Effects of β-Hydroxy β-Methylbutyric Supplementation in Combination with Conservative Non-Invasive Treatments in Athletes with Patellar Tendinopathy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
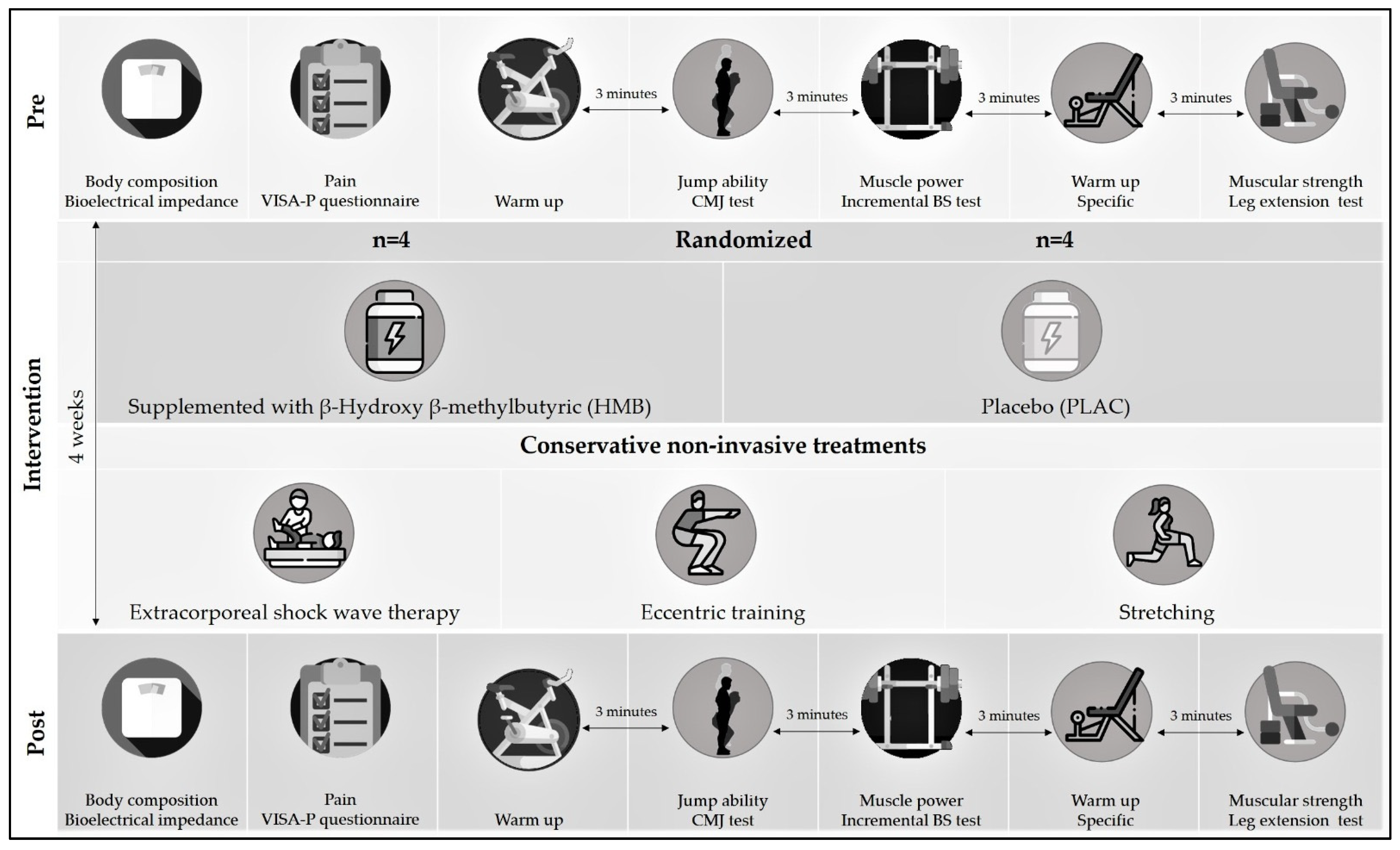
2.1. Experimental Design
2.2. Participants
2.3. Supplementation
2.4. Physical Rehabilitation
2.5. Body Composition Assessment
2.6. Pain Assessment
2.7. Muscular Function Assessment
2.8. Statistical Analysis
3. Results
4. Discussion
5. Practical Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lian, Ø.B.; Engebretsen, L.; Bahr, R. Prevalence of jumper’s knee among elite athletes from different sports: A cross-sectional study. Am. J. Sports Med. 2005, 33, 561–567. [Google Scholar] [CrossRef]
- Lian, Ø.; Refsnes, P.E.; Engebretsen, L.; Bahr, R. Performance characteristics of volleyball players with patellar tendinopathy. Am. J. Sports Med. 2003, 31, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Hannington, M.; Rio, E.; Padua, D.; Stanley, L.; Berkoff, D.; Edwards, S.; Rudavsky, A.; Cook, J.; Docking, S. Prevalence and impact of patellar tendinopathy on elite basketball athletes: Quantifying injury beyond the time-loss definition. J. Sci. Med. Sport 2017, 20, 17–18. [Google Scholar] [CrossRef]
- Blazina, M.E.; Kerlan, R.K.; Jobe, F.W.; Carter, V.S.; Carlson, G.J. Jumper’s knee. Orthop. Clin. North Am. 1973, 4, 665–678. [Google Scholar] [CrossRef]
- Peers, K.H.E.; Lysens, R.J.J. Patellar tendinopathy in athletes: Current diagnostic and therapeutic recommendations. Sport. Med. 2005, 35, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A. Epidemiology of Jumper’s Knee. Sport. Med. An Int. J. Appl. Med. Sci. Sport Exerc. 1986, 3, 289–295. [Google Scholar]
- Khan, K.M.; Cook, J.L.; Taunton, J.E.; Bonar, F. Overuse tendinosis, not tendinitis. Part 1: A new paradigm for a difficult clinical problem. Phys. Sportsmed. 2000, 28, 38–48. [Google Scholar] [CrossRef][Green Version]
- Childress, M.A.; Beutler, A. Management of chronic tendon injuries. Am. Fam. Physician 2013, 87, 486–490. [Google Scholar]
- Anitua, E.; Sánchez, M.; Orive, G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv. Drug Deliv. Rev. 2010, 62, 741–752. [Google Scholar] [CrossRef]
- Abat, F.; Diesel, W.J.; Gelber, P.E.; Polidori, F.; Monllau, J.C.; Sanchez-Ibañez, J.M. Effectiveness of the Intratissue Percutaneous Electrolysis (EPI®) technique and isoinertial eccentric exercise in the treatment of patellar tendinopathy at two years follow-up. Muscles. Ligaments Tendons J. 2014, 4, 188–193. [Google Scholar] [CrossRef]
