Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status
Abstract
:1. Introduction
2. Materials and Methods
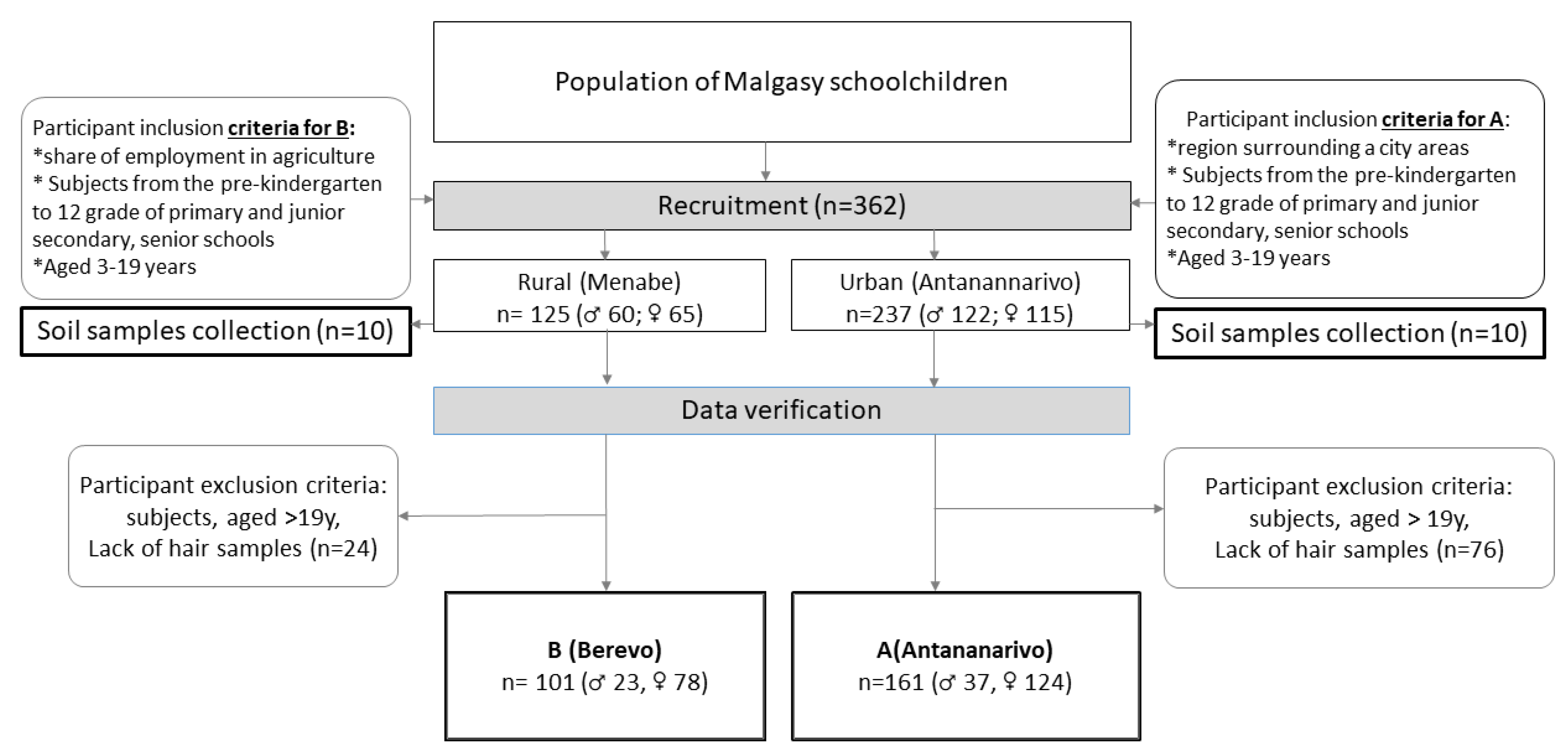
2.1. Study Design
2.2. Anthropometrics
2.3. Hair Sample Collection
2.4. Environmental Samples
2.5. Determination of Elemental Composition by Inductively Coupled Plasma-Optical Emission Spectrometers (ICP-OES)
2.5.1. Sample Preparation
2.5.2. Sample Analysis and Quality Control
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare Earth Elements in the Soil Environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef] [Green Version]
- Ram, R.; Becker, M.; Brugger, J.; Etschmann, B.; Burcher-Jones, C.; Howard, D.; Kooyman, P.J.; Petersen, J. Characterisation of a rare earth element- and zirconium-bearing ion-adsorption clay deposit in Madagascar. Chem. Geol. 2019, 522, 93–107. [Google Scholar] [CrossRef]
- Estrade, G.; Marquis, E.; Smith, M.; Goodenough, K.; Nason, P. REE concentration processes in ion adsorption deposits: Evidence from the Ambohimirahavavy alkaline complex in Madagascar. Ore Geol. Rev. 2019, 112, 103027. [Google Scholar] [CrossRef]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.; Suzuki, K.T. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ. Health Perspect. 1996, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [Green Version]
- Rashed, M.N.; Hossam, F. Heavy metals in fingernails and scalp hair of children, adults and workers from environmentally exposed areas at Aswan, Egypt. Environ. Bioindic. 2007, 2, 131–145. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Huang Yi, N.; Sheng, P.; Yang, B. Relationship between Rare Earth Elements, Lead and Intelligence of Children Aged 6 to 16 years: A Bayesian Structural Equation Modelling Method. Int. Arch. Nurs. Health Care 2019, 5, 1–9. [Google Scholar] [CrossRef]
- Wójciak, R.W.; Krejpcio, Z.; Czlapka-Matyasik, M.; Jeszka, J. Comparison of the hair bioelements in vegeterian and non-vegeterian women. Trace Elem. Electrolytes 2004, 21, 141–144. [Google Scholar] [CrossRef]
- Trojanowski, P.; Trojanowski, J.; Antonowicz, J.; Bokiniec, M. Lead and cadmium content in human hair in Central Pomerania (Northern Poland). J. Elemntology 2010, 15, 363–384. [Google Scholar] [CrossRef]
- Liang, G.; Pan, L.; Liu, X. Assessment of typical heavy metals in human hair of different age groups and foodstuffs in Beijing, China. Int. J. Environ. Res. Public Health 2017, 14, 914. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Li, Y.; Li, H.; Yu, J.; Ye, B.; Liang, T. Rare earth elements in human hair from a mining area of China. Ecotoxicol. Environ. Saf. 2013, 96, 118–123. