Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
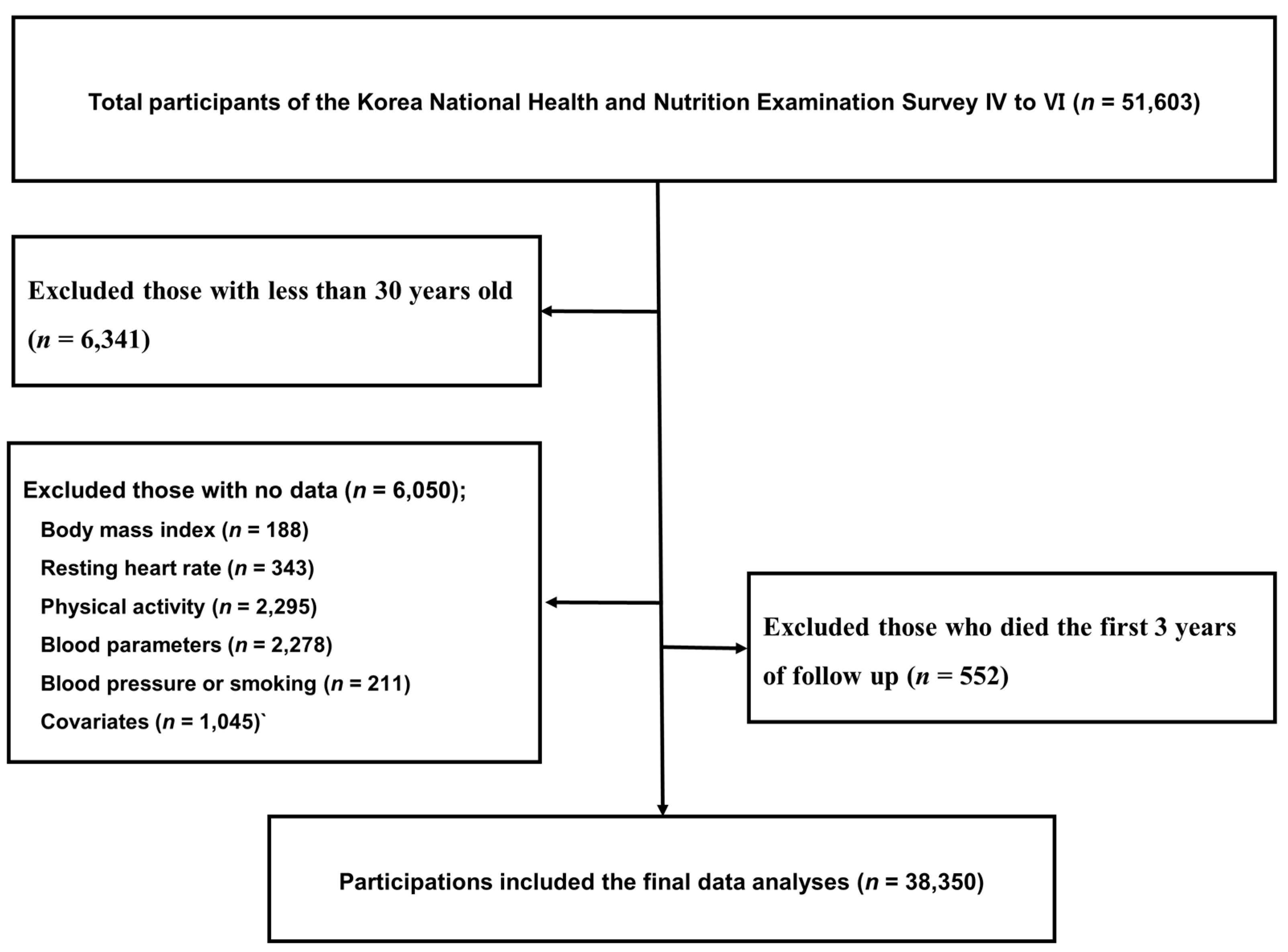
2.1. Study Population and Database Information
2.2. Determination of Anthropometrics and CVD Risk Factors
2.3. Determination of the 10-Year FRS and eCRF
2.4. Determination of Mortality
2.5. Determination of Covariates
2.6. Statistics
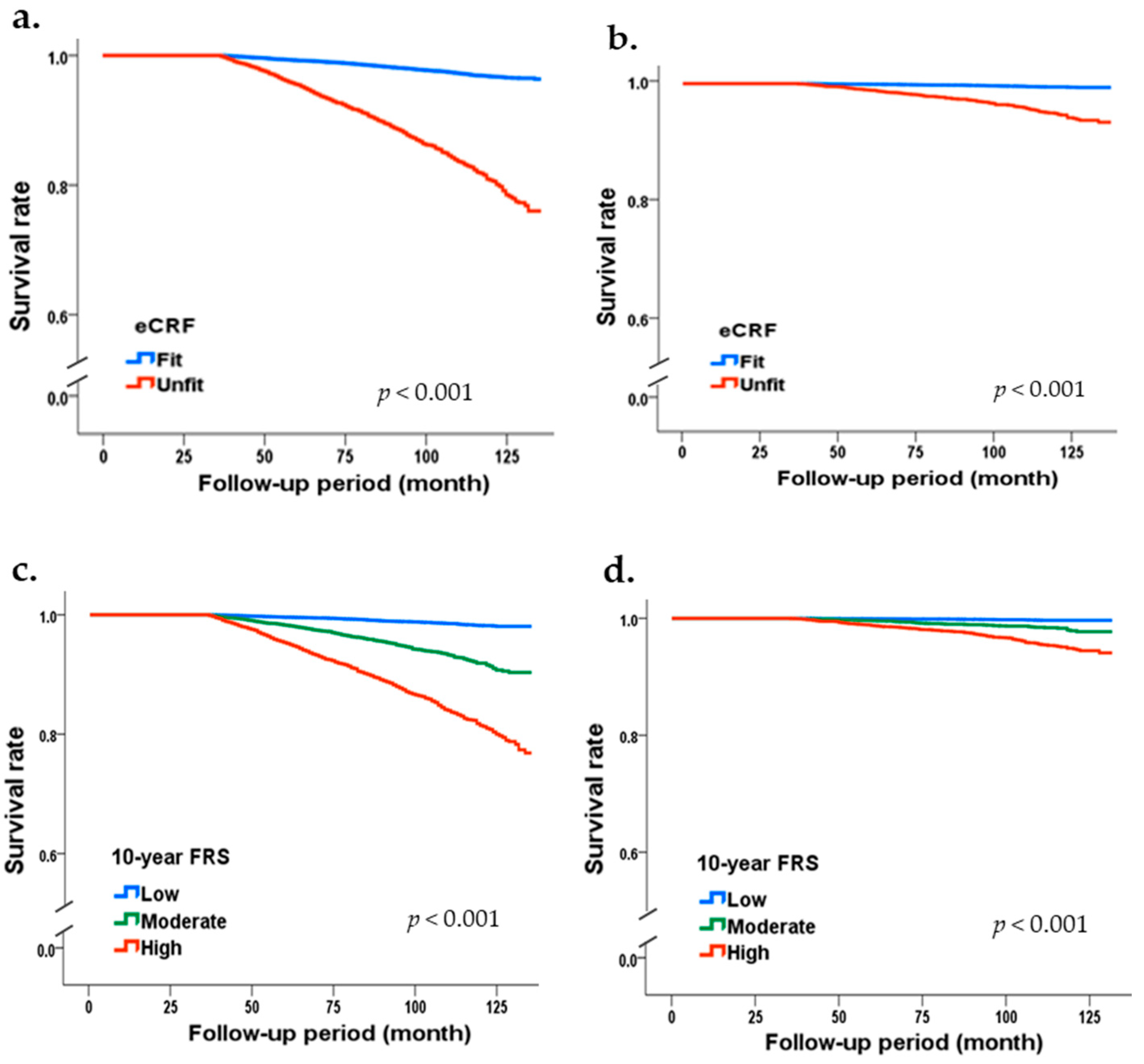
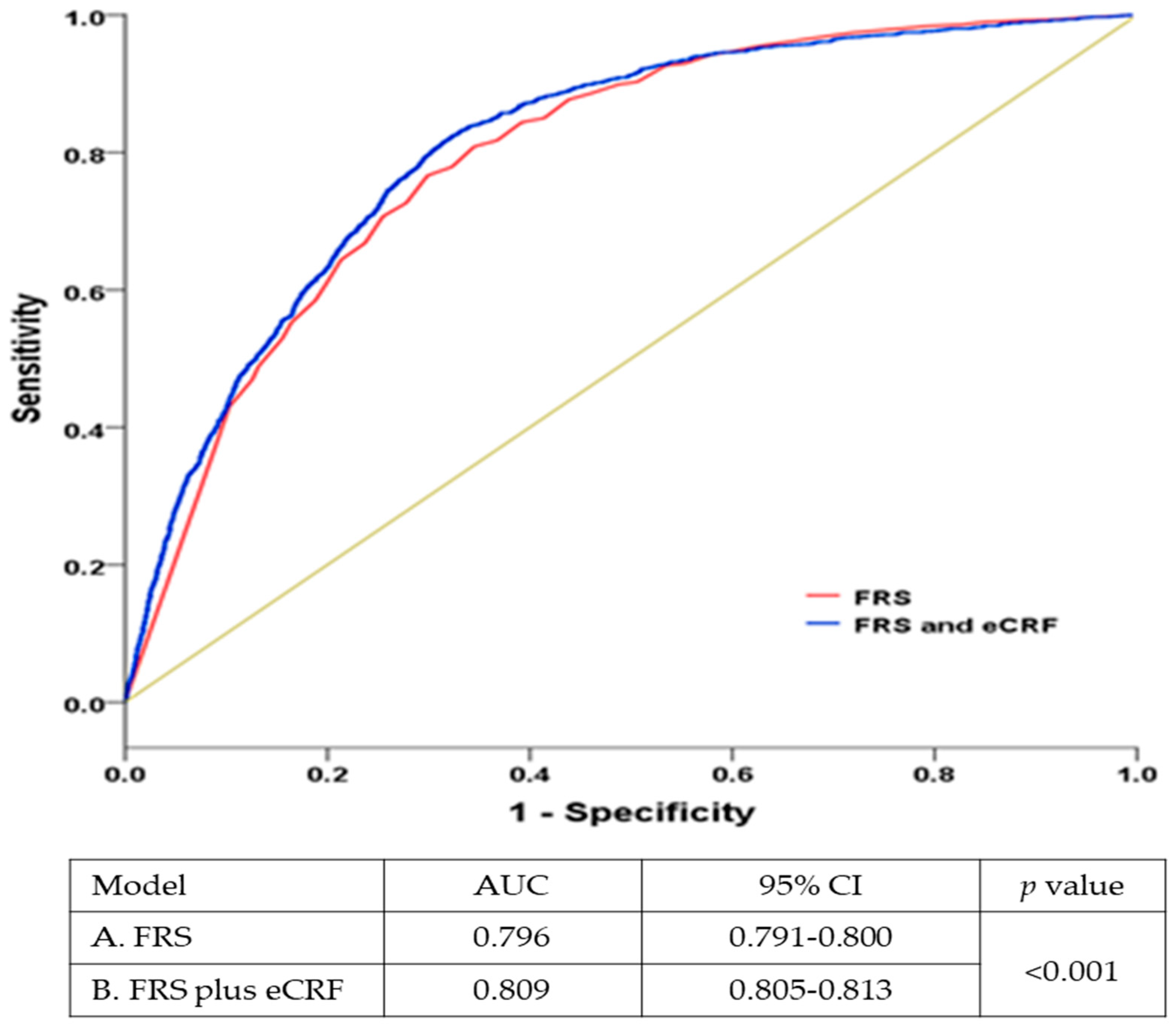
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Fact Sheets; Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Barlow, C.E.; Defina, L.F.; Radford, N.B.; Berry, J.D.; Cooper, K.H.; Haskell, W.L.; Jones, L.W.; Lakoski, S.G. Cardiorespiratory fitness and long-term survival in “low-risk” adults. J. Am. Heart Assoc. 2021, 1, e001354. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, S.M.; Lee, G.; Choi, S.; Kim, K.; Jeong, S.M.; Son, J.S.; Yun, J.M.; Kim, S.G.; Hwang, S.S.; et al. Association of early-onset diabetes, prediabetes and early glycaemic recovery with the risk of all-cause and cardiovascular mortality. Diabetologia 2020, 63, 2305–2314. [Google Scholar] [CrossRef]
- Li, T.C.; Li, C.I.; Liu, C.S.; Lin, W.Y.; Lin, C.H.; Yang, S.Y.; Lin, C.C. Derivation and validation of 10-year all-cause and cardiovascular disease mortality prediction model for middle-aged and elderly community-dwelling adults in Taiwan. PLoS ONE 2020, 15, e0239063. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Bitton, A.; Gaziano, T. The Framingham heart study’s impact on global risk assessment. Prog. Cardiovasc. Dis. 2020, 53, 68–78. [Google Scholar] [CrossRef]
- Collins, G.; Altman, D. An independent and external validation of QRISK2 cardiovascular disease risk score: A prospective open cohort study. BMJ 2010, 340, c2442. [Google Scholar] [CrossRef] [PubMed]
- Äijö, M.; Kauppinen, M.; Kujala, U.M.; Parkatti, T. Physical activity, fitness, and all-cause mortality: An 18-year follow-up among old people. J. Sport Health Sci. 2016, 5, 437–442. [Google Scholar] [CrossRef]