- Abat, F.; Sánchez-Sánchez, J.L.; Martín-Nogueras, A.M.; Calvo-Arenillas, J.I.; Yajeya, J.; Méndez-Sánchez, R.; Monllau, J.C.; Gelber, P.E. Randomized controlled trial comparing the effectiveness of the ultrasound-guided galvanic electrolysis technique (USGET) versus conventional electro-physiotherapeutic treatment on patellar tendinopathy. J. Exp. Orthop. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Cook, S.D.; Salkeld, S.L.; Popich-Patron, L.S.; Ryaby, J.P.; Jones, D.G.; Barrack, R.L. Improved cartilage repair after treatment with low-intensity pulsed ultrasound. In Proceedings of the Clinical Orthopaedics and Related Research; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Han, S.H.; Lee, J.W.; Guyton, G.P.; Parks, B.G.; Courneya, J.-P.; Schon, L.C.J. Leonard Goldner Award 2008: Effect of Extracorporeal Shock Wave Therapy on Cultured Tenocytes. Foot Ankle Int. 2009, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Cook, J. Treatment options for patellar tendinopathy: Critical review. Curr. Sports Med. Rep. 2011, 10, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.E.H.; Käll, I.; Nilsson-Helander, K. Treatment of patellar tendinopathy-a systematic review of randomized controlled trials. Knee Surgery Sport. Traumatol. Arthrosc. 2012, 20, 1632–1646. [Google Scholar] [CrossRef]
- Malliaras, P.; Barton, C.J.; Reeves, N.D.; Langberg, H. Achilles and patellar tendinopathy loading programmes: A systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sport. Med. 2013, 43, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.M.; Cini, A.; Sbruzzi, G.; Lima, C.S. Influence of static stretching on hamstring flexibility in healthy young adults: Systematic review and meta-analysis. Physiother. Theory Pract. 2016, 32, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Artrong, R.B.; Warren, G.L.; Warren, J.A. Mechanisms of Exercise-Induced Muscle Fibre Injury. Sport. Med. 1991, 12, 184–207. [Google Scholar] [CrossRef]
- Lieber, R.L. Skeletal Muscle Structure, Function, and Plasticity; Wolters Kluwer Health Adis (ESP): Philadelphia, PA, USA, 2011; ISBN 9780781775939. [Google Scholar]
- Nosaka, K.; Sakamoto, K.; Newton, M.; Sacco, P. The repeated bout effect of reduced-load eccentric exercise on elbow flexor muscle damage. Eur. J. Appl. Physiol. 2001, 85, 34–40. [Google Scholar] [CrossRef]
- Langberg, H.; Kongsgaard, M. Eccentric training in tendinopathy—More questions than answers: Editorial. Scand. J. Med. Sci. Sport. 2008, 18, 541–542. [Google Scholar] [CrossRef]
- Rees, J.D.; Wolman, R.L.; Wilson, A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br. J. Sports Med. 2009, 43, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Dimitrios, S.; Pantelis, M.; Kalliopi, S. Comparing the effects of eccentric training with eccentric training and static stretching exercises in the treatment of patellar tendinopathy. A controlled clinical trial. Clin. Rehabil. 2012, 26, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Watson, J.N.; Hutchinson, M.R. Patellar Tendinopathy. Sports Health 2015, 7, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.; Wiley, J.P. Extracorporeal shockwave therapy: A review. Sport. Med. 2002, 32, 851–865. [Google Scholar] [CrossRef]
