Changes in the Frequency of Moderate-to-Vigorous Physical Activity and Subsequent Risk of All-Cause and Cardiovascular Disease Mortality
Abstract
:1. Introduction
2. Methods
2.1. Data and Study Sample
2.2. Measurements for All-Cause and CVD Mortality
2.3. Measurements for MVPA
2.4. Measurement for Potential Confounding Factors
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, D.; Lawson, K.D.; Kolbe-Alexander, T.L.; Finkelstein, E.A.; Katzmarzyk, P.T.; van Mechelen, W.; Pratt, M.; Lancet Physical Activity Series 2 Executive Committee. The economic burden of physical inactivity: A global analysis of major non-communicable diseases. Lancet 2016, 388, 1311–1324. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- The Korea Centers for Disease Control and Prevention. 2018 Statistics for Korea National Health and Nutrition Examination Survey; KCDC: Sejong, Korea, 2020.
- Gebel, K.; Ding, D.; Chey, T.; Stamatakis, E.; Brown, W.J.; Bauman, A.E. Effect of Moderate to Vigorous Physical Activity on All-Cause Mortality in Middle-aged and Older Australians. JAMA Intern. Med. 2015, 175, 970–977. [Google Scholar] [CrossRef]
- Moore, S.C.; Patel, A.V.; Matthews, C.E.; Berrington de Gonzalez, A.; Park, Y.; Katki, H.A.; Linet, M.S.; Weiderpass, E.; Visvanathan, K.; Helzlsouer, K.J.; et al. Leisure time physical activity of moderate to vigorous intensity and mortality: A large pooled cohort analysis. PLoS Med. 2012, 9, e1001335. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Veeranki, S.P.; Li, S.; Steffen, L.M.; Xi, B. Beneficial associations of low and large doses of leisure time physical activity with all-cause, cardiovascular disease and cancer mortality: A national cohort study of 88,140 US adults. Br. J. Sports Med. 2019, 53, 1405–1411. [Google Scholar] [CrossRef] [Green Version]