- Min, C.; Yoo, D.M.; Wee, J.H.; Lee, H.J.; Byun, S.H.; Choi, H.G. Mortality and cause of death in physical activity and insufficient physical activity participants: A longitudinal follow-up study using a national health screening cohort. BMC Public Health 2020, 20, 1469. [Google Scholar] [CrossRef]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.L.; Kaminsky, L.A. Cardiorespiratory fitness and mortality in healthy men and women. J. Am. Coll. Cardiol. 2018, 72, 2283–2292. [Google Scholar] [CrossRef]
- Cao, C.; Yang, L.; Cade, W.T.; Racette, S.B.; Park, Y.; Cao, Y.; Friedenreich, C.M.; Hamer, M.; Stamatakis, E.; Smith, L. Cardiorespiratory fitness is associated with early death among healthy young and middle-aged baby boomers and generation Xers. Am. J. Med. 2020, 133, 961–968.e3. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Bornstein, D.B.; Sui, X.; Hooker, S.P.; Church, T.S.; Lee, C.D.; Lee, D.C.; Blair, S.N. The impact of combined health factors on cardiovascular disease mortality. Am. Heart J. 2010, 160, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Cardiorespiratory fitness as predictor of cancer mortality: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 272–278. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, Y.J.; Rhee, C.W.; Park, B.J.; Kim, D.H.; Bae, J.M.; Shin, M.H.; Lee, M.S.; Li, Z.M.; Ahn, Y.O. Cardiovascular health metrics and all-cause and cardiovascular disease mortality among middle-aged men in Korea: The Seoul male cohort study. J. Prev. Med. Public Health 2013, 46, 319–328. [Google Scholar] [CrossRef]
- Yun, J.E.; Won, S.; Kimm, H.; Jee, S.H. Effects of a combined lifestyle score on 10-year mortality in Korean men and women: A prospective cohort study. BMC Public Health 2012, 12, 673. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Chung, S.Y.; Chang, Y.; Kim, K. Physical activity and physical fitness as predictors of all-cause mortality in Korean men. J. Korean Med. Sci. 2009, 24, 13–19. [Google Scholar] [CrossRef][Green Version]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of Korean version of international physical activity questionnaire (IPAQ) short form. J. Korean Acad. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- D’Agostino, R.B.S.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Jurca, R.; Jackson, A.S.; LaMonte, M.J.; Morrow, J.R., Jr.; Blair, S.N.; Wareham, N.J.; Haskell, W.L.; van Mechelen, W.; Church, T.S.; Jakicic, J.M.; et al. Assessing cardiorespiratory fitness without performing exercise testing. Am. J. Prev. Med. 2005, 29, 185–193. [Google Scholar] [CrossRef]
- Gupta, S.; Rohatgi, A.; Ayers, C.R.; Willis, B.L.; Haskell, W.L.; Khera, A.; Drazner, M.H.; de Lemos, J.A.; Berry, J.D. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation 2011, 123, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Imboden, M.T.; Harber, M.P.; Whaley, M.H.; Finch, W.H.; Bishop, D.A.; Fleenor, B.S.; Kaminsky, L.A. The influence of change in cardiorespiratory fitness with short-term exercise training on mortality risk from the Ball State Adults Fitness Longitudinal Lifestyle Study (BALL ST). Mayo Clin. Proc. 2019, 94, 1406–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhou, J.; Ernstsen, L.; Lavie, C.J.; Hooker, S.P.; Sui, X. Nonexercise estimated cardiorespiratory fitness and mortality due to all causes and cardiovascular disease: The NHANES III study. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Zhang, J.; Zhang, Y.; Ernstsen, L.; Lavie, C.J.; Hooker, S.P.; Chen, Y.; Sui, X. Nonexercise estimated cardiorespiratory fitness and all-cancer mortality: The NHANES III Study. Mayo Clin. Proc. 2018, 93, 848–856. [Google Scholar] [CrossRef]
- Gander, J.C.; Sui, X.; Hébert, J.R.; Lavie, C.J.; Hazlett, L.J.; Cai, B.; Blair, S.N. Addition of estimated cardiorespiratory fitness to the clinical assessment of 10-year coronary heart disease risk in asymptomatic men. Prev. Med. Rep. 2017, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, I.; Kang, H. Cardiorespiratory Fitness without Exercise Testing Can Predict All-Cause Mortality Risk in a Representative Sample of Korean Older Adults. Int. J. Environ. Res. Public Health 2019, 16, 1633. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Nead, K.T.; Chang, P.; Abella, J.; Kokkinos, P.; Leeper, N.J. Improved reclassification of mortality risk by assessment of physical activity in patients referred for exercise testing. Am. J. Med. 2015, 128, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, M.; Tagawa, M.; Matsui, M.; Eriguchi, M.; Samejima, K.I.; Iseki, K.; Iseki, C.; Asahi, K.; Yamagata, K.; Konta, T.; et al. A prediction model with lifestyle in addition to previously known risk factors improves its predictive ability for cardiovascular death. Sci. Rep. 2019, 9, 12953. [Google Scholar] [CrossRef]
- Lee, W.J.; Peng, L.N.; Chiou, S.T.; Chen, L.K. Physical health indicators improve prediction of cardiovascular and all-cause mortality among middle-aged and older people: A national population-based study. Sci. Rep. 2017, 7, 40427. [Google Scholar] [CrossRef]
- Lau, K.; Malik, A.; Foroutan, F.; Buchan, T.A.; Daza, J.F.; Sekercioglu, N.; Orchanian-Cheff, A.; Alba, A.C. Resting heart rate as an important predictor of mortality and morbidity in ambulatory patients with heart failure: A systematic review and meta-analysis. J. Card. Fail. 2021, 27, 349–363. [Google Scholar] [CrossRef]
- Seccareccia, F.; Pannozzo, F.; Dima, F.; Minoprio, A.; Menditto, A.; Lo Noce, C.; Giampaoli, S.; Malattie Cardiovascolari Aterosclerotiche Istituto Superiore di Sanita Project. Heart rate as a predictor of mortality: The MATISS project. Am. J. Public Health 2001, 91, 1258–1263. [Google Scholar] [CrossRef]
- Jensen, M.T.; Suadicani, P.; Hein, H.O.; Gyntelberg, F. Elevated resting heart rate, physical fitness and all-cause mortality: A 16-year follow-up in the Copenhagen Male Study. Heart 2013, 99, 882–887. [Google Scholar] [CrossRef]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four countries. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Yang, Y.; Dugué, P.A.; Lynch, B.M.; Hodge, A.M.; Karahalios, A.; MacInnis, R.J.; Milne, R.L.; Giles, G.G.; English, D.R. Trajectories of body mass index in adulthood and all-cause and cause-specific mortality in the Melbourne collaborative cohort study. BMJ Open 2019, 9, e030078. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Agostinis-Sobrinho, C.A.; Ruiz, J.R.; Moreira, C.; Abreu, S.; Luís, L.; Oliveira-Santos, J.; Mota, J.; Santos, R. Cardiorespiratory fitness and inflammatory profile on cardiometabolic risk in adolescents from the LabMed Physical Activity Study. Eur. J. Appl. Physiol. 2017, 117, 2271–2279. [Google Scholar] [CrossRef]
- Oliveira, C.; Silveira, E.A.; Rosa, L.; Santos, A.; Rodrigues, A.P.; Mendonça, C.; Silva, L.; Gentil, P.; Rebelo, A.C. Risk factors associated with cardiac autonomic modulation in obese individuals. J. Obes. 2020, 7185249. [Google Scholar] [CrossRef]
- Haufe, S.; Engeli, S.; Budziarek, P.; Utz, W.; Schulz-Menger, J.; Hermsdorf, M.; Wiesner, S.; Otto, C.; Haas, V.; de Greiff, A.; et al. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes 2001, 59, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Breneman, C.B.; Polinski, K.; Sarzynski, M.A.; Lavie, C.J.; Kokkinos, P.F.; Ahmed, A.; Sui, X. The impact of cardiorespiratory fitness levels on the risk of developing atherogenic dyslipidemia. Am. J. Med. 2016, 129, 1060–1066. [Google Scholar] [CrossRef]
- González-Gil, E.M.; Santaliestra-Pasías, A.M.; Buck, C.; Gracia-Marco, L.; Lauriam, F.; Pala, V.; Molnar, D.; Veidebaum, T.; Iacoviello, L.; Tornaritis, M.; et al. Improving cardiorespiratory fitness protects against inflammation in children: The IDEFICS study. Pediatr. Res. 2021. [Google Scholar] [CrossRef]
- Agostinis-Sobrinho, C.; Ruiz, J.R.; Moreira, C.; Abreu, S.; Lopes, L.; Oliveira-Santos, J.; Mota, J.; Santos, R. Cardiorespiratory fitness and blood pressure: A longitudinal analysis. J. Pediatr. 2018, 192, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Röhling, M.; Strom, A.; Bönhof, G.J.; Roden, M.; Ziegler, D. Cardiorespiratory Fitness and Cardiac Autonomic Function in Diabetes. Curr. Diab. Rep. 2017, 17, 125. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.S.; Krogh-Madsen, R.; Petersen, G.L.; Hansen, Å.M.; Pedersen, B.K.; Lund, R.; Bruunsgaard, H. Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. PLoS ONE 2018, 13, e0194991. [Google Scholar] [CrossRef]