- Di Meglio, F.; Sacco, A.M.; Belviso, I.; Romano, V.; Sirico, F.; Loiacono, C.; Palermi, S.; Pempinello, C.; Montagnani, S.; Nurzynska, D.; et al. Influence of supplements and drugs used for the treatment of musculoskeletal disorders on adult human tendon-derived stem cells. Muscles. Ligaments Tendons J. 2020, 10, 376–384. [Google Scholar] [CrossRef]
- Loiacono, C.; Palermi, S.; Massa, B.; Belviso, I.; Romano, V.; Di Gregorio, A.; Sirico, F.; Sacco, A.M. Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review. Medicina 2019, 55, 447. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D. Dietary strategies to attenuate muscle loss during recovery from injury. Nestle Nutr. Inst. Workshop Ser. 2013, 75, 51–61. [Google Scholar]
- Tipton, K.D. Nutritional Support for Exercise-Induced Injuries. Sport. Med. 2015, 45, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary supplements for health, adaptation, and recovery in athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Jeszka, J. The efficacy of a β-hydroxy-β-methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: A randomised, double-blind, placebocontrolled crossover study. J. Int. Soc. Sports Nutr. 2015, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Baxter, J.H.; Mukerji, P.; Tisdale, M.J. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E923–E931. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Kornasio, R.; Riederer, I.; Butler-Browne, G.; Mouly, V.; Uni, Z.; Halevy, O. β-hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 755–763. [Google Scholar] [CrossRef]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by {beta}-hydroxy-{beta}-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [PubMed]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Sanchez-Martinez, J.; Santos-Lozano, A.; Garcia-Hermoso, A.; Sadarangani, K.P.; Cristi-Montero, C. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: A meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2018, 21, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.M.; Roberts, B.M.; Krieger, J.W.; Schoenfeld, B.J. Does HMB Enhance Body Composition in Athletes? A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2019. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.C.; Wilson, S.M.C.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A.; et al. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef]
