Examining the Use of Antidepressants for Adolescents with Depression/Anxiety Who Regularly Use Cannabis: A Narrative Review
Abstract
:1. Introduction
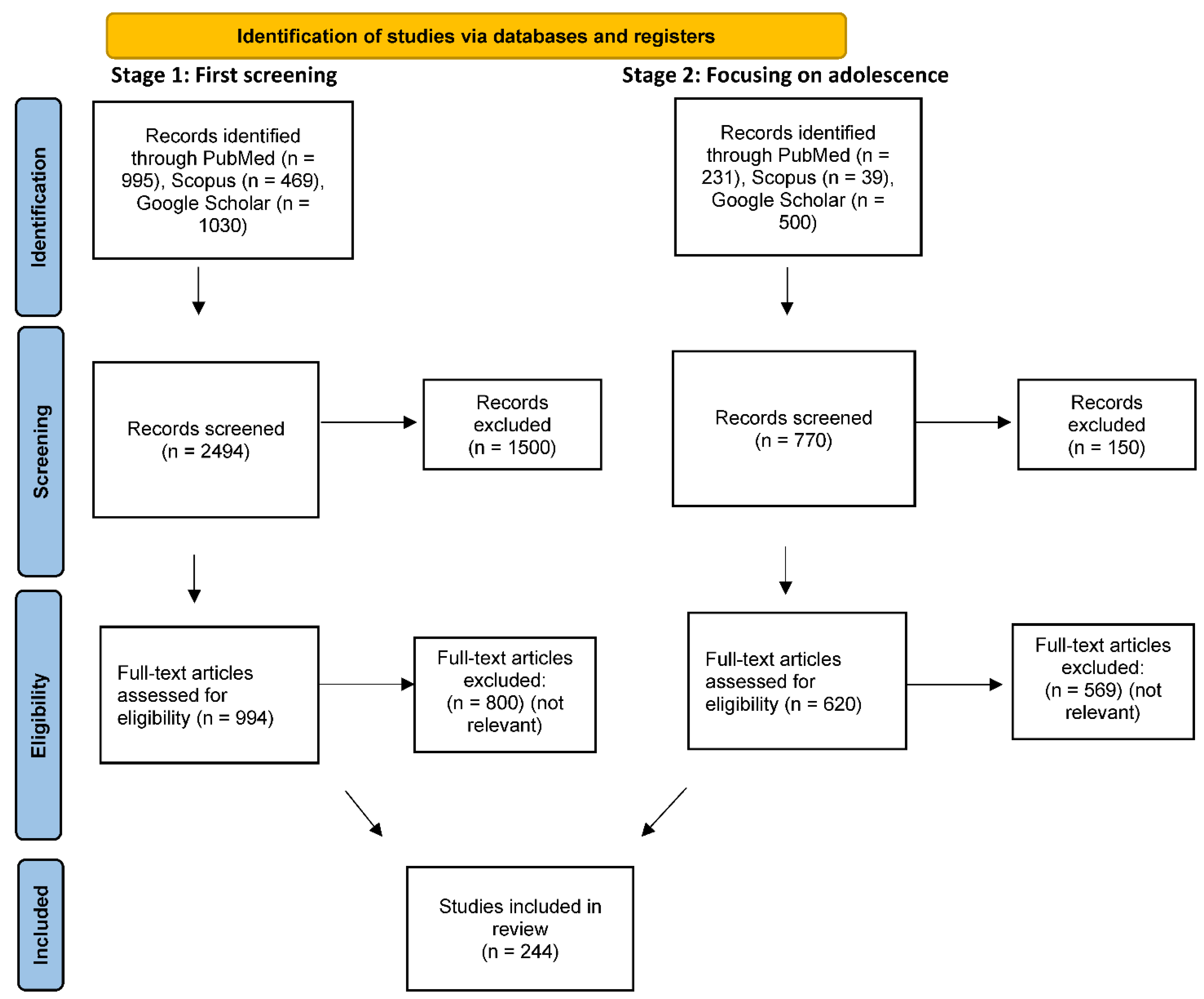
2. Method
Search Strategy and Paper Selection
3. The Efficacy of Antidepressants: The Importance of Adherence
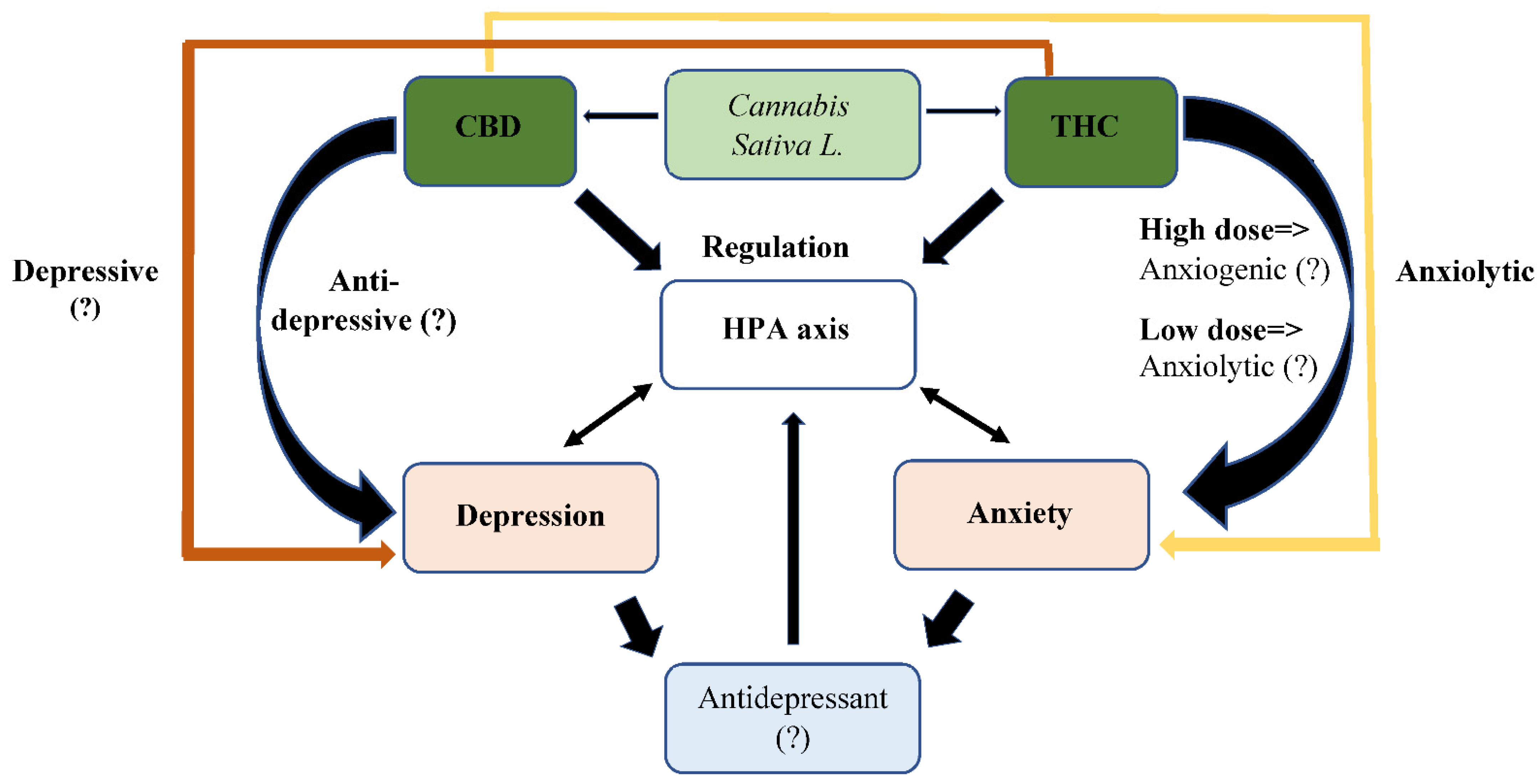
4. Cannabinoids for Treatment of Depression and Anxiety: Changing Perceptions throughout the Years
5. Antidepressant Treatment Combined with Cannabis Use: A Gap in Knowledge
6. Summary: What Is Known and What Needs to Be Studied about Antidepressants and Cannabinoids?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smithson, S.; Pignone, M.P. Screening Adults for Depression in Primary Care. Med. Clin. N. Am. 2017, 101, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Bernaras, E.; Jaureguizar, J.; Garaigordobil, M. Child and adolescent depression: A review of theories, evaluation instruments, prevention programs, and treatments. Front. Psychol. 2019, 10, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Diffusion of eHealth: Making Universal Health Coverage Achievable: Report of the Third Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Lee, J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc. Health 2020, 4, 421. [Google Scholar] [CrossRef]
- Berndt, E.R.; Koran, L.M.; Finkelstein, S.N.; Gelenberg, A.J.; Kornstein, S.G.; Miller, I.M.; Keller, M.B. Lost human capital from early-onset chronic depression. Am. J. Psychiatry 2000, 157, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. Researchers probe depression in children. JAMA 2003, 289, 3078–3079. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M.; Wolk, S.; Goldstein, R.B.; Moreau, D.; Adams, P.; Greenwald, S.; Wickramaratne, P. Depressed adolescents grown up. JAMA 1994, 281, 1707–1713. [Google Scholar] [CrossRef]
- Weissman, M.M.; Wolk, S.; Wickramaratne, P.; Goldstein, R.B.; Adams, P.; Greenwald, S.; Steinberg, D. Children with prepubertal-onset major depressive disorder and anxiety grown up. Arch. Gen. Psychiatry 1999, 56, 794–801. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. Interoception in anxiety and depression. Brain Struct. Funct. 2010, 214, 451–463. [Google Scholar] [CrossRef] [Green Version]
- Angold, A.; Costello, E.J.; Erkanli, A. Comorbidity. J. Child Psychol. Psychiatry Allied Discip. 1999, 40, 57–87. [Google Scholar] [CrossRef]
- Angold, A.; Erkanli, A.; Farmer, E.M.; Fairbank, J.A.; Burns, B.J.; Keeler, G.; Costello, E.J. Psychiatric disorder, impairment, and service use in rural African American and white youth. Arch. Gen. Psychiatry 2002, 59, 893–901. [Google Scholar] [CrossRef]
- Paris, J. Recent research in personality disorders. Preface. Psychiatr. Clin. N. Am. 2008, 31, xi–xii. [Google Scholar] [CrossRef]
- Renouf, A.G.; Kovacs, M.; Mukerji, P. Relationship of depressive, conduct, and comorbid disorders and social functioning in childhood. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 998–1004. [Google Scholar] [CrossRef]
- Oquendo, M.A.; Mann, J.J. Suicidal behavior: A developmental perspective. Psychiatr. Clin. N. Am. 2008, 31, xiii–xvi. [Google Scholar] [CrossRef] [Green Version]
- Sher, L.; Sperling, D.; Stanley, B.H.; Carballo, J.J.; Shoval, G.; Zalsman, G.; Burke, A.K.; Mann, J.J.; Oquendo, M.A. Triggers for suicidal behavior in depressed older adolescents and young adults: Do alcohol use disorders make a difference? Int. J. Adolesc. Med. Health 2007, 19, 91–98. [Google Scholar] [CrossRef]
- Shoval, G.; Sever, J.; Sher, L.; Diller, R.; Apter, A.; Weizman, A.; Zalsman, G. Substance use, suicidality, and adolescent-onset schizophrenia: An Israeli 10-year retrospective study. J. Child Adolesc. Psychopharmacol. 2006, 16, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Shoval, G.; Shmulewitz, D.; Wall, M.M.; Aharonovich, E.; Spivak, B.; Weizman, A.; Hasin, D. Alcohol dependence and suicide-related ideation/behaviors in an Israeli household sample, with and without major depression. Alcohol. Clin. Exp. Res. 2014, 38, 820–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoval, G.; Zalsman, G.; Apter, A.; Diller, R.; Sher, L.; Weizman, A. A 10-year retrospective study of inpatient adolescents with schizophrenia/schizoaffective disorder and substance use. Compr. Psychiatry 2007, 48, 1–7. [Google Scholar] [CrossRef]
- Zalsman, G.; Misgav, S.; Sommerfeld, E.; Kohn, Y.; Brunstein-Klomek, A.; Diller, R.; Sher, L.; Schwartz, J.; Shoval, G.; Ben-Dor, D.H.; et al. Children’s Depression Inventory (CDI) and the Children’s Depression Rating Scale-Revised (CDRS-R): Reliability of the Hebrew version. Int. J. Adolesc. Med. Health 2005, 17, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Sepede, G.; Farano, M.; Santacroce, R.; Santoro, R.; Marini, S.; Mangifesta, R.; Salerno, R.M. Depressive symptoms in adolescence: The role of gender and personality traits. Res. Adv. Psychiatry 2015, 2, 9–16. [Google Scholar]
- Nardi, B.; Francesconi, G.; Catena-Dell’osso, M.; Bellantuono, C. Adolescent depression: Clinical features and therapeutic strategies. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1546–1551. [Google Scholar] [PubMed]
- Hollingworth, S.A.; Burgess, P.M.; Whiteford, H.A. Affective and anxiety disorders: Prevalence, treatment and antidepressant medication use. Aust. N. Z. J. Psychiatry 2010, 44, 513–519. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Egger, M. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Bachmann, C.J.; Aagaard, L.; Burcu, M.; Glaeske, G.; Kalverdijk, L.J.; Petersen, I.; Hoffmann, F. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005–2012. Eur. Neuropsychopharmacol. 2016, 26, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Hamrin, V.; Iennaco, J.D. Evaluation of motivational interviewing to improve psychotropic medication adherence in adolescents. J. Child Adolesc. Psychopharmacol. 2017, 27, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Cuijpers, P. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Schroder, C.M.; Reynaud, E.; Bourgin, P. Efficacy of light therapy versus antidepressant drugs, and of the combination versus monotherapy, in major depressive episodes: A systematic review and meta-analysis. Sleep Med Rev. 2019, 48, 101213. [Google Scholar] [CrossRef]