- Barengo, N.C.; Hu, G.; Lakka, T.A.; Pekkarinen, H.; Nissinen, A.; Tuomilehto, J. Low physical activity as a predictor for total and cardiovascular disease mortality in middle-aged men and women in Finland. Eur. Heart J. 2004, 25, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.B.; Gronbaek, M.; Helge, J.W.; Thygesen, L.C.; Schnohr, P.; Tolstrup, J.S. Changes in physical activity in leisure time and the risk of myocardial infarction, ischemic heart disease, and all-cause mortality. Eur. J. Epidemiol 2012, 27, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Schnohr, P.; Scharling, H.; Jensen, J.S. Changes in leisure-time physical activity and risk of death: An observational study of 7,000 men and women. Am. J. Epidemiol. 2003, 158, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Byberg, L.; Melhus, H.; Gedeborg, R.; Sundstrom, J.; Ahlbom, A.; Zethelius, B.; Berglund, L.G.; Wolk, A.; Michaelsson, K. Total mortality after changes in leisure time physical activity in 50 year old men: 35 year follow-up of population based cohort. BMJ 2009, 338, b688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnohr, P.; O’Keefe, J.H.; Lange, P.; Jensen, G.B.; Marott, J.L. Impact of persistence and non-persistence in leisure time physical activity on coronary heart disease and all-cause mortality: The Copenhagen City Heart Study. Eur. J. Prev. Cardiol. 2017, 24, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, S.K.; Croci, I.; Wisloff, U.; Nauman, J. Temporal Changes in a Novel Metric of Physical Activity Tracking (Personal Activity Intelligence) and Mortality: The HUNT Study, Norway. Prog. Cardiovasc. Dis. 2019, 62, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Kim, D.Y.; Kang, D.W.; Kim, B.J.; Kim, C.K.; Kim, Y.; Yang, W.; Park, E.S.; Lee, S.H. Physical Activity Frequency and the Risk of Stroke: A Nationwide Cohort Study in Korea. J. Am. Heart Assoc. 2017, 6, e005671. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Agarwal, D.P. Cardioprotective effects of light-moderate consumption of alcohol: A review of putative mechanisms. Alcohol Alcohol. 