- Rossi, A.P.; D’Introno, A.; Rubele, S.; Caliari, C.; Gattazzo, S.; Zoico, E.; Mazzali, G.; Fantin, F.; Zamboni, M. The Potential of β-Hydroxy-β-Methylbutyrate as a New Strategy for the Management of Sarcopenia and Sarcopenic Obesity. Drugs Aging 2017, 34, 833–840. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Purdam, C.; Gaida, J.; Moseley, G.L.; Pearce, A.J.; Cook, J. Isometric exercise induces analgesia and reduces inhibition in patellar tendinopathy. Br. J. Sports Med. 2015, 49, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kaczka, P.; Michalczyk, M.M.; Jastrzab, R.; Gawelczyk, M.; Kubicka, K. Mechanism of action and the effect of beta-hydroxy-beta-methylbutyrate (HMB) supplementation on different types of physical performance-A systematic review. J. Hum. Kinet. 2019, 68, 211–222. [Google Scholar] [CrossRef]
- Holden, S.; Lyng, K.; Graven-Nielsen, T.; Riel, H.; Olesen, J.L.; Larsen, L.H.; Rathleff, M.S. Isometric exercise and pain in patellar tendinopathy: A randomized crossover trial. J. Sci. Med. Sport 2020, 23, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef]
- Al-Abbad, H.; Simon, J.V. The effectiveness of extracorporeal shock wave therapy on chronic Achilles tendinopathy: A systematic review. Foot Ankle Int. 2013, 34, 33–41. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Visentini, P.J.; Khan, K.M.; Cook, J.L.; Kiss, Z.S.; Harcourt, P.R.; Wark, J.D. The VISA score: An index of severity of symptoms in patients with jumper’s knee (Patellar Tendinosis). J. Sci. Med. Sport 1998, 1, 22–28. [Google Scholar] [CrossRef]
- Balsalobre-Fernández, C.; Tejero-González, C.M.; Del Campo-Vecino, J.; Bavaresco, N. The concurrent validity and reliability of a low-cost, high-speed camera-based method for measuring the flight time of vertical jumps. J. Strength Cond. Res. 2014, 28, 528–533. [Google Scholar] [CrossRef]
- Pérez-Castilla, A.; Piepoli, A.; Delgado-García, G.; Garrido-Blanca, G.; García-Ramos, A. Reliability and concurrent validity of seven commercially available devices for the assessment of movement velocity at different intensities during the bench press. J. Strength Cond. Res. 2019, 33, 1258–1265. [Google Scholar] [CrossRef]
- Maté-Muñoz, J.L.; Lougedo, J.H.; Garnacho-Castaño, M.V.; Veiga-Herreros, P.; Lozano-Estevan, M.D.C.; García-Fernández, P.; de Jesús, F.; Guodemar-Pérez, J.; San Juan, A.F.; Domínguez, R. Effects of β-alanine supplementation during a 5-week strength training program: A randomized, controlled study. J. Int. Soc. Sports Nutr. 2018, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Sascha, G.; Künzell, S. Reliabilität eines 5-RM krafttests für den gesundheits- und fitnesssport. Dtsch. Z. Sportmed. 2014, 65, 314–317. [Google Scholar]
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Bahr, R.; Fossan, B.; Løken, S.; Engebretsen, L. Surgical treatment compared with eccentric training for patellar tendinopathy (jumper’s knee): A randomized, controlled trial. J. Bone Jt. Surg. Ser. A 2006, 88, 1689–1698. [Google Scholar] [CrossRef]