- Kirsch, I.; Deacon, B.J.; Huedo-Medina, T.B.; Scoboria, A.; Moore, T.J.; Johnson, B.T. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008, 5, e45. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Geddes, J.R.; Higgins, J.P.; Churchill, R.; Tansella, M. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet 2009, 373, 746–758. [Google Scholar] [CrossRef]
- Mulder, R.T.; Joyce, P.R.; Frampton, C.M.; Luty, S.E. Antidepressant treatment is associated with a reduction in suicidal ideation and suicide attempts. Acta Psychiatr. Scand. 2008, 118, 116–122. [Google Scholar] [CrossRef]
- Price, C.; Hemmingsson, T.; Lewis, G.; Zammit, S.; Allebeck, P. Cannabis and suicide: Longitudinal study. Br. J. Psychiatry 2009, 195, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Weller, A. The “entourage effect”: Terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Hurst, T. World drug report. In The Encyclopedia of Women and Crime; Wiley on line: New York, NY, USA, 2019; pp. 1–2. [Google Scholar]
- Hall, W.; Degenhardt, L. Adverse health effects of non-medical cannabis use. Lancet 2010, 374, 1383–1391. [Google Scholar] [CrossRef]
- Camchong, J.; Lim, K.O.; Kumra, S. Adverse effects of cannabis on adolescent brain development: A longitudinal study. Cereb. Cortex 2017, 27, 1922–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonomo, Y.; Souza, J.D.S.; Jackson, A.; Crippa, J.A.S.; Solowij, N. Clinical issues in cannabis use. Br. J. Clin. Pharmacol. 2018, 84, 2495–2498. [Google Scholar] [CrossRef] [Green Version]
- Krediet, E.; Janssen, D.G.; Heerdink, E.R.; Egberts, T.C.; Vermetten, E. Experiences with medical cannabis in the treatment of veterans with PTSD: Results from a focus group discussion. Eur. Neuropsychopharmacol. 2020, 36, 244–254. [Google Scholar] [CrossRef]
- Lintzeris, N.; Mills, L.; Suraev, A.; Bravo, M.; Arkell, T.; Arnold, J.C.; McGregor, I.S. Medical cannabis use in the Australian community following introduction of legal access: The 2018–2019 Online Cross-Sectional Cannabis as Medicine Survey (CAMS-18). Harm. Reduct. J. 2020, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. Linking molecules to mood: New insight into the biology of depression. Am. J. Psychiatry 2010, 167, 1305–1320. [Google Scholar] [CrossRef] [Green Version]
- De Mello Schier, A.R.; de Oliveira Ribeiro, N.P.; Coutinho, D.S.; Machado, S.; Arias-Carrión, O.; Crippa, J.A.; Silva, A.C. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurol. Disord. Drug Targets 2014, 13, 953–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resstel, L.B.; Tavares, R.F.; Lisboa, S.F.; Joca, S.R.; Correa, F.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Shbiro, L.; Hen-Shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Pro-hedonic effect of cannabidiol in a rat model for depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimaraes, F.S.; Joca, S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, A.H.; Zhu, J.; Lee, J.; Anastasiou, E.; Copeland, J.; Goodwin, R.D. Cannabis use among youth in the United States, 2004–2016: Faster rate of increase among youth with depression. Drug Alcohol Depend. 2020, 209, 107894. [Google Scholar] [CrossRef]
- Shoval, G.; Zalsman, G.; Sher, L.; Apter, A.; Weizman, A. Clinical characteristics of inpatient adolescents with severe obsessive-compulsive disorder. Depress. Anxiety 2006, 23, 62–70. [Google Scholar] [CrossRef]
- Fournier, J.C.; DeRubeis, R.J.; Hollon, S.D.; Dimidjian, S.; Amsterdam, J.D.; Shelton, R.C.; Fawcett, J. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA 2010, 303, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbregts, K.M.; Hoogendoorn, A.; Slottje, P.; van Balkom, A.J.; Batelaan, N.M. Long-term and short-term antidepressant use in general practice: Data from a large cohort in the Netherlands. Psychother. Psychosom. 2017, 86, 362–369. [Google Scholar] [CrossRef]
- Kellner, M. Drug treatment of obsessive-compulsive disorder. Dialogues Clin. Neurosci. 2010, 12, 187. [Google Scholar] [CrossRef]
- Krivoy, A.; Stubbs, B.; Balicer, R.D.; Weizman, S.; Feldman, B.; Hoshen, M.; Shoval, G. Low adherence to antidepressants is associated with increased mortality following stroke: A large nationally representative cohort study. Eur. Neuropsychopharmacol. 2017, 27, 970–976. [Google Scholar] [CrossRef]
- Rubio, G.; Jiménez-Arriero, M.A.; Martínez-Gras, I.; Manzanares, J.; Palomo, T. The effects of topiramate adjunctive treatment added to antidepressants in patients with resistant obsessive-compulsive disorder. J. Clin. Psychopharmacol. 2006, 26, 341–344. [Google Scholar] [CrossRef]
- De Graaf, R.; Ten Have, M.; Van Gool, C.; Van Dorsselaer, S. Prevalence of mental disorders, and trends from 1996 to 2009. Results from NEMESIS-2. Tijdschr. Psychiatr. 2012, 54, 27. [Google Scholar] [PubMed]
- Öztürk, G.; Yetkiner, H.; Özden, E. Macroeconomic determinants of antidepressant use. J. Policy. Model. 2020, 42, 1394–1407. [Google Scholar] [CrossRef]
- Noordam, R.; Aarts, N.; Verhamme, K.M.; Sturkenboom, M.C.; Stricker, B.H.; Visser, L.E. Prescription and indication trends of antidepressant drugs in the Netherlands between 1996 and 2012: A dynamic population-based study. Eur. J. Clin. Pharmacol. 2015, 71, 369–375. [Google Scholar] [CrossRef]
- Pacher, P.; Kecskemeti, V. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr. Med. Chem. 2004, 11, 925–943. [Google Scholar] [CrossRef] [Green Version]
- Insel, T.R.; Wang, P.S. The STAR* D trial: Revealing the need for better treatments. Psychiatr. Serv. 2009, 60, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Bschor, T.; Ising, M.; Erbe, S.; Winkelmann, P.; Ritter, D.; Uhr, M.; Lewitzka, U. Impact of citalopram on the HPA system. A study of the combined DEX/CRH test in 30 unipolar depressed patients. J. Psychiatr. Res. 2012, 46, 111–117. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S. When should you move beyond first-line therapy for depression? J. Clin. Psychiatry 2010, 71 (Suppl. 1), 16–20. [Google Scholar] [CrossRef]
- Fava, M.; McGrath, P.J.; Sheu, W.P.; Reboxetine Study Group. Switching to reboxetine: An efficacy and safety study in patients with major depressive disorder unresponsive to fluoxetine. J. Clin. Psychopharmacol. 2003, 23, 365–369. [Google Scholar] [CrossRef]