2002, 37, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, H.; Iso, H.; Toyoshima, H.; Date, C.; Yamamoto, A.; Kikuchi, S.; Koizumi, A.; Kondo, T.; Watanabe, Y.; Wada, Y.; et al. Walking and sports participation and mortality from coronary heart disease and stroke. J. Am. Coll. Cardiol. 2005, 46, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Sofi, F.; Capalbo, A.; Cesari, F.; Abbate, R.; Gensini, G.F. Physical activity during leisure time and primary prevention of coronary heart disease: An updated meta-analysis of cohort studies. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 247–257. [Google Scholar] [CrossRef]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W.; Haskell, W.; Lee, I.M. Dose Response Between Physical Activity and Risk of Coronary Heart Disease A Meta-Analysis. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koba, S.; Tanaka, H.; Maruyama, C.; Tada, N.; Birou, S.; Teramoto, T.; Sasaki, J. Physical activity in the Japan population: Association with blood lipid levels and effects in reducing cardiovascular and all-cause mortality. J. Atheroscler. Thromb. 2011, 18, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.M.; Paffenbarger, R.S., Jr. Preventing coronary heart disease: The role of physical activity. Phys. Sportsmed. 2001, 29, 37–52. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Despres, J.P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R.; et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, J.L.; Whooley, M.A.; Regan, M.C.; Sin, N.L.; Cohen, B.E. Effect of physical activity level on biomarkers of inflammation and insulin resistance over 5 years in outpatients with coronary heart disease (from the Heart and Soul Study). Am. J. Cardiol. 2014, 114, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borjesson, M.; Onerup, A.; Lundqvist, S.; Dahlof, B. Physical activity and exercise lower blood pressure in individuals with hypertension: Narrative review of 27 RCTs. Br. J. Sports Med. 2016, 50, 356–361. [Google Scholar] [CrossRef]