- Romero-Rodriguez, D.; Gual, G.; Tesch, P.A. Efficacy of an inertial resistance training paradigm in the treatment of patellar tendinopathy in athletes: A case-series study. Phys. Ther. Sport 2011, 12, 43–48. [Google Scholar] [CrossRef]
- Biernat, R.; Trzaskoma, Z.; Trzaskoma, Ł.; Czaprowski, D. Rehabilitation protocol for patellar tendinopathy applied among 16-to 19-year old volleyball players. J. Strength Cond. Res. 2014, 28, 43–52. [Google Scholar] [CrossRef]
- Frohm, A.; Halvorsen, K.; Thorstensson, A. Patellar tendon load in different types of eccentric squats. Clin. Biomech. 2007, 22, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Nance, S.; Moore, M. The Load That Maximizes the Average Mechanical Power Output during Jump Squats in Power-Trained Athletes. J. Strength Cond. Res. 2001, 15, 92–97. [Google Scholar]
- Cronin, J.; Sleivert, G. Challenges in understanding the influence of maximal power training on improving athletic performance. Sport. Med. 2005, 35, 213–234. [Google Scholar] [CrossRef]
- Astrom, M.; Arvidson, T. Alignment and joint motion in the normal foot. J. Orthop. Sports Phys. Ther. 1995, 22, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Helland, C.; Bojsen-Møller, J.; Raastad, T.; Seynnes, O.R.; Moltubakk, M.M.; Jakobsen, V.; Visnes, H.; Bahr, R. Mechanical properties of the patellar tendon in elite volleyball players with and without patellar tendinopathy. Br. J. Sports Med. 2013, 47, 862–868. [Google Scholar] [CrossRef]
- Lee, W.C.; Zhang, Z.J.; Masci, L.; Ng, G.Y.F.; Fu, S.N. Alterations in mechanical properties of the patellar tendon is associated with pain in athletes with patellar tendinopathy. Eur. J. Appl. Physiol. 2017, 117, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Onaimbele, G.N.L. Influence of time of day on tendon compliance and estimations of voluntary activation levels. Muscle and Nerve 2006, 33, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Yilmaz, S.; Onur, M.R.; Soylu, A.R.; Altuntaş, O.; Korkusuz, F. Patellar tendon mechanical properties change with gender, body mass index and quadriceps femoris muscle strength. Acta Orthop. Traumatol. Turc. 2017, 51, 54–59. [Google Scholar] [CrossRef]
- Troy Blackburn, J.; Bell, D.R.; Norcross, M.F.; Hudson, J.D.; Engstrom, L.A. Comparison of hamstring neuromechanical properties between healthy males and females and the influence of musculotendinous stiffness. J. Electromyogr. Kinesiol. 2009, 19, e362–e369. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Kubo, K.; Kanehisa, H.; Fukunaga, T. Effect of series elasticity on isokinetic torque-angle relationship in humans. Eur. J. Appl. Physiol. 2002, 87, 381–387. [Google Scholar] [PubMed]