- De Vries, Y.A.; de Jonge, P.; Kalverdijk, L.; Bos, J.H.; Schuiling-Veninga, C.C.; Hak, E. Poor guideline adherence in the initiation of antidepressant treatment in children and adolescents in the Netherlands: Choice of antidepressant and dose. Eur. Child Adolesc. Psychiatry 2016, 25, 1161–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, A.J.; Fava, M.; Wisniewski, S.R.; Lavori, P.W.; Trivedi, M.H.; Sackeim, H.A.; Kupfer, D.J. Sequenced treatment alternatives to relieve depression (STAR* D): Rationale and design. Control. Clin. Trials 2004, 25, 119–142. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Stewart, J.W.; Nierenberg, A.A.; Thase, M.E.; Shores-Wilson, K. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1231–1242. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, C.; Flores-Ramos, M. Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J. Affect. Disord. 2006, 95, 119–123. [Google Scholar] [CrossRef]
- Bigos, K.L.; Pollock, B.G.; Stankevich, B.A.; Bies, R.R. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: An updated review. Gend. Med. 2009, 6, 522–543. [Google Scholar] [CrossRef]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 2010, 106, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Guasti, A.; Fiedler, J.L.; Herrera, L.; Handa, R.J. Sex, stress, and mood disorders: At the intersection of adrenal and gonadal hormones. Horm. Metab. Res. 2012, 44, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeGates, T.A.; Kvarta, M.D.; Thompson, S.M. Sex differences in antidepressant efficacy. Neuropsychopharmacology 2019, 44, 140–154. [Google Scholar] [CrossRef] [Green Version]
- Bylund, D.B.; Reed, A.L. Childhood and adolescent depression: Why do children and adults respond differently to antidepressant drugs? Neurochem. Int. 2007, 51, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, P.R.; Mulder, R.T.; Luty, S.E.; McKenzie, J.M.; Miller, A.L.; Rogers, G.R.; Kennedy, M.A. Age-dependent antidepressant pharmacogenomics: Polymorphisms of the serotonin transporter and G protein β3 subunit as predictors of response to fluoxetine and nortriptyline. Int. J. Neuropsychopharmacol. 2003, 6, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Mulder, R.T.; Watkins, W.G.; Joyce, P.R.; Luty, S.E. Age may affect response to antidepressants with serotonergic and noradrenergic actions. J. Affect. Disord. 2003, 76, 143–149. [Google Scholar] [CrossRef]
- Tedeschini, E.; Levkovitz, Y.; Iovieno, N.; Ameral, V.E.; Nelson, J.C.; Papakostas, G.I. Efficacy of antidepressants for late-life depression: A meta-analysis and meta-regression of placebo-controlled randomized trials. J. Clin. Psychiatry 2011, 72, 1660–1668. [Google Scholar] [CrossRef]
- Bauer, M.; Severus, E.; Koehler, S.; Whybrow, P.C.; Angst, J.; Moeller, H.J.; Wfsbp Task Force on Treatment Guidelines for Unipolar Depressive Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: Maintenance treatment of major depressive disorder-update 2015. World J. Biol. Psychiatry 2015, 16, 76–95. [Google Scholar] [CrossRef]
- Burchi, E.; Hollander, E.; Pallanti, S. From treatment response to recovery: A realistic goal in OCD. Int. J. Neuropsychopharmacol. 2018, 21, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kjernisted, K.D.; Bleau, P. Long-term goals in the management of acute and chronic anxiety disorders. Can. J. Psychiatry 2004, 49, 51–63. [Google Scholar]
- Lam, R.W.; Kennedy, S.H. Evidence-based strategies for achieving and sustaining full remission in depression: Focus on metaanalyses. Can. J. Psychiatry 2004, 49, 17–26. [Google Scholar]
- Cheung, A.H.; Emslie, G.J.; Mayes, T.L. Review of the efficacy and safety of antidepressants in youth depression. J. Child Psychol. Psychiatry 2005, 46, 735–754. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Kendall, P.C.; Sakolsky, D.; Compton, S.N.; Piacentini, J.; Albano, A.M.; March, J. Remission after acute treatment in children and adolescents with anxiety disorders: Findings from the CAMS. J. Consult. Clin. Psychol. 2011, 79, 806. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Cook, E.H.; Chung, H.; Messig, M. Remission status after long-term sertraline treatment of pediatric obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2003, 13 (Suppl. 1), 53–60. [Google Scholar] [CrossRef]
- Whiskey, E.; Taylor, D. A review of the adverse effects and safety of noradrenergic antidepressants. J. Psychopharmacol. 2013, 27, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Nahshoni, E.; Spitzer, S.; Berant, M.; Shoval, G.; Zalsman, G.; Weizman, A. QT interval and dispersion in very young children treated with antipsychotic drugs: A retrospective chart review. J. Child Adolesc. Psychopharmacol. 2007, 17, 187–194. [Google Scholar] [CrossRef]
- Hu, X.H.; Bull, S.A.; Hunkeler, E.M.; Ming, E.; Lee, J.Y.; Fireman, B.; Markson, L.E. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry 2004, 65, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Kostev, K.; Rex, J.; Eith, T.; Heilmaier, C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. Ger. Med. Sci. 2014, 12, Doc15. [Google Scholar] [CrossRef] [PubMed]
- Burra, T.A.; Chen, E.; McIntyre, R.S.; Grace, S.L.; Blackmore, E.R.; Stewart, D.E. Predictors of self-reported antidepressant adherence. Behav. Med. 2007, 32, 127–134. [Google Scholar] [CrossRef]
- De las Cuevas, C.; Peñate, W.; Sanz, E.J. Risk factors for non-adherence to antidepressant treatment in patients with mood disorders. Eur. J. Clin. Pharmacol. 2014, 70, 89–98. [Google Scholar] [CrossRef]
- Milan, R.; Vasiliadis, H.M. The association between side effects and adherence to antidepressants among primary care community-dwelling older adults. Aging Ment. Health 2020, 24, 1229–1236. [Google Scholar] [CrossRef]
- Aljumah, K.; Hassali, A.A.; AlQhatani, S. Examining the relationship between adherence and satisfaction with antidepressant treatment. Neuropsychiatr. Dis. Treat. 2014, 10, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Geddes, J.R.; Carney, S.M.; Davies, C.; Furukawa, T.A.; Kupfer, D.J.; Frank, E.; Goodwin, G.M. Relapse prevention with antidepressant drug treatment in depressive disorders: A systematic review. Lancet 2003, 361, 653–661. [Google Scholar] [CrossRef]
- Sawada, N.; Uchida, H.; Suzuki, T.; Watanabe, K.; Kikuchi, T.; Handa, T.; Kashima, H. Persistence and compliance to antidepressant treatment in patients with depression: A chart review. BMC Psychiatry 2009, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.H.; Erickson, S.R.; Piette, J.D.; Balkrishnan, R. The association of race, comorbid anxiety, and antidepressant adherence among Medicaid enrollees with major depressive disorder. Res. Soc. Adm. Pharm. 2012, 8, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Treglia, M.; Obenchain, R.L.; Dulisse, B.; Melfi, C.A.; Croghan, T.W. Determinants of antidepressant treatment outcome. Am. J. Manag. Care 2000, 6, 1327–1339. [Google Scholar]