- Shigdel, R.; Dalen, H.; Sui, X.; Lavie, C.J.; Wisløff, U.; Ernstsen, L. Cardiorespiratory fitness and the risk of first acute myocardial infarction: The HUNT study. J. Am. Heart Assoc. 2019, 8, e010293. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 38,350) | Men (n = 16,505) | Women (n = 21,845) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | 52.5 ± 13.7 | 52.7 ± 13.6 | 52.4 ± 13.8 | 0.027 | |
| BMI (kg/m2) | 23.9 ± 3.3 | 24.2 ± 3.1 | 23.7 ± 3.4 | <0.001 | |
| Income (10,000/won) | 329.2 ± 271.2 | 338.2 ± 270.4 | 322.3 ± 271.7 | <0.001 | |
| Educational background, n (%) | <0.001 | ||||
| Elementary or less | 10,460 (27.3) | 3155 (19.1) | 7305 (33.4) | ||
| Middle | 4670 (12.2) | 2132 (12.9) | 2538 (11.6) | ||
| High | 12,237 (31.9) | 5407 (32.8) | 6830 (31.3) | ||
| College or higher | 10,983 (28.6) | 5811 (35.2) | 5172 (23.7) | ||
| Marital status, n (%) | <0.001 | ||||
| Married | 31,121 (81.1) | 14,405 (87.3) | 16,716 (76.5) | ||
| Widowed/divorced | 5383 (14.0) | 971 (5.9) | 4412 (20.2) | ||
| Unmarried | 1846 (4.9) | 1129 (6.8) | 717 (3.3) | ||
| Residence area, n (%) | <0.001 | ||||
| Urban | 29,550 (77.1) | 12,573 (76.2) | 16,977 (77.7) | ||
| Rural | 8800 (22.9) | 3932 (23.8) | 4868 (22.3) | ||
| CVD risk factors | |||||
| HDL-C (mg/dL) | 49.1 ± 11.7 | 46.1 ± 10.9 | 51.4 ± 11.8 | <0.001 | |
| LDL-C (mg/dL) | 114.1 ± 33.6 | 110.4 ± 3.9 | 117.0 ± 32.3 | <0.001 | |
| TC (mg/dL) | 190.9 ± 35.9 | 189.0 ± 35.4 | 192.4 ± 36.3 | <0.001 | |
| SBP (mmHg) | 119.8 ± 17.3 | 122.2 ± 16.0 | 118.1 ± 18.1 | <0.001 | |
| SBP treat, n (%) | 8209 (21.4) | 3486 (21.1) | 4723 (21.6) | 0.238 | |
| Smoking, n (%) | 15,004 (39.1) | 13,251 (80.3) | 1753 (8.0) | <0.001 | |
| Diabetes, n (%) | 2885 (7.5) | 1530 (9.3) | 1352 (6.2) | <0.001 | |
| Resting heart rate (beats/min) | 69.3 ± 9.7 | 68.5 ± 10.1 | 69.9 ± 9.3 | <0.001 | |
| Physically inactive, n (%) | 25,146 (65.6) | 9741 (59.0) | 15,405 (70.5) | <0.001 | |