- Crossley, K.M.; Thancanamootoo, K.; Metcalf, B.R.; Cook, J.L.; Purdam, C.R.; Warden, S.J. Clinical features of patellar tendinopathy and their implications for rehabilitation. J. Orthop. Res. 2007, 25, 1164–1175. [Google Scholar] [CrossRef]
- Kannus, P. Etiology and pathophysiology of chronic tendon disorders in sports. Scand. J. Med. Sci. Sport. 1997, 7, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Gravare Silbernagel, K.; Siljeholm, C.; Di Iorio, A.; De Amicis, D.; Salini, V.; Werner, S.; Paganelli, R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Kjaer, M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011, 11, 115–123. [Google Scholar]
- Kongsgaard, M.; Kovanen, V.; Aagaard, P.; Doessing, S.; Hansen, P.; Laursen, A.H.; Kaldau, N.C.; Kjaer, M.; Magnusson, S.P. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand. J. Med. Sci. Sports 2009, 19, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Gómez Díaz, J. Effectiveness of eccentric exercise in patellar tendinopathy. Literature review. Arch. Med. Deport. 2016, 33, 59–66. [Google Scholar]
- Witvrouw, E.; Bellemans, J.; Lysens, R.; Danneels, L.; Cambier, D. Intrinsic risk factors for the development of patellar tendinitis in an athletic population: A two-year prospective study. Am. J. Sports Med. 2001, 29, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Kiss, Z.S.; Khan, K.M.; Purdam, C.R.; Webster, K.E. Anthropometry, physical performance, and ultrasound patellar tendon abnormality in elite junior basketball players: A cross-sectional study. Br. J. Sports Med. 2004, 38, 206–209. [Google Scholar] [CrossRef]
- Piva, S.R.; Goodnite, E.A.; Childs, J.D. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J. Orthop. Sports Phys. Ther. 2005, 35, 793–801. [Google Scholar] [CrossRef]
- Palermi, S.; Massa, B.; Vecchiato, M.; Mazza, F.; De Blasiis, P.; Romano, A.M.; Di Salvatore, M.G.; Della Valle, E.; Tarantino, D.; Ruosi, C.; et al. Indirect Structural Muscle Injuries of Lower Limb: Rehabilitation and Therapeutic Exercise. J. Funct. Morphol. Kinesiol. 2021, 6, 75. [Google Scholar] [CrossRef]
- Mann, K.J.; Edwards, S.; Drinkwater, E.J.; Bird, S.P. A lower limb assessment tool for athletes at risk of developing patellar tendinopathy. Med. Sci. Sports Exerc. 2013, 45, 527–533. [Google Scholar] [CrossRef]
- Mendonça, L.D.; Verhagen, E.; Bittencourt, N.F.N.; Gonçalves, G.G.P.; Ocarino, J.M.; Fonseca, S.T. Factors associated with the presence of patellar tendon abnormalities in male athletes. J. Sci. Med. Sport 2016, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Ko, J.Y.; Chan, Y.S.; Weng, L.H.; Hsu, S.L. Extracorporeal shockwave for chronic patellar tendinopathy. Am. J. Sports Med. 2007, 35, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Visco, V.; Vulpiani, M.C.; Torrisi, M.R.; Ferretti, A.; Pavan, A.; Vetrano, M. Experimental studies on the biological effects of extracorporeal shock wave therapy on tendon models. A review of the literature. Muscles. Ligaments Tendons J. 2014, 4, 357–361. [Google Scholar] [CrossRef]