- Posternak, M.A.; Baer, L.; Nierenberg, A.A.; Fava, M. Response rates to fluoxetine in subjects who initially show no improvement. J. Clin. Psychiatry 2011, 72, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E. Evaluating antidepressant therapies: Remission as the optimal outcome. J. Clin. Psychiatry 2003, 64 (Suppl. 13), 18–25. [Google Scholar]
- Birmaher, B.; Brent, D.; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 1503–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krivoy, A.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Zalsman, G.; Weizman, A.; Shoval, G. Adherence to antidepressants is associated with lower mortality: A 4-year population-based cohort study. J. Clin. Psychiatry 2016, 77, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Krivoy, A.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Zalsman, G.; Weizman, A.; Shoval, G. Adherence to antidepressant therapy and mortality rates in ischaemic heart disease: Cohort study. Br. J. Psychiatry 2015, 206, 297–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoval, G.; Stubbs, B.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Zalsman, G.; Krivoy, A. Low adherence to antidepressants is associated with increased mortality in Parkinson disease patients. Parkinsonism Relat. Disord. 2017, 43, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoval, G.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Eger, G.; Weizman, A.; Krivoy, A. Adherence to antidepressant medications is associated with reduced premature mortality in patients with cancer: A nationwide cohort study. Depress. Anxiety 2019, 36, 921–929. [Google Scholar] [CrossRef]
- Bambauer, K.Z.; Adams, A.S.; Zhang, F.; Minkoff, N.; Grande, A.; Weisblatt, R.; Ross-Degnan, D. Physician alerts to increase antidepressant adherence: Fax or fiction? Arch. Intern. Med. 2006, 166, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunot, V.M.; Horne, R.; Leese, M.N.; Churchill, R.C. A cohort study of adherence to antidepressants in primary care: The influence of antidepressant concerns and treatment preferences. Prim. Care Companion. J. Clin. Psychiatry 2007, 9, 91. [Google Scholar] [CrossRef]
- Olfson, M.; Marcus, S.C. National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry 2009, 66, 848–856. [Google Scholar] [CrossRef]
- Adhikari, K.; Patten, S.B.; Lee, S.; Metcalfe, A. Adherence to and persistence with antidepressant medication during pregnancy: Does it differ by the class of antidepressant medication prescribed? Can J. Psychiatry 2019, 64, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Fontanella, C.A.; Bridge, J.A.; Marcus, S.C.; Campo, J.V. Factors associated with antidepressant adherence for Medicaid-enrolled children and adolescents. Ann. Pharmacother. 2011, 45, 898–909. [Google Scholar] [CrossRef]
- Rossom, R.C.; Shortreed, S.; Coleman, K.J.; Beck, A.; Waitzfelder, B.E.; Stewart, C.; Simon, G.E. Antidepressant adherence across diverse populations and healthcare settings. Depress. Anxiety 2016, 33, 765–774. [Google Scholar] [CrossRef]
- Krivoy, A.; Balicer, R.D.; Feldman, B.; Hoshen, M.; Zalsman, G.; Weizman, A.; Shoval, G. The impact of age and gender on adherence to antidepressants: A 4-year population-based cohort study. Psychopharmacology 2015, 232, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Shalit, N.; Barzilay, R.; Shoval, G.; Shlosberg, D.; Mor, N.; Zweigenhaft, N.; Krivoy, A. Characteristics of synthetic cannabinoid and cannabis users admitted to a psychiatric hospital: A comparative study. J. Clin. Psychiatry 2016, 77, 989–995. [Google Scholar] [CrossRef]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef] [Green Version]
- Achterberg, E.M.; van Swieten, M.M.; Driel, N.V.; Trezza, V.; Vanderschuren, L.J. Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharmacol. Res. 2016, 110, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; De Wit, H.; Van Cauter, E. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep 2016, 39, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morera-Herreras, T.; Miguelez, C.; Aristieta, A.; Torrecilla, M.; Ruiz-Ortega, J.A.; Ugedo, L. Cannabinoids and motor control of the basal ganglia: Therapeutic potential in movement disorders. In Cannabinoids in Health and Disease; Meccariello, R., Chianese, R., Eds.; InTech: Rijeka, Croatia, 2016; pp. 59–92. [Google Scholar]
- Kruk-Slomka, M.; Dzik, A.; Budzynska, B.; Biala, G. Endocannabinoid system: The direct and indirect involvement in the memory and learning processes—A short review. Mol. Neurobiol. 2017, 54, 8332–8347. [Google Scholar] [CrossRef] [Green Version]
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The endocannabinoid system in cardiovascular function: Novel insights and clinical implications. Clin. Auton. Res. 2018, 28, 35–52. [Google Scholar] [CrossRef]
- Soria-Gómez, E.; Bellocchio, L.; Reguero, L.; Lepousez, G.; Martin, C.; Bendahmane, M.; Wiesner, T. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 2014, 17, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Gertsch, J.; Pertwee, R.G.; Di Marzo, V. Phytocannabinoids beyond the Cannabis plant–do they exist? Br. J. Pharmacol. 2010, 160, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Gaoni, Y.; Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.A.; Warner, P.; Wang, H. Antibacterial properties of hemp and other natural fibre plants: A review. BioResources 2014, 9, 3642–3659. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batalla, A.; Crippa, J.A.; Busatto, G.F.; Guimaraes, F.S.; Zuardi, A.W.; Valverde, O.; Martin-Santos, R. Neuroimaging studies of acute effects of THC and CBD in humans and animals: A systematic review. Curr. Pharm. Des. 2014, 20, 2168–2185. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Mehta, M.A. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bhattacharyya, S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Dewey, W.L. Cannabinoid pharmacology. Pharmacol. Rev. 1986, 38, 151–178. [Google Scholar] [CrossRef]