| Follow-up time (years) | 7.3 ± 2.4 | 7.3 ± 2.4 | 7.3 ± 2.4 | 0.241 | |
| Death, n | 1474 | 875 | 599 | <0.001 | |
| Person/years (PY) | 280,315 | 120,368 | 159,946 | <0.001 | |
| Death rate per 1000 PY | 5.3 | 7.3 | 3.8 | <0.001 | |
| eCRF | p-Value | |||
|---|---|---|---|---|
| Fit (n = 29,339) | Unfit n = 9011) | |||
| eCRF (METs) | 10.1 ± 2.1 | 6.4 ± 1.7 | <0.001 | |
| Women, n (%) | 16,692 (56.9) | 5153 (57.2) | 0.624 | |
| Age (years) | 47.7 ± 11.4 | 68.1 ± 7.6 | <0.001 | |
| BMI (kg/m2) | 23.6 ± 3.1 | 25.0 ± 3.4 | <0.001 | |
| Income (10,000/won) | 367.6 ± 271.2 | 203.9 ± 230.4 | <0.001 | |
| Education, n (%) | <0.001 | |||
| Elementary or less | 4861 (16.6) | 5599 (62.1) | ||
| Middle | 3394 (11.6) | 1276 (14.2) | ||
| High | 10,771 (36.7) | 1466 (16.3) | ||
| College or higher | 10,313 (35.1) | 670 (7.4) | ||
| Marital status, n (%) | <0.001 | |||
| Married | 24,841 (84.7) | 6280 (69.7) | ||
| Widowed/divorced | 2718 (9.3) | 2665 (29.6) | ||
| Unmarried | 1780 (6.0) | 66 (0.7) | ||
| Residence area, n (%) | <0.001 | |||
| Urban | 23,331 (79.5) | 6219 (69.0) | ||
| Rural | 6008 (20.5) | 2792 (31.0) | ||
| CVD risk factors | ||||
| HDL-C (mg/dL) | 49.9 ± 11.8 | 46.6 ± 11.1 | <0.001 | |
| LDL-C (mg/dL) | 113.8 ± 33.0 | 115.3 ± 35.6 | <0.001 | |
| TC (mg/dL) | 190.6 ± 35.2 | 192.0 ± 38.4 | 0.001 | |
| SBP (mmHg) | 117.1 ± 16.4 | 128.9 ± 17.2 | <0.001 | |
| SBP treat, n (%) | 3940 (13.4) | 4269 (47.4) | <0.001 | |
| Smoking, n (%) | 11,477 (39.1) | 3527 (39.1) | 0.970 | |
| Diabetes, n (%) | 1611 (5.5) | 1274 (14.1) | <0.001 | |
| 10-year FRS (%) | 8.3 ± 8.7 | 19.4 ± 9.4 | <0.001 | |
| N | Number of Deaths | Death Rate a | HR (95% CI) for All-Cause Mortality b | HR (95% CI) for All-Cause Mortality c | HR (95% CI) for CVD Mortality b | HR (95% CI) for CVD Mortality c | ||
|---|---|---|---|---|---|---|---|---|
| eCRF | ||||||||
| Fit | 29,339 | 562 | 2.6 | 1 | 1 | 1 | 1 | |
| Unfit | 9011 | 912 | 15.1 | 1.35 (1.20–1.52) | 1.31 (1.16–1.47) | 1.62 (1.24–2.12) | 1.57 (1.20–2.05) | |
| p-value | <0.001 | <0.001 | <0.001 | 0.001 | ||||