- Seals, D.R.; Desouza, C.A.; Donato, A.J.; Tanaka, H. Habitual exercise and arterial aging. J. Appl. Physiol. 2008, 105, 1323–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roque, F.R.; Hernanz, R.; Salaices, M.; Briones, A.M. Exercise training and cardiometabolic diseases: Focus on the vascular system. Curr. Hypertens. Rep. 2013, 15, 204–214. [Google Scholar] [CrossRef]
- Sattar, N.; Preiss, D. Reverse Causality in Cardiovascular Epidemiological Research: More Common Than Imagined? Circulation 2017, 135, 2369–2372. [Google Scholar] [CrossRef]
- Strain, T.; Wijndaele, K.; Sharp, S.J.; Dempsey, P.C.; Wareham, N.; Brage, S. Impact of follow-up time and analytical approaches to account for reverse causality on the association between physical activity and health outcomes in UK Biobank. Int. J. Epidemiol. 2020, 49, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korea Institute for Health and Social Affairs. The Fifth Korea National Health and Nutrition Examination Survey (KNHANES V), 2011—Health Behaviors of Adults; Korea Institute for Health and Social Affairs: Sejong, Korea, 2012. [Google Scholar]
- Pratt, M.; Varela, A.R.; Salvo, D.; Kohl, H.W., III; Ding, D. Attacking the pandemic of physical inactivity: What is holding us back? Br. Assoc. Sport Exerc. Med. 2020, 54, 760–762. [Google Scholar] [CrossRef] [PubMed]
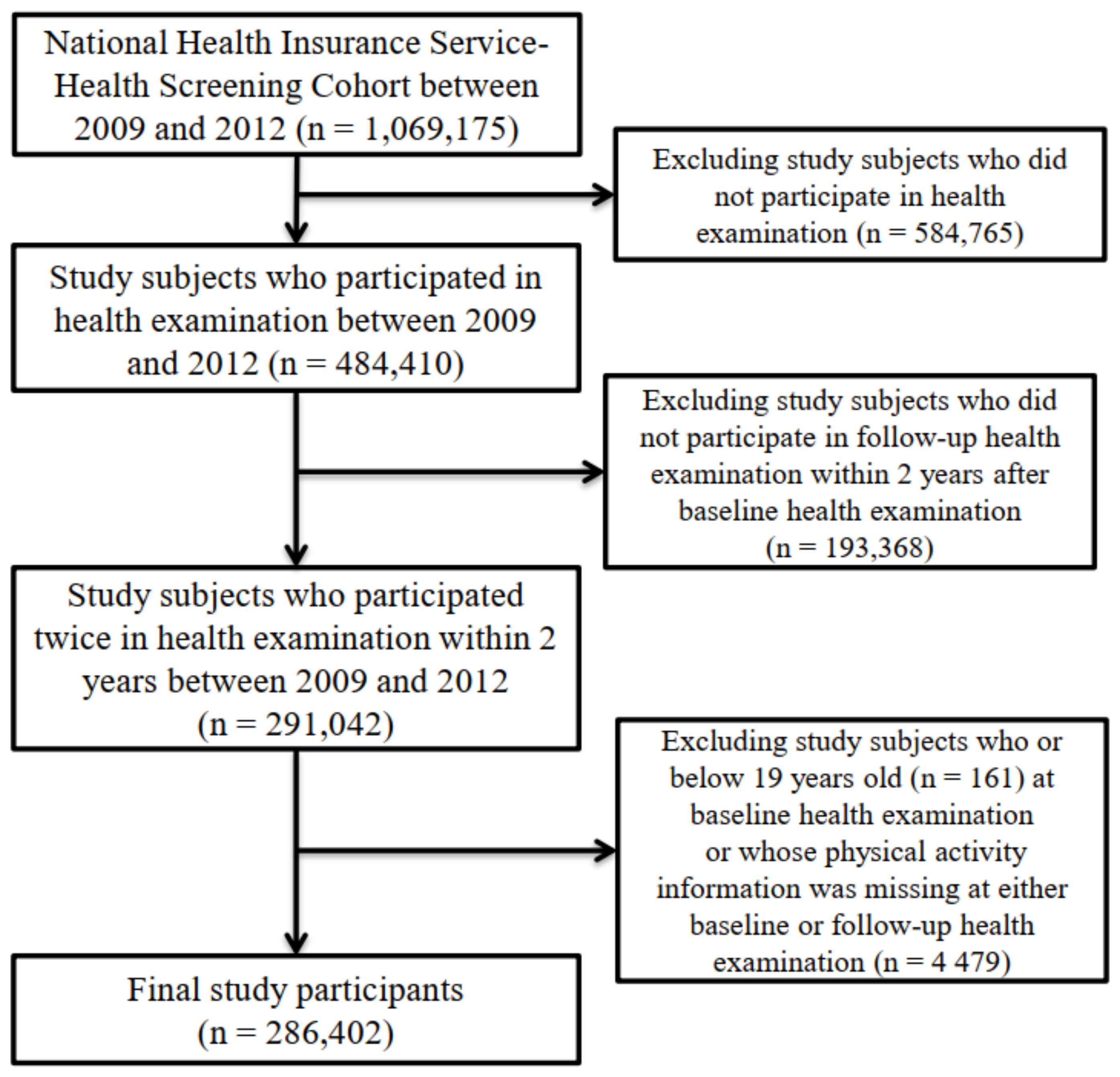
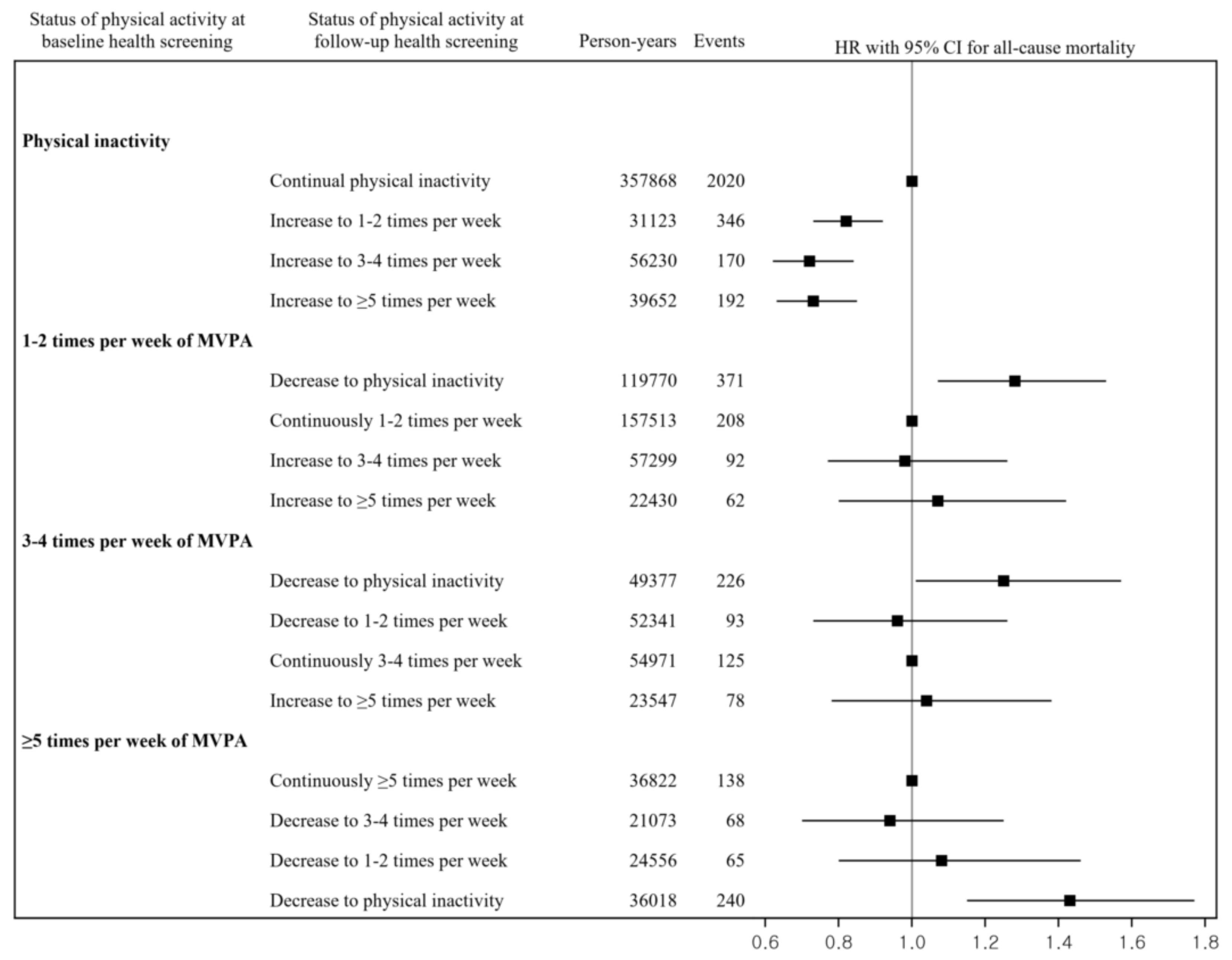
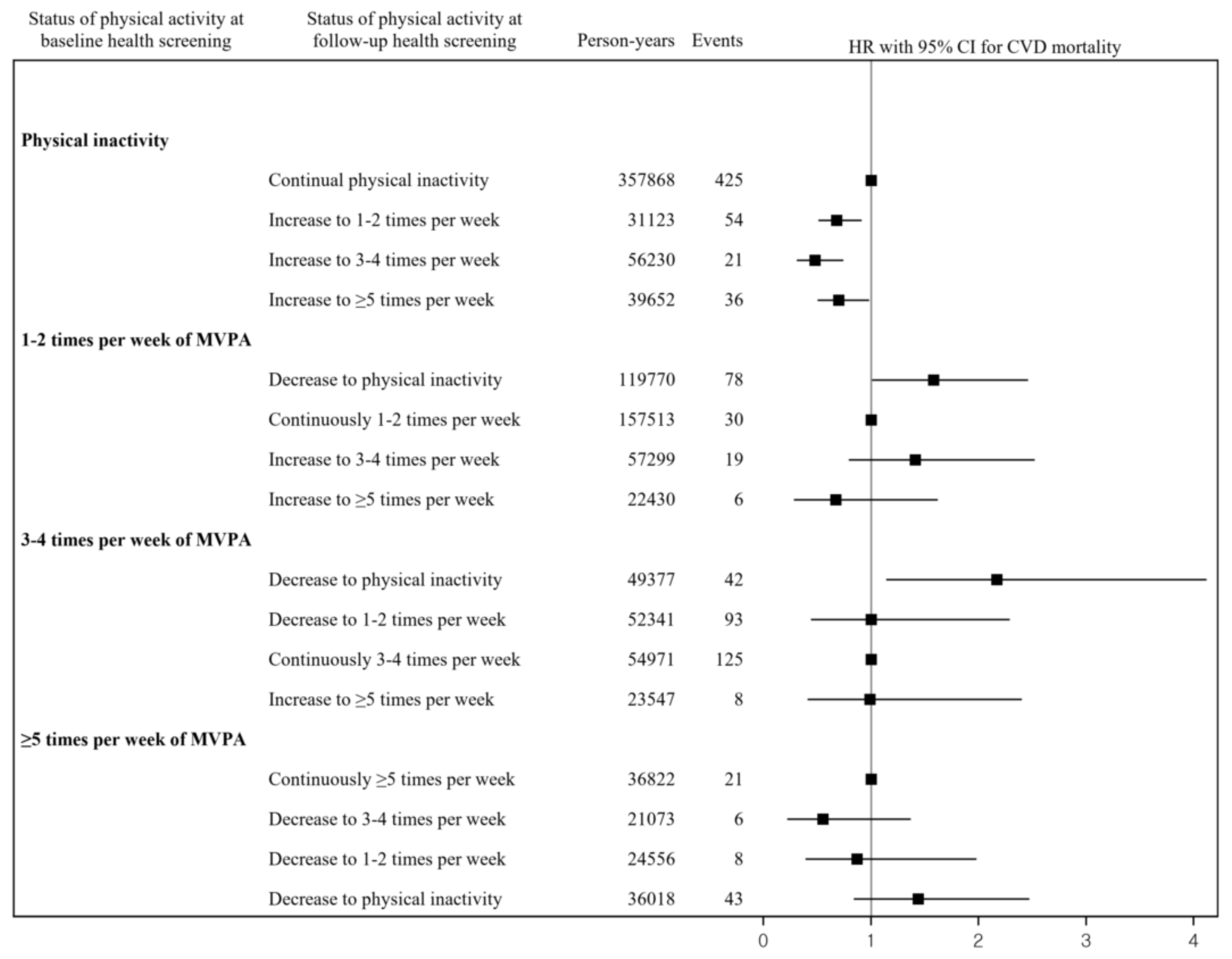
| Variables | Baseline Health Examination (2009–2011) | Follow-Up Health Examination (2010–2012) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Total | 286,402 | 100.0 | 286,402 | 100.0 | |
| Physical activity | |||||
| Physically inactive | 137,181 | 47.9 | 131,286 | 45.8 | |
| 1–2 sessions of MVPA per week | 80,598 | 28.1 | 82,822 | 28.9 | |