- Guimarães, F.S.; Chiaretti, T.M.; Graeff, F.G.; Zuardi, A.W. Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology 1990, 100, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Luginbuhl, A.M. Industrial hemp (Cannabis sativa L.): The geography of a controversial plant. Calif. Geogr. 2001, 41, 1–14. [Google Scholar]
- Mead, A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 2017, 70, 288–291. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.T. Tetrahydrocannabinol and the marijuana-induced social “high,” or the effects of the mind on marijuana. Ann. N. Y. Acad. Sci. 1971, 191, 155–165. [Google Scholar] [CrossRef]
- Murray, R.M.; Hall, W. Will legalization and commercialization of cannabis use increase the incidence and prevalence of psychosis? JAMA Psychiatry 2020, 77, 777–778. [Google Scholar] [CrossRef] [PubMed]
- DeFleur, L.B.; Garrett, G.R. Dimensions of marijuana usage in a land-grant university. J. Couns. Psychol. 1970, 17, 468. [Google Scholar] [CrossRef]
- Hall, W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction 2015, 110, 19–35. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.; Degenhardt, L. Prevalence and correlates of cannabis use in developed and developing countries. Curr. Opin. Psychiatry 2007, 20, 393–397. [Google Scholar] [CrossRef]
- Woodruff, S.I.; Shillington, A.M. Sociodemographic and drug use severity differences between medical marijuana users and non-medical users visiting the emergency department. Am. J. Addict. 2016, 25, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Patton, G.C.; Coffey, C.; Carlin, J.B.; Degenhardt, L.; Lynskey, M.; Hall, W. Cannabis use and mental health in young people: Cohort study. BMJ 2002, 325, 1195–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abush, H.; Akirav, I. Cannabinoids ameliorate impairments induced by chronic stress to synaptic plasticity and short-term memory. Neuropsychopharmacology 2013, 38, 1521–1534. [Google Scholar] [CrossRef] [Green Version]
- Fattore, L.; Fadda, P.; Spano, M.S.; Pistis, M.; Fratta, W. Neurobiological mechanisms of cannabinoid addiction. Mol. Cell. Endocrinol. 2008, 286, S97–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganon-Elazar, E.; Akirav, I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology 2012, 37, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Di Marzo, V. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.A.; Wotjak, C.T. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 2008, 170, 397–432. [Google Scholar] [CrossRef]
- Hall, W. Cannabis use and psychosis. Drug. Alcohol Rev. 1998, 17, 433–444. [Google Scholar] [CrossRef]
- Linszen, D.H.; Dingemans, P.M.; Lenior, M.E. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch. Gen. Psychiatry 1994, 51, 273–279. [Google Scholar] [CrossRef]
- Machielsen, M.; van der Sluis, S.; de Haan, L. Cannabis use in patients with a first psychotic episode and subjects at ultra high risk of psychosis: Impact on psychotic-and pre-psychotic symptoms. Aust. N. Z. J. Psychiatry 2010, 44, 721–728. [Google Scholar] [CrossRef]
- Schoeler, T.; Petros, N.; Di Forti, M.; Pingault, J.B.; Klamerus, E.; Foglia, E.; Bhattacharyya, S. Association between continued cannabis use and risk of relapse in first-episode psychosis: A quasi-experimental investigation within an observational study. JAMA Psychiatry 2016, 73, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Buckner, J.D.; Crosby, R.D.; Wonderlich, S.A.; Schmidt, N.B. Social anxiety and cannabis use: An analysis from ecological momentary assessment. J. Anxiety Disord. 2012, 26, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Cheung, J.T.; Mann, R.E.; Ialomiteanu, A.; Stoduto, G.; Chan, V.; Ala-Leppilampi, K.; Rehm, J. Anxiety and mood disorders and cannabis use. Am. J. Drug Alcohol Abuse 2010, 36, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kedzior, K.K.; Laeber, L.T. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population-a meta-analysis of 31 studies. BMC Psychiatry 2014, 14, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, G.; Atkin, T.; Zytynski, T.; Wang, S.; Askari, S.; Boruff, J.; Mayo, N. Association of cannabis use in adolescence and risk of depression, anxiety, and suicidality in young adulthood: A systematic review and meta-analysis. JAMA Psychiatry 2019, 76, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Shalit, N.; Shoval, G.; Shlosberg, D.; Feingold, D.; Lev-Ran, S. The association between cannabis use and suicidality among men and women: A population-based longitudinal study. J. Affect. Disord. 2016, 205, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Duperrouzel, J.; Hawes, S.W.; Lopez-Quintero, C.; Pacheco-Colón, I.; Comer, J.; Gonzalez, R. The association between adolescent cannabis use and anxiety: A parallel process analysis. Addict. Behav. 2018, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L. Marihuana: The First Twelve Thousand Years; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Larsen, C.; Shahinas, J. Dosage, efficacy and safety of cannabidiol administration in adults: A systematic review of human trials. J. Clin. Med. Res. 2020, 12, 129. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Spagnolo, E.V.; Busardò, F.P.; Cas, R.D.; Calapai, G. Neurological aspects of medical use of cannabidiol. CNS Neurol. Disord. Drug Targets 2017, 16, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Vuolo, F.; Petronilho, F.; Sonai, B.; Ritter, C.; Hallak, J.E.; Zuardi, A.W.; Dal-Pizzol, F. Evaluation of serum cytokines levels and the role of cannabidiol treatment in animal model of asthma. Mediat. Inflamm. 2015, 2015, 538670. [Google Scholar] [CrossRef]
- Morgan, C.J.A.; Gardener, C.; Schafer, G.; Swan, S.; Demarchi, C.; Freeman, T.P.; Wingham, G. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 2012, 42, 391. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacological and therapeutic targets for Δ 9 tetrahydrocannabinol and cannabidiol. Euphytica 2014, 140, 73–82. [Google Scholar] [CrossRef]