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L.; Li, Y.; Li, H.; Xu, Y. Distribution Characteristics of Rare Earth Elements and Selenium in Hair of Centenarians Living in China Longevity Region. Biol. Trace Elem. Res. 2020, 197, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, M.K.; Lichota, M.; Górniak, K. Relationship between the arches of feet and the Cole’s index. Adv. Rehabil. 2019, 2019, 29–35. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and Obesity Worldwide: International Survey. BMJ 2000, 320, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Serfor-Armah, Y.; Nyarko, B.J.B.; Adotey, D.K.; Dampare, S.B.; Adomako, D. Levels of arsenic and antimony in water and sediment from Prestea, a gold mining town in Ghana and its environs. Water Air Soil Pollut. 2006, 175, 181–192. [Google Scholar] [CrossRef]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Kozak, L.; Niedzielski, P. The evolution of December 2004 tsunami deposits: Temporal and spatial distribution of potentially toxic metalloids. Chemosphere 2013, 93, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hao, F.; Ouyang, W.; Liu, S.; Chunye, L.; Wenjing, Y. Vertical distribution of rare earth elements in a wetland soil core from the Sanjiang Plain in China. J. Rare Earths 2012, 30, 731–738. [Google Scholar] [CrossRef]
- Chang, C.; Li, F.; Liu, C.; Gao, J.; Tong, H.; Chen, M. Fractionation characteristics of rare earth elements (REEs) linked with secondary Fe, Mn, and Al minerals in soils. Acta Geochim. 2016, 35, 329–339. [Google Scholar] [CrossRef]
- Xu, N.; Morgan, B.; Rate, A.W. From source to sink: Rare-earth elements trace the legacy of sulfuric dredge spoils on estuarine sediments. Sci. Total Environ. 2018, 637–638, 1537–1549. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.; Karayannis, V.; Baklavaridis, A. Environmental Defects and Economic Impact on Global Market of Rare Earth Metals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 161, 012069. [Google Scholar] [CrossRef] [Green Version]
- Behbahaninia, A.; Mirbagheri, S.A.; Khorasani, N.; Nouri, J.; Javid, A.H. Heavy metal contamination of municipal effluent in soil and plants. J. Food Agric. Environ. 2009, 7, 851–856. [Google Scholar]
- d’Aquino, L.; de Pinto, M.C.; Nardi, L.; Morgana, M.; Tommasi, F. Effect of some light rare earth elements on seed germination, seedling growth and antioxidant metabolism in Triticum durum. Chemosphere 2009, 75, 900–905. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Effects of lanthanum and cerium on the growth and mineral nutrition of corn and mungbean. Ann. Bot. 2008, 101, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Zhu, J.G.; Cheng, H.L.; Xie, Z.B.; Chu, H.Y. Phytotoxicity of lanthanum in rice in haplic acrisols and cambisols. Ecotoxicol. Environ. Saf. 2006, 64, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Zhang, M.; Zhang, N.; Li, M. Effects of rare earth elements on growth and metabolism of medicinal plants. Acta Pharm. Sin. B 2013, 3, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, M.; Wang, L.; Wu, G.; Wang, K.; Jiang, X.; Liu, T.; Xiao, P.; Yu, L.; Jiang, Y.; Song, J.; et al. Health risk assessment of rare earth elements in cereals from mining area in Shandong, China. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Zielińska-Dawidziak, M.; Czlapka-Matyasik, M.; Wojciechowska, Z.; Proch, J.; Niedzielski, P. Concentration of selected elements in the hair of Madagascar girls in relation to nutritional status and place of residence. Br. J. Nutr. 2021, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Magnin, M.; Stoll, B.; Voahangy, R.; Jeannot, E. Most children who took part in a comprehensive malnutrition programme in Madagascar reached and maintained the recovery threshold. Acta Paediatr. Int. J. Paediatr. 2017, 106, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.C.; Garchitorena, A.; Rabemananjara, F.; Cordier, L.; Randriamanambintsoa, M.; Rabeza, V.; Razanadrakoto, H.T.R.; Rakoto Ramakasoa, R.; Ramahefarisontiana, O.; Ratsimbazafy, B.N.; et al. Factors associated with risk of developmental delay in preschool children in a setting with high rates of malnutrition: A cross-sectional analysis of data from the IHOPE study, Madagascar. BMC Pediatr. 2020, 20, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tippairote, T.; Temviriyanukul, P.; Benjapong, W.; Trachootham, D. Prevalence and Factors Associated with High Levels of Aluminum, Arsenic, Cadmium, Lead, and Mercury in Hair Samples of Well-Nourished Thai Children in Bangkok and Perimeters. Biol. Trace Elem. Res. 2019, 188, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.R.S. Nutritional Factors that May Influence Bioavailability of Cadmium. J. Environ. Qual. 1988, 17, 175–180. [Google Scholar] [CrossRef]
| Parameter (Mean ± SD) 1 | Total Sample (n = 262) | East of Madagascar (Close to Antananarivo) (n = 161) | West of Madagascar (Menabe Region) (n = 101) | |||
|---|---|---|---|---|---|---|
| x ± SD | Min–Max | x ± SD | Min–Max | x ± SD | Min–Max | |
| Age (years) | 11 ± 3 | 5–19 | 12 ± 4 a,1 | 5–19 | 10 ± 3 a | 5–16 |
| Weight (kg) | 29.2 ± 10.6 | 13.9–60.7 | 30.9 ± 11.6 a | 13.9–56.1 | 27.1 ± 8.8 b | 14.7–60.7 |
| Height (cm) | 134 ± 13 | 102–167 | 136 ± 12 a | 115–167 | 133 ± 12 a | 102–162 |
| BMI (kg/m2) | 17.0 ± 2.9 | 12.1–24.6 | 17.5 ± 3.1 a | 13.3–24.6 | 16.0 ± 2.1 b | 12.1–23.9 |
| Cole index (%) | 97 ± 10 | 79–124 | 99 ± 10 a | 79–124 | 94 ± 9 b | 79–112 |
| Cole index distribution n (%) (p < 0.001) 2: | ||||||
| Underweight | 60 (23) | 25 (16) | 35 (35) | |||
| Recommended values | 174 (66) | 113 (70) | 61 (60) | |||
| First degree obesity | 20 (8) | 16 (10) | 4 (4) | |||
