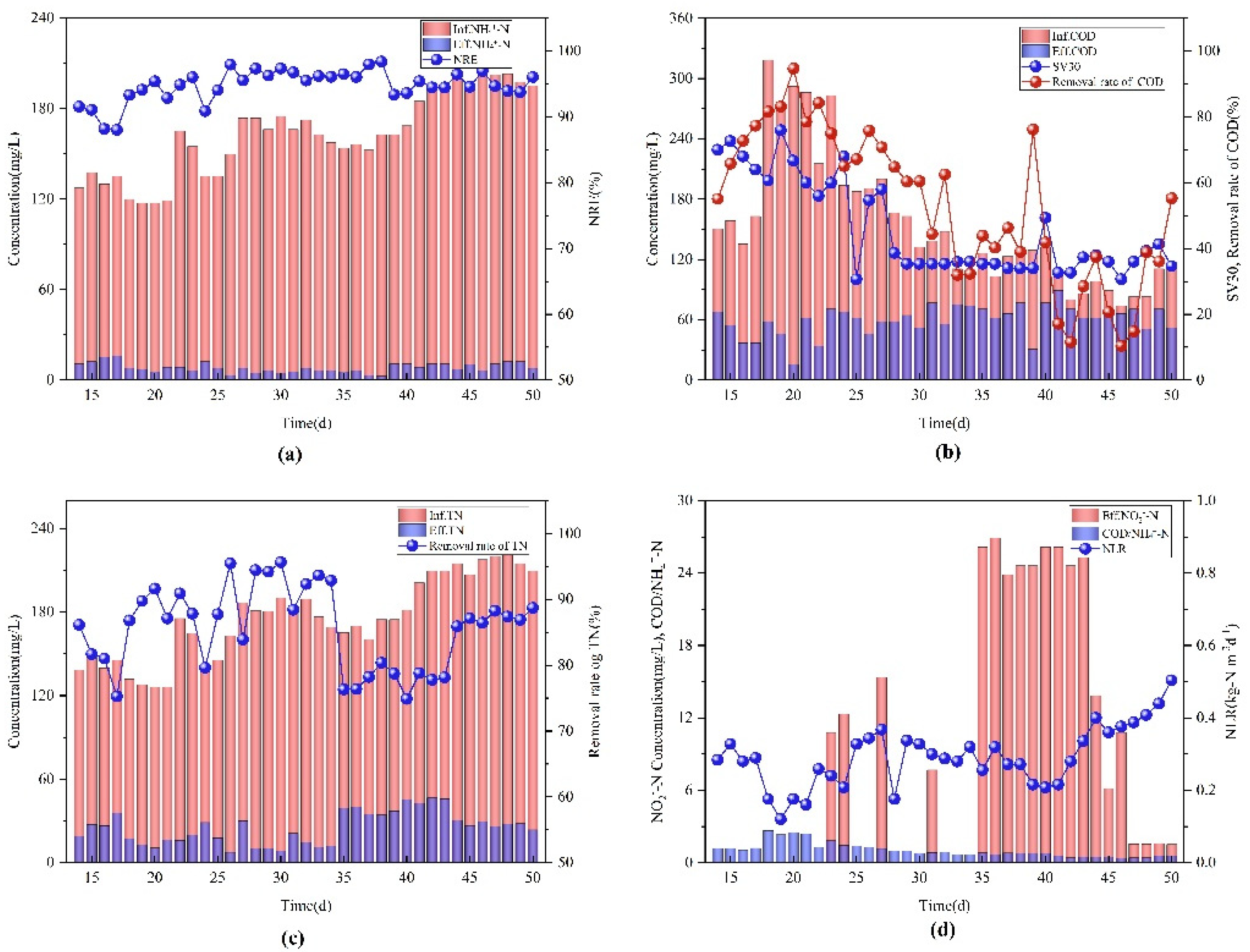
3.2. NLR Domestication Stage
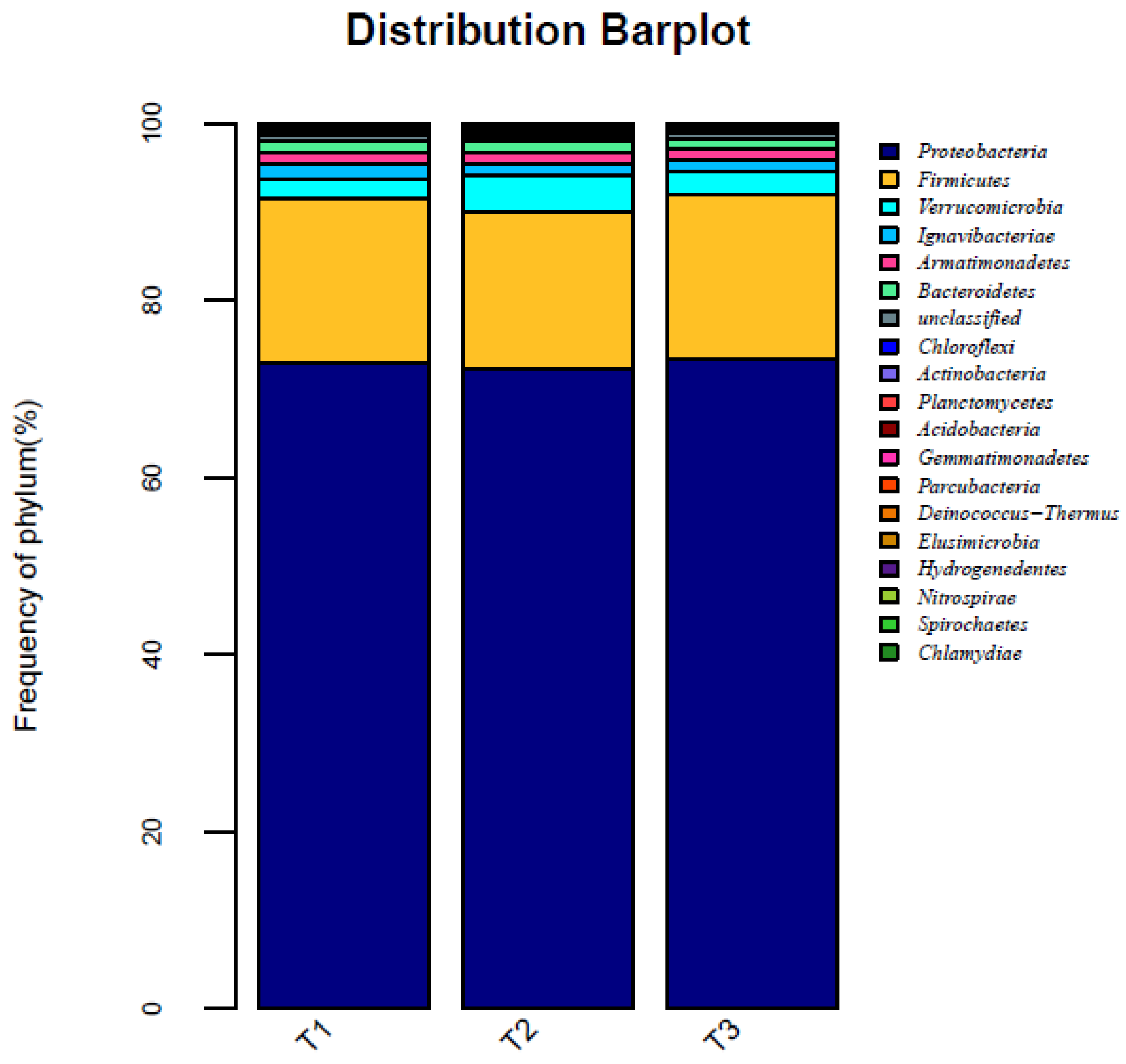
3.3. Microbial Analysis
3.4. Engineering Benefit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brase, L.; Sanders, T.; Dahnke, K. Anthropogenic changes of nitrogen loads in a small river: External nutrient sources vs. internal turnover processes. Isot. Environ. Health Stud. 2018, 54, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Ai, G.; Miao, L.; Zheng, H.; Liu, Z. The characteristics of a novel heterotrophic nitrification-aerobic denitrification bacterium, Bacillus methylotrophicus strain L7. Bioresour. Technol. 2012, 108, 35–44. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, M.; An, Q.; Ye, J.; Guo, J. Characteristics of a heterotrophic nitrogen removal bacterium and its potential application on treatment of ammonium-rich wastewater. Bioresour. Technol. 2017, 226, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, S.; Hao, H.; Chen, J. Characteristics and metabolic pathway of Alcaligenes sp. TB for simultaneous heterotrophic nitrification-aerobic denitrification. Appl. Microbiol. Biotechnol. 2016, 100, 9787–9794. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Jia, Y.; Chen, Y.; Hu, Y. Simultaneous nitrification and denitrification without nitrite accumulation by a novel isolated Ochrobactrum anthropic LJ81. Bioresour. Technol. 2019, 272, 442–450. [Google Scholar] [CrossRef]
- Liu, T.; He, X.; Jia, G.; Xu, J.; Quan, X.; You, S. Simultaneous nitrification and denitrification process using novel surface-modified suspended carriers for the treatment of real domestic wastewater. Chemosphere 2020, 247, 125831. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.; Zhang, W. Effect of nitrite concentration on the growth and microbial diversity of anaerobic ammonia oxidation (anammox) sludge. Desalin. Water Treat. 2020, 179, 54–62. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Xiang, B.; Zhang, W. Effect of Hydraulic Retention Time on Carbon Sequestration during the Two-Stage Anammox Process. Processes 2019, 7, 717. [Google Scholar] [CrossRef] [Green Version]
- Wen, R.; Wei, Y.; Zhang, W. Recovery of nitrogen removal by N2H4 after nitrite inhibited anammox reaction. Global NEST J. 2021, 23, 249–256. [Google Scholar]
- Liu, X.; Wang, D.; Zhang, W. Rapid start-up of anammox reactor using granular sludge supported on activated carbon. Global NEST J. 2020, 22, 289–296. [Google Scholar]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, W. Nitrous oxide emissions from an anammox reactor from the startup to stable-running period. Desalin. Water Treat. 2021, 212, 43–50. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Li, H.; Jin, Y.; Zhang, W. Carbon sequestration pathway of inorganic carbon in partial nitrification sludge. Bioresour. Technol. 