- Maier, M.; Averbeck, B.; Milz, S.; Refior, H.J.; Schmitz, C. Substance P and prostaglandin E2 release after shock wave application to the rabbit femur. Clin. Orthop. Relat. Res. 2003, 406, 237–245. [Google Scholar] [CrossRef]
- Knitter, A.E.; Panton, L.; Rathmacher, J.A.; Petersen, A.; Sharp, R. Effects of β-hydroxy-β-methylbutyrate on muscle damage after a prolonged run. J. Appl. Physiol. 2000, 89, 1340–1344. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Keech, A.; Jenkins, D. Short-term β-hydroxy-β-methylbutyrate supplementation does not reduce symptoms of eccentric muscle damage. Int. J. Sport Nutr. 2001, 11, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, K.A.; Edwards, A.J.; Howatson, G. Supplementation with β-hydroxy- β-methylbutyrate (hmb) and α-ketoisocaproic acid (KIC) reduces signs and symptoms of exercise-induced muscle damage in man. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.A.; Rice, D.; Fuller, J.C.; Connelly, A.S.; Abumrad, N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef]
- Allen, D.G.; Whitehead, N.P.; Yeung, E.W. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: Role of ionic changes. J. Physiol. 2005, 567, 723–735. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Hirayama, K.; Ueda, H.; Ochi, E. Two and Four Weeks of β-Hydroxy-β-Methylbutyrate (HMB) Supplementations Reduce Muscle Damage Following Eccentric Contractions. J. Am. Coll. Nutr. 2019, 38, 373–379. [Google Scholar] [PubMed]
- Reeves, N.D.; Maganaris, C.N.; Ferretti, G.; Narici, M.V. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J. Appl. Physiol. 2005, 98, 2278–2286. [Google Scholar] [CrossRef]
- Seynnes, O.R.; De Boer, M.; Narici, M.V. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J. Appl. Physiol. 2007, 102, 368–373. [Google Scholar] [CrossRef]
- Jonsson, P.; Alfredson, H. Superior results with eccentric compared to concentric quadriceps training in patients with jumper’s knee: A prospective randomised study. Br. J. Sports Med. 2005, 39, 847–850. [Google Scholar] [CrossRef] [PubMed]
| Variable | Supplementation | Intervention | p-Value Intervention | p-Value Supplementation | p-Value Intervention· Supplementation | |
|---|---|---|---|---|---|---|
| PRE | POST | |||||
| Body mass (kg) | HMBG | 79.7 ± 9.3 | 80.3 ± 10.7 | 0.950 | 0.924 | 0.456 |
| PLACG | 79.5 ± 7.3 | 78.8 ± 8.4 | ||||
| BMI | HMBG | 24.3 ± 2.7 | 25.1 ± 2.1 | 0.387 | 0.927 | 0.367 |
| PLACG | 24.9 ± 1.9 | 24.7 ± 2.1 | ||||