| 10-year FRS | ||||||||
| Low | 22,653 | 230 | 1.4 | 1 | 1 | 1 | 1 | |
| Moderate | 7443 | 344 | 6.4 | 1.14 (0.95–1.36) | 1.08 (0.91–1.30) | 1.31 (0.87–1.96) | 1.25 (0.83–1.87) | |
| High | 8254 | 900 | 15.5 | 1.31 (1.07–1.60) | 1.26 (1.03–1.54) | 2.16 (1.41–3.30) | 2.07 (1.36–3.16) | |
| p-value for trend | 0.025 | 0.046 | <0.001 | <0.001 | ||||
| HR (95% CI) for All-Cause Mortality | HR (95% CI) for CVD Mortality | ||||||
|---|---|---|---|---|---|---|---|
| Total | Number of Deaths | Death Rate a | Model 1 | Model 2 | Model 1 | Model 2 | |
| Fit | |||||||
| Low FRS | 20,635 | 169 | 1.1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Moderate FRS | 4586 | 126 | 3.6 | 0.89 (0.67–1.14) | 0.84 (0.66–1.07) | 1.05 (0.58–1.90) | 0.98 (0.54–1.77) |
| High FRS | 3556 | 267 | 9.4 | 1.03 (0.80–1.31) | 0.98 (0.77–1.26) | 1.62 (0.92–2.86) | 1.53 (0.87–2.69) |
| Unfit | |||||||
| Low FRS | 1788 | 61 | 4.9 | 0.84 (0.61–1.15) | 0.79 (0.58–1.08) | 1.07 (0.54–2.09) | 0.98 (0.50–1.93) |
| Moderate FRS | 2513 | 218 | 11.9 | 1.32 (1.04–1.67) | 1.21 (0.95–1.54) | 1.70 (0.98–2.93) | 1.55 (0.90–2.67) |
| High FRS | 3798 | 633 | 21.4 | 1.44 (1.13–1.84) | 1.34 (1.05–1.71) | 2.73 (1.58–4.72) | 2.50 (1.45–4.31) |
| P for interaction | <0.001 | <0.001 | <0.001 | <0.001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.; Kim, J.; Kang, H. Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 510. https://doi.org/10.3390/ijerph19010510
Lee I, Kim J, Kang H. Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(1):510. https://doi.org/10.3390/ijerph19010510
Chicago/Turabian StyleLee, Inhwan, Jeonghyeon Kim, and Hyunsik Kang. 2022. "Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study" International Journal of Environmental Research and Public Health 19, no. 1: 510. https://doi.org/10.3390/ijerph19010510
APA StyleLee, I., Kim, J., & Kang, H. (2022). Adding Estimated Cardiorespiratory Fitness to the Framingham Risk Score and Mortality Risk in a Korean Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 19(1), 510. https://doi.org/10.3390/ijerph19010510