| Second degree obesity | 8 (3) | 7 (4) | 1 (1) | |||
| The frequency of meals n (%): | ||||||
| Everyday breakfast | 161 (62) | 161 (100) | 0 (0) | |||
| Regular lunch 3 | 90 (34) | 90 (56) | 0 (0) | |||
| Random meals | 133 (51) | 32 (20) | 101 (100) | |||
| Economic situation of the family 4 | ||||||
| Below average | 199 (76) | 101 (63) | 98 (94) | |||
| Average | 60 (23) | 57 (35) | 3 (6) | |||
| Above average: | 3 (1) | 3 (2) | 0 (0) | |||
| Caregiver completed education level: | ||||||
| Primary or lower | 143 (55) | 48 (30) | 95 (90) | |||
| Secondary | 114 (43) | 109 (68) | 5 (10) | |||
| Upper secondary | 5 (2) | 5 (3) | 1 (0) | |||
| Sources of drinking water: | ||||||
| Water supply network | 161 (62) | 161 (100) | 0(0) | |||
| Surface water (rivers, lakes, wells) | 101 (38) | 0 (0) | 101 (100) | |||
| Toilets availability at home: | ||||||
| Yes | 161 (62) | 161 (100) | 0 (0) | |||
| No | 101 (38) | 0 (0) | 101(100) | |||
| Region | Sample | Geographic Coordinates |
|---|---|---|
| A | 1 | 18°49′ S; 47°26′ E |
| 2 | 18°48′ S; 47°26′ E | |
| 3 | 18°47′ S; 47°23′ E | |
| 4 | 18°45′ S; 47°33′ E | |
| 5 | 18°49′ S; 47°26′ E | |
| 6 | 18°56′ S; 48°25′ E | |
| 7 | 18°55′ S; 47°32′ E | |
| 8 | 18°55′ S; 47°32′ E | |
| 9 | 18°55′ S; 47°32′ E | |
| 10 | 19°39′ S; 46°32′ E | |
| B | 11 | 19°32′ S; 45°27′ E |
| 12 | 19°40′ S; 45°23′ E | |
| 13 | 19°42′ S; 45°20′ E | |
| 14 | 19°45′ S; 45°2′ E | |
| 15 | 19°43′ S; 44°58′ E | |
| 16 | 19°16′ S; 44°57′ E | |
| 17 | 19°43′ S; 44°57′ E | |
| 18 | 19°43′ S; 44°57′ E | |
| 19 | 19°42′ S; 44°34′ E | |
| 20 | 20°17′ S; 44°16′ E |
| Element | Place | Content (mg/kg) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | 25th | 75th | Mean | SD | |||
| Ce | A | 21.82 | 14.48 | 50.34 | 17.62 | 22.12 | 25.28 | 14.37 | 0.09 |
| B | 13.91 | 3.62 | 32.22 | 8.98 | 15.42 | 14.25 | 8.11 | ||
| Dy | A | 2.06 | 1.23 | 4.41 | 1.29 | 2.83 | 2.37 | 1.32 | 0.13 |
| B | 0.99 | 0.40 | 3.64 | 0.69 | 1.68 | 1.37 | 0.98 | ||
| Er | A | 0.67 | 0.44 | 1.24 | 0.58 | 0.94 | 0.77 | 0.32 | 0.07 |
| B | 0.41 | 0.20 | 0.88 | 0.27 | 0.60 | 0.47 | 0.25 | ||
| Eu | A | 0.28 | 0.10 | 0.48 | 0.12 | 0.33 | 0.26 | 0.16 | 0.21 |
| B | 0.14 | 0.08 | 0.36 | 0.09 | 0.24 | 0.17 | 0.10 | ||
| Gd | A | 2.50 | 0.87 | 4.58 | 1.27 | 4.24 | 2.69 | 1.68 | 0.11 |
| B | 0.73 | 0.07 | 4.32 | 0.63 | 1.89 | 1.31 | 1.29 | ||
| Ho | A | 0.03 | <LOD | 0.16 | <LOD | 0.12 | 0.11 | 0.07 | 0.35 |
| B | 0.02 | <LOD | 0.11 | <LOD | 0.06 | 0.04 | 0.35 | ||
| La | A | 10.51 | 6.79 | 26.48 | 7.98 | 11.42 | 12.64 | 7.96 | 0.06 |
| B | 7.16 | 1.8 | 11.89 | 4.61 | 8.10 | 6.73 | 2.89 | ||
| Lu | A | <LOD | <LOD | 0.19 | <LOD | 0.16 | 0.08 | 0.09 | 0.44 |
| B | <LOD | <LOD | 0.26 | <LOD | <LOD | 0.04 | 0.08 | ||