| 3–4 sessions of MVPA per week | 41,178 | 14.4 | 44,087 | 15.4 | |
| ≥5 sessions of MVPA per week | 27,445 | 9.6 | 28,207 | 9.8 | |
| Sex | |||||
| Men | 152,572 | 53.3 | 152,572 | 53.3 | |
| Women | 133,830 | 46.7 | 133,830 | 46.7 | |
| Age (years), mean ± SD | 48.9 | 14.0 | 50.4 | 14.2 | |
| BMI (kg/m2) | |||||
| ≤18.5 | 10,045 | 3.5 | 9802 | 3.4 | |
| 18.5–23 | 110,139 | 38.5 | 108,920 | 38.0 | |
| 23–25 | 71,339 | 24.9 | 71,832 | 25.1 | |
| 25–30 | 84,671 | 29.6 | 85,226 | 29.8 | |
| ≥30 | 10,092 | 3.5 | 10,571 | 3.7 | |
| SBP (mmHg), mean ± SD | 122.4 | 14.9 | 122.4 | 14.8 | |
| DBP (mmHg), mean ± SD | 76.2 | 10.0 | 76.2 | 9.9 | |
| Fasting glucose (mg/dL), mean ± SD | 97.3 | 23.2 | 97.8 | 22.9 | |
| Total cholesterol (mg/dL), mean ± SD | 195.3 | 40.9 | 195.0 | 38.3 | |
| Alcohol consumption | |||||
| None | 148,456 | 51.8 | 151,314 | 52.8 | |
| Heavy drinking | 18,574 | 6.5 | 17,663 | 6.2 | |
| Non-heavy drinking | 118,284 | 41.3 | 117,293 | 41.0 | |
| Cigarette smoking status | |||||
| Never smoking | 174,559 | 60.9 | 175,051 | 61.1 | |
| Ex-smoking | 42,294 | 14.8 | 45,212 | 15.8 | |
| Current smoking | 68,804 | 24.0 | 65,955 | 23.0 | |
| Household income | |||||
| Low | 90,640 | 31.6 | 89,532 | 31.3 | |
| Middle | 122,187 | 42.7 | 121,647 | 42.5 | |
| High | 73,575 | 25.7 | 75,223 | 26.3 | |
| Location of residence | |||||
| Metropolitan | 53,995 | 18.9 | 53,090 | 18.5 | |
| Urban | 75,644 | 26.4 | 75,180 | 26.2 | |
| Rural | 156,763 | 54.7 | 158,132 | 55.2 | |
| Disability | 14,816 | 5.2 | 16,461 | 5.7 | |
| Comorbidities | |||||
| Diabetes mellitus | 60,883 | 21.3 | 71,404 | 24.9 | |
| Hypertension | 78,817 | 27.5 | 89,089 | 31.1 | |
| Dyslipidemia | 90,092 | 31.5 | 110,562 | 38.6 | |
| Stroke | 12,235 | 4.3 | 15,223 | 5.3 | |
| Ischemic heart disease | 36,348 | 12.7 | 43,019 | 15.0 | |
| Cancer | 20,510 | 7.2 | 25,521 | 8.9 | |
| Variable | Number of Participants | Person-Years | Cases | HR a | 95% CI a | p-Value | |