- Zuardi, A.W. Cannabidiol: From an inactive cannabinoid to a drug with wide spectrum of action. Braz. J. Psychiatry 2008, 30, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Casarotto, P.C.; Gomes, F.V.; Resstel, L.B.; Guimarães, F.S. Cannabidiol inhibitory effect on marble-burying behaviour: Involvement of CB1 receptors. Behav. Pharmacol. 2010, 21, 353–358. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Platt, B. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Nardo, M.; Casarotto, P.C.; Gomes, F.V.; Guimaraes, F.S. Cannabidiol reverses the mCPP-induced increase in marble-burying behavior. Fundam. Clin. Pharmacol. 2014, 28, 544–550. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Lawn, W.; Freeman, T.P.; Pope, R.A.; Joye, A.; Harvey, L.; Hindocha, C.; Das, R.K. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: An evaluation of the cannabis ‘amotivational’ hypotheses. Psychopharmacology 2016, 233, 3537–3552. [Google Scholar] [CrossRef] [Green Version]
- Selvarajah, D.; Gandhi, R.; Emery, C.J.; Tesfaye, S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: Depression is a major confounding factor. Diabetes Care 2010, 33, 128–130. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136. [Google Scholar] [CrossRef]
- Hen-Shoval, D.; Amar, S.; Shbiro, L.; Smoum, R.; Haj, C.G.; Mechoulam, R.; Shoval, G. Acute oral cannabidiolic acid methyl ester reduces depression-like behavior in two genetic animal models of depression. Behav. Brain Res. 2018, 351, 1–3. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Rock, E.M.; Guenther, K.; Limebeer, C.L.; Stevenson, L.A.; Haj, C.; Mechoulam, R. Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT1A receptor-mediated suppression of nausea and anxiety in rats. Br. J. Pharmacol. 2018, 175, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Rock, E.M.; Limebeer, C.L.; Petrie, G.N.; Williams, L.A.; Mechoulam, R.; Parker, L.A. Effect of prior foot shock stress and Δ 9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology 2017, 234, 2207–2217. [Google Scholar] [CrossRef]
- Ware, M.A.; Adams, H.; Guy, G.W. The medicinal use of cannabis in the UK: Results of a nationwide survey. Int. J. Clin. Pract. 2005, 59, 291–295. [Google Scholar] [CrossRef]
- Binder, E.B.; Künzel, H.E.; Nickel, T.; Kern, N.; Pfennig, A.; Majer, M.; Holsboer, F. HPA-axis regulation at in-patient admission is associated with antidepressant therapy outcome in male but not in female depressed patients. Psychoneuroendocrinology 2009, 34, 99–109. [Google Scholar] [CrossRef]
- De Kloet, E.R.; DeRijk, R.H.; Meijer, O.C. Therapy insight: Is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 168–179. [Google Scholar] [CrossRef]
- Lam, V.Y.; Raineki, C.; Wang, L.Y.; Chiu, M.; Lee, G.; Ellis, L.; Weinberg, J. Role of corticosterone in anxiety-and depressive-like behavior and HPA regulation following prenatal alcohol exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 1–15. [Google Scholar] [CrossRef]
- Morilak, D.A.; Frazer, A. Antidepressants and brain monoaminergic systems: A dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol. 2004, 7, 193–218. [Google Scholar] [CrossRef]
- Cota, D. The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J. Neuroendocrinol. 2008, 20, 35–38. [Google Scholar] [CrossRef]
- Gobbi, G.; Bambico, F.R.; Mangieri, R.; Bortolato, M.; Campolongo, P.; Solinas, M.; Tontini, A. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. USA 2005, 102, 18620–18625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.N.; Gorzalka, B.B. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur. Neuropsychopharmacol. 2005, 15, 593–599. [Google Scholar] [CrossRef]
- Witkin, J.M.; Tzavara, E.T.; Nomikos, G.G. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav. Pharmacol. 2005, 16, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Gorzalka, B.B. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol. Disord. Drug Targets 2009, 8, 451–458. [Google Scholar] [CrossRef]
- Viveros, M.P.; Marco, E.M.; File, S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005, 81, 331–342. [Google Scholar] [CrossRef]
- Lev-Ran, S.; Roerecke, M.; Le Foll, B.; George, T.P.; McKenzie, K.; Rehm, J. The association between cannabis use and depression: A systematic review and meta-analysis of longitudinal studies. Psychol. Med. 2014, 44, 797. [Google Scholar] [CrossRef]
- Feingold, D.; Weiser, M.; Rehm, J.; Lev-Ran, S. The association between cannabis use and mood disorders: A longitudinal study. J. Affect. Disord. 2015, 172, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, M.; Braley, G.; Pittman, B.; Cooper, T.; Perry, E.; Krystal, J.; D’Souza, D.C. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology 2009, 203, 737. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, S.E.; Strawn, J.R.; Poweleit, E.A.; Sarangdhar, M.; Ramsey, L.B. The impact of marijuana on antidepressant treatment in adolescents: Clinical and pharmacologic considerations. J. Pers. Med. 2021, 11, 615. [Google Scholar] [CrossRef]
- Dinan, T.G. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996, 58, 1683–1694. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect. Disord. 1998, 51, 215–235. [Google Scholar] [CrossRef]
- Leonard, B.E. The HPA and immune axes in stress: The involvement of the serotonergic system. Eur. Psychiatry 2005, 20, S302–S306. [Google Scholar] [CrossRef]
- Stahl, S. 5HT1A receptors and pharmacotherapy. Is serotonin receptor down-regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol. Bull. 1994, 30, 39–43. [Google Scholar]
- Blier, P. Pharmacology of rapid-onset antidepressant treatment strategies. J. Clin. Psychiatry 2001, 62, 12. [Google Scholar] [PubMed]
- Cryan, J.F.; Leonard, B.E. 5-HT1A and beyond: The role of serotonin and its receptors in depression and the antidepressant response. Hum. Psychopharmacol. 2000, 15, 113–135. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Chuzi, S.E.; Sousa, J.L.; Fava, M. 5HT1A-mediated stimulation of cortisol release in major depression: Use of non-invasive cortisol measurements to predict clinical response. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Braida, D.; Limonta, V.; Malabarba, L.; Zani, A.; Sala, M. 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats. Eur. J. Pharmacol. 2007, 555, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Malone, D.T.; Taylor, D.A. Involvement of somatodendritic 5-HT1A receptors in Δ9-tetrahydrocannabinol-induced hypothermia in the rat. Pharmacol. Biochem. Behav. 2001, 69, 595–601. [Google Scholar] [CrossRef]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology 2008, 199, 223. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Ogata, A.; Hazekawa, M.; Liu, A.X.; Iwasaki, K. Repeated treatment with cannabidiol but not Δ9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance. Neuropharmacology 2007, 52, 1079–1087. [Google Scholar] [CrossRef]