2019, 293, 122101. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, Y.; Zhang, W. Domestic Sewage Treatment Using a One-Stage ANAMMOX Process. Int. J. Environ. Res. Public Health 2020, 17, 3284. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Zhang, W. Effects of NH4+-N and NO2−-N on carbon fixation in an anaerobic tor ammonium oxidation reactor. J. Environ. Manag. 2019, 241, 450–457. [Google Scholar] [CrossRef]
- Ding, S.; Bao, P.; Wang, B.; Zhang, Q.; Peng, Y. Long-term stable simultaneous partial nitrification, anammox and denitrification (SNAD) process treating real domestic sewage using suspended activated sludge. Chem. Eng. J. 2018, 339, 180–188. [Google Scholar] [CrossRef]
- He, Q.; Song, Q.; Zhang, S.; Zhang, W.; Wang, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: Reactor performance, extracellular polymeric substances and microbial successions. Chem. Eng. J. 2018, 331, 841–849. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Yang, F.; Xue, Y.; Wang, T. The development of simultaneous partial nitrification, ANAMMOX and denitrification (SNAD) process in a single reactor for nitrogen removal. Bioresour. Technol. 2009, 100, 1548–1554. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 1966, 56, 387–388. [Google Scholar]
- Bi, Z.; Zhang, W.; Song, G.; Huang, Y. Iron-dependent nitrate reduction by anammox consortia in continuous-flow reactors: A novel prospective scheme for autotrophic nitrogen removal. Sci. Total Environ. 2019, 692, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Chen, H.; Lyu, Y.K. Ammonium removal characteristics of an acid-resistant bacterium Acinetobacter sp. JR1 from pharmaceutical wastewater capable of heterotrophic nitrification-aerobic denitrification. Bioresour. Technol. 2019, 274, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Zhao, X.; Senbati, Y.; Song, K.; He, X. Nitrogen removal by heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas sp. DM02: Removal performance, mechanism and immobilized application for real aquaculture wastewater treatment. Bioresour. Technol. 2021, 322, 124555. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Q.; Zhang, G.; Li, Q. Operational Performance and Microbial Community Structure in a Completely Mixed Aeration System. Environ. Sci. 2017, 38, 665–671. [Google Scholar]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.J.; Wei, J.; Wu, Z.; El-Din, M.G. Heterotrophic nitrification and aerobic denitrification process: Promising but a long way to go in the wastewater treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef]