|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||
| Physical inactivity | 137,181 | 807,247 | 2728 | 1.00 | |||
| 1–2 sessions of MVPA per week | 80,598 | 476,581 | 733 | 0.82 | 0.76 | 0.89 | <0.001 |
| 3–4 sessions of MVPA per week | 41,178 | 243,791 | 522 | 0.87 | 0.79 | 0.96 | 0.005 |
| ≥5 sessions of MVPA per week | 27,445 | 162,719 | 511 | 0.80 | 0.73 | 0.88 | <0.001 |
| CVD mortality | |||||||
| Physical inactivity | 137,181 | 807,247 | 536 | 1.00 | |||
| 1–2 sessions of MVPA per week | 80,598 | 476,581 | 133 | 0.88 | 0.73 | 1.07 | 0.206 |
| 3–4 sessions of MVPA per week | 41,178 | 243,791 | 73 | 0.70 | 0.55 | 0.90 | 0.005 |
| ≥5 sessions of MVPA per week | 27,445 | 162,719 | 78 | 0.69 | 0.54 | 0.88 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Choi, J.W. Changes in the Frequency of Moderate-to-Vigorous Physical Activity and Subsequent Risk of All-Cause and Cardiovascular Disease Mortality. Int. J. Environ. Res. Public Health 2022, 19, 504. https://doi.org/10.3390/ijerph19010504
Choi Y, Choi JW. Changes in the Frequency of Moderate-to-Vigorous Physical Activity and Subsequent Risk of All-Cause and Cardiovascular Disease Mortality. International Journal of Environmental Research and Public Health. 2022; 19(1):504. https://doi.org/10.3390/ijerph19010504
Chicago/Turabian StyleChoi, Young, and Jae Woo Choi. 2022. "Changes in the Frequency of Moderate-to-Vigorous Physical Activity and Subsequent Risk of All-Cause and Cardiovascular Disease Mortality" International Journal of Environmental Research and Public Health 19, no. 1: 504. https://doi.org/10.3390/ijerph19010504
APA StyleChoi, Y., & Choi, J. W. (2022). Changes in the Frequency of Moderate-to-Vigorous Physical Activity and Subsequent Risk of All-Cause and Cardiovascular Disease Mortality. International Journal of Environmental Research and Public Health, 19(1), 504. https://doi.org/10.3390/ijerph19010504