- Resstel, L.B.; Joca, S.R.; Moreira, F.A.; Corrêa, F.M.; Guimarães, F.S. Effects of cannabidiol and diazepam on behavioral and cardiovascular responses induced by contextual conditioned fear in rats. Behav. Brain Res. 2006, 172, 294–298. [Google Scholar] [CrossRef]
- Castrén, E.; Rantamäki, T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev. Neurobiol. 2010, 70, 289–297. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkholm, C.; Monteggia, L.M. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Butovsky, E.; Juknat, A.; Goncharov, I.; Elbaz, J.; Eilam, R.; Zangen, A.; Vogel, Z. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Δ9-tetrahydrocannabinol. J. Neurochem. 2005, 93, 802–811. [Google Scholar] [CrossRef]
- Derkinderen, P.; Valjent, E.; Toutant, M.; Corvol, J.C.; Enslen, H.; Ledent, C.; Girault, J.A. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 2003, 23, 2371–2382. [Google Scholar] [CrossRef] [PubMed]
- Maj, P.F.; Collu, M.; Fadda, P.; Cattaneo, A.; Racagni, G.; Riva, M.A. Long-term reduction of brain-derived neurotrophic factor levels and signaling impairment following prenatal treatment with the cannabinoid receptor 1 receptor agonist (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinyl-methyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone. Eur. J. Neurosci. 2007, 25, 3305–3311. [Google Scholar] [CrossRef]
- Rubino, T.; Viganò, D.; Premoli, F.; Castiglioni, C.; Bianchessi, S.; Zippel, R.; Parolaro, D. Changes in the expression of G protein-coupled receptor kinases and β-arrestins in mouse brain during cannabinoid tolerance. Mol. Neurobiol. 2006, 33, 199–213. [Google Scholar] [CrossRef]
- Giacoppo, S.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Target regulation of PI3K/Akt/mTOR pathway by cannabidiol in treatment of experimental multiple sclerosis. Fitoterapia 2017, 116, 77–84. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.C.; Pittman, B.; Perry, E.; Simen, A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology 2009, 202, 569. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.A.; Neufeld, K.A.M. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Sanchez-Labraca, N.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200. [Google Scholar] [CrossRef]
- Macedo, D.; Chaves Filho, A.J.M.; de Sousa, C.N.S.; Quevedo, J.; Barichello, T.; Júnior, H.V.N.; de Lucena, D.F. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef]
- Yang, C.; Qu, Y.; Fujita, Y.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Possible role of the gut microbiota–brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl. Psychiatry 2017, 7, 1294. [Google Scholar] [CrossRef]
- Neufeld, K.A.M.; Bienenstock, J.; Bharwani, A.; Champagne-Jorgensen, K.; Mao, Y.; West, C.; Forsythe, P. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci. Rep. 2019, 9, 14290. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Wiley, J.W. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Zoppi, S.; Madrigal, J.L.; Pérez-Nievas, B.G.; Marín-Jiménez, I.; Caso, J.R.; Alou, L.; Menchén, L. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G565–G571. [Google Scholar] [CrossRef]
- Sherwin, E.; Dinan, T.G.; Cryan, J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2017, 1420, 5–25. [Google Scholar] [CrossRef]
- Karoly, H.C.; Mueller, R.L.; Bidwell, L.C.; Hutchison, K.E. Cannabinoids and the Microbiota–Gut–Brain Axis: Emerging Effects of Cannabidiol and Potential Applications to Alcohol Use Disorders. Alcohol Clin. Exp. Res. 2020, 44, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Pettit, D.A.D. Drugs and immunity: Cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmunol. 1998, 83, 116–123. [Google Scholar] [CrossRef]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the immune system: Potential for the treatment of inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Klein, T.W.; Friedman, H.; Specter, S. Marijuana, immunity and infection. J. Neuroimmunol. 1998, 83, 102–115. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Parolaro, D. Cannabinoids, immune system and cytokine network. Curr. Pharm. Des. 2006, 12, 3135–3146. [Google Scholar] [CrossRef] [Green Version]
- Roth, M.D.; Baldwin, G.C.; Tashkin, D.P. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem. Phys. Lipids 2002, 1211, 229–239. [Google Scholar] [CrossRef]
- Eyre, H.; Baune, B.T. Neuroplastic changes in depression: A role for the immune system. Psychoneuroendocrinology 2012, 37, 1397–1416. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B. Stress, depression and the activation of the immune system. World J. Biol. Psychiatry 2000, 1, 17–25. [Google Scholar] [CrossRef]
- Leonard, B.E. The immune system, depression and the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 767–780. [Google Scholar] [CrossRef]
- Leonard, B.E.; Song, C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol. Biochem. Behav. 1996, 54, 299–303. [Google Scholar] [CrossRef]
- Linn, B.S.; Linn, M.W.; Jensen, J. Anxiety and immune responsiveness. Psychol. Rep. 1981, 49, 969–970. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Ribeiro, A.C.; Pfaff, D.W.; Silver, R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 18053–18057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H. Neuroendocrine and immune system interactions in stress and depression. Psychiatr. Clin. N. Am. 1998, 21, 443–463. [Google Scholar] [CrossRef]
- Stein, M.; Keller, S.E.; Schleifer, S.J. Immune system: Relationship to anxiety disorders. Psychiatr. Clin. N. Am. 1988, 11, 349–360. [Google Scholar] [CrossRef]
- Fischer, J.A.; Clavarino, A.M.; Plotnikova, M.; Najman, J.M. Cannabis use and quality of life of adolescents and young adults: Findings from an Australian birth cohort. J. Psychoact. Drugs 2015, 47, 107–116. [Google Scholar] [CrossRef]
- Volkow, N.D.; Hampson, A.J.; Baler, R.D. Don’t worry, be happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 285–308. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, L.; Hall, W.; Lynskey, M. Alcohol, cannabis and tobacco use among Australians: A comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction 2001, 96, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). World Drug Report, United Nations Office on Drugs and Crime. 2015. Available online: https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf (accessed on 21 October 2020).