- Zhou, M.; Ye, H.; Zhao, X. Isolation and characterization of a novel heterotrophic nitrifying and aerobic denitrifying bacterium Pseudomonas stutzeri KTB for bioremediation of wastewater. Biotechnol. Bioprocess Eng. 2014, 19, 231–238. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, X.; Wang, E.; Li, B.; He, R.; Yuan, H. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a phosphate accumulating bacterium Pseudomonas stutzeri YG-24. Bioresour. Technol. 2015, 182, 18–25. [Google Scholar] [CrossRef]
- Wen, R.; Jin, Y.; Zhang, W. Application of the anammox in china—A review. Int. J. Environ. Res. Public Health 2020, 17, 1090. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; He, S.; Wu, C.; Du, D. Characteristics of heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter sp. t1 and its application for pig farm wastewater treatment—Sciencedirect. J. Biosci. Bioeng. 2019, 127, 201–205. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Piggery wastewater treatment using alcaligenes faecalis strain no. 4 with heterotrophic nitrification and aerobic denitrification. Water Res. 2006, 40, 3029–3036. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.X.; Liang, X.; Zhao, S.Q.; Wang, J.P.; Xia, Z.H. Nitrogen removal characteristics of a heterotrophic nitrifier acinetobacter junii yb and its potential application for the treatment of high-strength nitrogenous wastewater. Bioresour. Technol. 2015, 193, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, W. NaH2PO4 as pH buffer in an anaerobic ammonium oxidation (anammox) reactor treating high-strength livestock manure digester liquor. Desalin. Water Treat. 2016, 57, 27028–27034. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, J. Landfill leachate treatment with a novel process: Anaerobic ammonium oxidation (Anammox) combined with soil infiltration system. J. Hazard. Mater. 2008, 151, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Ashbolt, N. Adaptation of anaerobic ammonium-oxidising consortium to synthetic coke-ovens wastewater. Appl. Microbiol. Biotechnol. 2002, 59, 344–352. [Google Scholar] [PubMed]
- Daverey, A.; Su, S.H.; Huang, Y.T.; Chen, S.S.; Sung, S.; Lin, J.G. Partial nitrification and anammox process: A method for high strength optoelectronic industrial wastewater treatment. Water Res. 2013, 47, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shao, M.F.; Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J. 2012, 6, 1137–1147. [Google Scholar] [CrossRef]
- Zi, Z.; Bing, T. Research progress in the microbial community and its functional characteristics in activated sludge systems. Ind. Water Treat. 2015, 35, 5–9. [Google Scholar]


| Unit | Wastewater Quality | Post-Treatment Water Quality | |
|---|---|---|---|
| NH4+-N | mg/L | 79.08~208.3 | 15 |
| COD | mg/L | 76~368.5 | 100 |
| NO2−-N | mg/L | <2 | - |