| Body fat mass (kg) | HMBG | 13.9 ± 7.2 | 14.8 ± 6.9 | 0.223 | 0.984 | 0.168 |
| PLACG | 18.2 ± 5.6 | 18.6 ± 5.4 | ||||
| %Body fat mass | HMBG | 18.2 ± 11.0 | 18.9 ± 10.0 | 0.160 | 0.876 | 0.371 |
| PLACG | 22.8 ± 7.0 | 23.6 ± 7.0 | ||||
| Body muscle mass (kg) | HMBG | 63.3 ± 14.3 | 62.2 ± 13.1 | 0.460 | 0.621 | 0.277 |
| PLACG | 57.8 ± 7.9 | 57.7 ± 7.1 | ||||
| %Body muscle mass | HMBG | 77.7 ± 10.6 | 77.1 ± 9.8 | 0.710 | 0.544 | 0.888 |
| PLACG | 73.1 ± 7.0 | 72.8 ± 6.4 | ||||
| Variable | Supplementation | Intervention | p-Value Intervention | p-Value Supplementation | p-Value Intervention· Supplementation | |
|---|---|---|---|---|---|---|
| PRE | POST | |||||
| CMJ (cm) | HMBG | 38.1 ± 10.4 | 41.1 ± 11.7 | 0.850 | 0.694 | 0.049λ |
| PLACG | 37.0 ± 6.0 | 35.6 ± 4.7 | ||||
| PPKG (kg) | HMBG | 59.4 ± 13.0 | 71.5 ± 17.0 | 0.028 * | 0.948 | 0.335 |
| PLACG | 65.0 ± 17.8 | 64.4 ± 11.6 | ||||
| PPMV (m·s−1) | HMBG | 0.78 ± 0.12 | 0.81 ± 0.05 | 0.296 | 0.268 | 0.796 |
| PLACG | 0.74 ± 0.04 | 0.79 ± 0.03 | ||||
| PPPP (W) | HMBG | 455.5 ± 105.5 # | 575.3 ± 138.8 | 0.002 * | 0.842 | 0.142 |
| PLACG | 479.0 ± 125.3 | 514.4 ± 107.0 | ||||
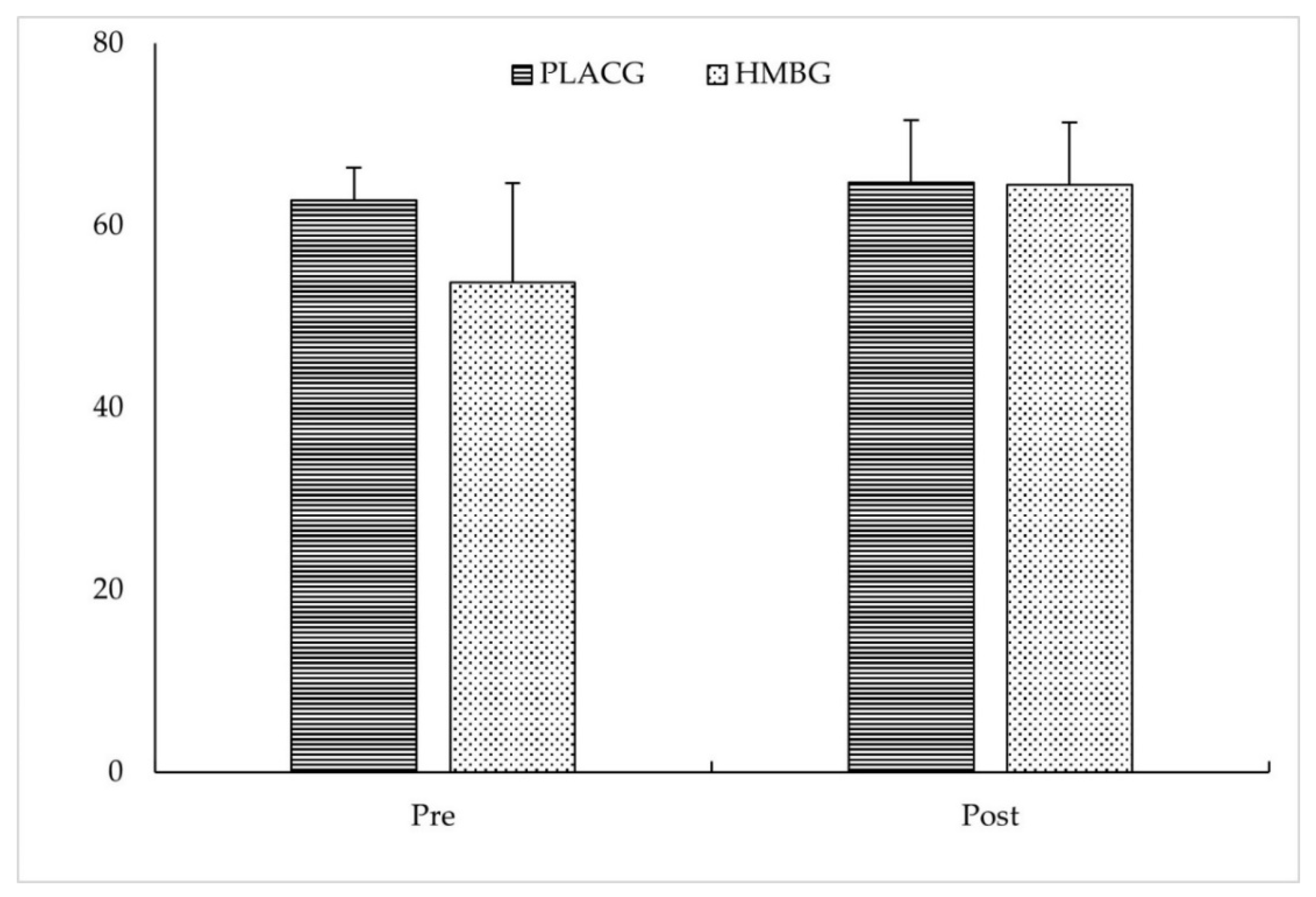
| 5-RM test (kg) | HMBG | 55.0 ± 4.1 # | 68.1 ± 3.1 | 0.001 * | 0.081 | 0.184 |
| PLACG | 73.8 ± 10.5 # | 80.0 ± 9.4 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Gómez, Á.; Jurado-Castro, J.M.; Mata, F.; Sánchez-Oliver, A.J.; Domínguez, R. Effects of β-Hydroxy β-Methylbutyric Supplementation in Combination with Conservative Non-Invasive Treatments in Athletes with Patellar Tendinopathy: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 471. https://doi.org/10.3390/ijerph19010471
Sánchez-Gómez Á, Jurado-Castro JM, Mata F, Sánchez-Oliver AJ, Domínguez R. Effects of β-Hydroxy β-Methylbutyric Supplementation in Combination with Conservative Non-Invasive Treatments in Athletes with Patellar Tendinopathy: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(1):471. https://doi.org/10.3390/ijerph19010471
Chicago/Turabian StyleSánchez-Gómez, Ángela, Jose Manuel Jurado-Castro, Fernando Mata, Antonio Jesús Sánchez-Oliver, and Raúl Domínguez. 2022. "Effects of β-Hydroxy β-Methylbutyric Supplementation in Combination with Conservative Non-Invasive Treatments in Athletes with Patellar Tendinopathy: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 1: 471. https://doi.org/10.3390/ijerph19010471
APA StyleSánchez-Gómez, Á., Jurado-Castro, J. M., Mata, F., Sánchez-Oliver, A. J., & Domínguez, R. (2022). Effects of β-Hydroxy β-Methylbutyric Supplementation in Combination with Conservative Non-Invasive Treatments in Athletes with Patellar Tendinopathy: A Pilot Study. International Journal of Environmental Research and Public Health, 19(1), 471. https://doi.org/10.3390/ijerph19010471