- Leung, L. Cannabis and its derivatives: Review of medical use. J. Am. Board Fam. Med. 2011, 24, 452–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotan, I.; Treves, T.A.; Roditi, Y.; Djaldetti, R. Cannabis (medical marijuana) treatment for motor and non–motor symptoms of Parkinson disease: An open-label observational study. Clin. Neuropharmacol. 2014, 37, 41–44. [Google Scholar] [CrossRef]
- Woolridge, E.; Barton, S.; Samuel, J.; Osorio, J.; Dougherty, A.; Holdcroft, A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manag. 2005, 29, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Helzer, J.E. Syndromes of Drug Abuse and Dependence. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study; The Free Press: New York, NY, USA, 1991; pp. 116–154. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub.: Washington, DC, USA, 2013. [Google Scholar]
- Hollister, L.E.; Kanter, S.L. Laboratory verification of “heavy” and “light” users of cannabis. Drug Alcohol Depend. 1980, 5, 151–152. [Google Scholar] [CrossRef]
- Tait, R.J.; Mackinnon, A.; Christensen, H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction 2011, 106, 2195–2203. [Google Scholar] [CrossRef]
- Taylor, M.; Lees, R.; Henderson, G.; Lingford-Hughes, A.; Macleod, J.; Sullivan, J.; Hickman, M. Comparison of cannabinoids in hair with self-reported cannabis consumption in heavy, light and non-cannabis users. Drug Alcohol Rev. 2017, 36, 220–226. [Google Scholar] [CrossRef] [Green Version]
| Drug Name | Active Principle | Main Targets | Mechanisms of Action | Main Side Effects |
|---|---|---|---|---|
| Tricyclic antidepressants (TCAs) | The chemical structure of a TCA consists of a three-ringed structure with an attached secondary or tertiary amine | Serotonin, norepinephrine, and acetyl choline | Act on approximately five different neurotransmitter pathways to achieve their effects: block the reuptake of Serotonin and Norepinephrine in presynaptic terminals which leads to increased concentration of these neurotransmitters in the synaptic cleft. act as competitive antagonists on post-synaptic alpha cholinergic (alpha1 and alpha2), muscarinic, and histaminergic receptors (H1). | Constipation, dizziness, blurred vision, confusion, urinary retention, and tachycardia |
| Monoamine oxidase inhibitors (MAOIs) | Blocking monoamine oxidase | Norepinephrine and serotonin | Breaks down different types of neurotransmitters from the brain: norepinephrine, serotonin, dopamine, and tyramine. MAOIs inhibit the breakdown of these neurotransmitters thus, increasing their levels. | Dry mouth, nausea, diarrhea, constipation, drowsiness, insomnia, dizziness, and/or lightheadedness |
| Selective Serotonin Reuptake inhibitors (SSRIs) | Inhibit the reuptake of serotonin | Serotonin | Block the reuptake of serotonin into the presynaptic nerve terminal via the serotonin uptake site, thus increasing the synaptic concentration of serotonin. | Flatulence, somnolence, memory impairment, decreased concentration, yawning, fatigue, dry mouth, weight gain, light headedness, adverse sexual effects, and sweating |
| Selective Norepinephrine | Inhibit reuptake of norepinephrine | Norepinephrine | Block the reuptake of norepinephrine into the presynaptic nerve terminal via somatodendritic 2a-adrenoceptors, thus increasing the synaptic concentration of norepinephrine | Dry mouth, constipation, insomnia, increased sweating, tachycardia, vertigo, urinary hesitancy and/or retention, and impotence |
| Dual Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs) | Inhibit the uptake of serotonin and norepinephrine | Norepinephrine and serotonin | Bind to serotonin and norepinephrine transporters to selectively inhibit the reuptake of these neurotransmitters from the synaptic cleft | Nausea, hypertension, somnolence, dizziness, and dry mouth |
| Norepinephrine/Dopamine Reuptake Inhibitor (NDRIs) | Inhibit the uptake of dopamine and norepinephrine | Norepinephrine and dopamine | Block the reuptake of norepinephrine and dopamine into the presynaptic nerve terminal thus increasing the synaptic concentration of norepinephrine and dopamine | Fatigue, sleepiness, and somnolence |
| Noradrenergic and Specific Serotonergic antidepressants (NaSSAs) | Enhance serotonergic and noradrenergic neurotransmission | Norepinephrine and serotonin | Potent antagonism of central α2-adrenergic autoreceptors and heteroreceptors and antagonism of both 5-HT2 and 5-HT, receptors with low affinity for muscarinic, cholinergic, and dopaminergic receptors | Somnolence, increased appetite, weight gain, dry mouth, constipation, and dizziness |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hen-Shoval, D.; Weller, A.; Weizman, A.; Shoval, G. Examining the Use of Antidepressants for Adolescents with Depression/Anxiety Who Regularly Use Cannabis: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 523. https://doi.org/10.3390/ijerph19010523
Hen-Shoval D, Weller A, Weizman A, Shoval G. Examining the Use of Antidepressants for Adolescents with Depression/Anxiety Who Regularly Use Cannabis: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(1):523. https://doi.org/10.3390/ijerph19010523
Chicago/Turabian StyleHen-Shoval, Danielle, Aron Weller, Abraham Weizman, and Gal Shoval. 2022. "Examining the Use of Antidepressants for Adolescents with Depression/Anxiety Who Regularly Use Cannabis: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 1: 523. https://doi.org/10.3390/ijerph19010523
APA StyleHen-Shoval, D., Weller, A., Weizman, A., & Shoval, G. (2022). Examining the Use of Antidepressants for Adolescents with Depression/Anxiety Who Regularly Use Cannabis: A Narrative Review. International Journal of Environmental Research and Public Health, 19(1), 523. https://doi.org/10.3390/ijerph19010523