| NO3−-N | mg/L | <2 | - |
| pH | - | 6.4~8.5 | 6~9 |
| Stage | Operation Days (d) | Inlet Pump (m3/h) | NLR (kg-N m−3d−1) | OLR (kg-COD m−3d−1) | pH Limits | NH4+-N Limits (mg/L) |
|---|---|---|---|---|---|---|
| Start-up Period | 1–13 | 50 | 0.144~0.368 | 0.48~1.18 | 7.3~7.9 | 6~9 |
| NLR Domestication Stage | 14–51 | 25 | 0.127~0.501 | 0.21~0.88 | 7.2~7.4 | 11~12 |
| Seed Sludge | Wastewater | Reactor | Scale | Reference |
|---|---|---|---|---|
| Acinetobacter sp. T1 | Real piggery wastewater | A2O | Full-scale | [29] |
| A. faecalis No.4 strains | Real piggery wastewater | Aerobic reactor | Lab-scale | [30] |
| Acinetobacter junii YB | High-strength ammonium wastewater (synthetic) | SBR | Lab-scale | [31] |
| Kuenenia stuttgartiensis | livestock manure digester liquor | - | - | [32] |
| Anaerobic ammonia oxidation seed sludge and activated sludge | Landfill leachate treatment | UFR | Lab-scale | [33] |
| Activated sludge | Synthetic coke-ovens wastewater | - | Lab-scale | [34] |
| Sludge from reactor treating a synthetic wastewater | High-strength optoelectronic industrial wastewater | SBR | Lab-scale | [35] |
| Functional Bacteria | T1 | T2 | T3 |
|---|---|---|---|
| Acinetobacter | 68.32 | 67.20 | 69.01 |
| Sporosarcina | 5.23 | 5.22 | 5.03 |
| Planococcaceae | 4.36 | 3.91 | 4.41 |
| Bacillus | 2.92 | 2.63 | 2.81 |
| Armatimonadetes_gp5 | 1.23 | 1.41 | 1.44 |
| Candidatus_Kuenrnia | 0.08 | 0.10 | 0.06 |
| Nitrosomonas | 0.05 | 0.05 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Zhang, W.; Wang, D.; Jin, Y. Full-Scale Application of One-Stage Simultaneous Nitrification and Denitrification Coupled with Anammox Process for Treating Collagen Casing Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 5787. https://doi.org/10.3390/ijerph19105787
Yu D, Zhang W, Wang D, Jin Y. Full-Scale Application of One-Stage Simultaneous Nitrification and Denitrification Coupled with Anammox Process for Treating Collagen Casing Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(10):5787. https://doi.org/10.3390/ijerph19105787
Chicago/Turabian StyleYu, Dayan, Wenjie Zhang, Dunqiu Wang, and Yue Jin. 2022. "Full-Scale Application of One-Stage Simultaneous Nitrification and Denitrification Coupled with Anammox Process for Treating Collagen Casing Wastewater" International Journal of Environmental Research and Public Health 19, no. 10: 5787. https://doi.org/10.3390/ijerph19105787
APA StyleYu, D., Zhang, W., Wang, D., & Jin, Y. (2022). Full-Scale Application of One-Stage Simultaneous Nitrification and Denitrification Coupled with Anammox Process for Treating Collagen Casing Wastewater. International Journal of Environmental Research and Public Health, 19(10), 5787. https://doi.org/10.3390/ijerph19105787