| Nd | A | 8.52 | 4.63 | 20.47 | 5.42 | 10.28 | 9.86 | 6.36 | 0.08 |
| B | 6.33 | 1.72 | 8.52 | 3.92 | 6.96 | 5.59 | 2.11 | ||
| Pr | A | 4.21 | 2.13 | 8.12 | 2.36 | 4.38 | 4.24 | 2.40 | 0.08 |
| B | 1.95 | 0.55 | 5.47 | 1.60 | 2.64 | 2.31 | 1.40 | ||
| Sc | A | 1.73 | 0.83 | 3.01 | 1.00 | 2.93 | 1.90 | 1.03 | 0.31 |
| B | 0.61 | 0.37 | 4.22 | 0.43 | 1.53 | 1.21 | 1.23 | ||
| Sm | A | 2.59 | 1.53 | 4.31 | 1.87 | 2.60 | 2.58 | 1.07 | 0.86 |
| B | 1.64 | 0.64 | 2.54 | 1.47 | 2.15 | 1.76 | 0.59 | ||
| Tb | A | 0.3 | 0.01 | 0.62 | 0.11 | 0.60 | 0.33 | 0.28 | 0.17 |
| B | <LOD | <LOD | 0.67 | <LOD | 0.24 | 0.14 | 0.22 | ||
| Tm | A | 1.77 | 1.29 | 2.59 | 1.76 | 2.13 | 1.91 | 0.49 | 0.31 |
| B | 1.02 | 0.38 | 3.03 | 0.78 | 1.89 | 1.43 | 0.92 | ||
| Y | A | 1.09 | 1.30 | 6.68 | 1.85 | 4.74 | 3.34 | 2.29 | 0.06 |
| B | 2.12 | 0.71 | 2.86 | 1.046 | 2.15 | 1.60 | 0.82 | ||
| Yb | A | 0.46 | 0.15 | 0.73 | 0.26 | 0.52 | 0.42 | 0.22 | 0.08 |
| B | 0.15 | 0.06 | 0.64 | 0.07 | 0.28 | 0.21 | 0.19 | ||
| Element | Content (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| Median | Min | Max | 25th | 75th | Mean | SD | |
| Ce | 1.54 | <LOD | 101 | 0.43 | 4.04 | 3.02 | 3.04 |
| Dy | <LOD | <LOD | 1.24 | <LOD | 0.17 | 0.14 | 0.22 |
| Er | 0.02 | <LOD | 0.37 | <LOD | 0.07 | 0.05 | 0.06 |
| Eu | 0.029 | <LOD | 0.22 | <LOD | 0.05 | 0.04 | 0.04 |
| Gd | 0.26 | <LOD | 1.42 | 0.15 | 0.48 | 0.35 | 0.28 |
| Ho | <LOD | <LOD | 0.67 | <LOD | 0.07 | 0.06 | 0.09 |
| La | 0.75 | <LOD | 9.29 | 0.27 | 2.09 | 1.38 | 1.53 |
| Lu | <LOD | <LOD | 0.08 | <LOD | <LOD | 0.39 | 0.01 |
| Nd | 0.64 | <LOD | 7.44 | 0.32 | 1.75 | 1.17 | 1.28 |
| Pr | 0.35 | <LOD | 3.19 | 0.08 | 0.76 | 0.52 | 0.56 |
| Sc | 0.13 | <LOD | 1.36 | 0.05 | 0.35 | 0.23 | 0.27 |
| Sm | 0.20 | <LOD | 2.13 | <LOD | 0.49 | 0.32 | 0.39 |
| Tb | 0.07 | <LOD | 0.34 | <LOD | 0.14 | 0.09 | 0.0.7 |
| Tm | <LOD | <LOD | 0.29 | <LOD | <LOD | <LOD | 0.03 |
| Y | 0.20 | <LOD | 2.71 | 0.09 | 0.53 | 0.40 | 0.47 |
| Yb | 0.03 | <LOD | 0.33 | <LOD | 0.07 | 0.05 | 0.05 |
| Element | Correlation Coefficient (R) between the Concentration of REEs in Subjects’ Hair and | |
|---|---|---|
| Age | Cole’ Index | |
| Ce | −0.388 | −0.256 |
| Dy | −0.364 | −0.216 |
| Er | −0.282 | −0.174 |
| Eu | −0.328 | −0.216 |
| Gd | −0.384 | −0.208 |
| Ho | 0.135 | 0.066 |
| La | −0.368 | −0.256 |
| Lu | −0.178 | 0.114 |
| Nd | −0.357 | −0.232 |
| Pr | −0.303 | −0.234 |
| Sc | −0.467 | −0.283 |
| Sm | −0.071 | −0.074 |
| Tb | −0.073 | −0.166 |
| Tm | −0.178 | 0.086 |
| Y | −0.346 | −0.265 |
| Yb | −0.375 | −0.277 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska-Dawidziak, M.; Czlapka-Matyasik, M.; Wojciechowska, Z.; Proch, J.; Kowalski, R.; Niedzielski, P. Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status. Int. J. Environ. Res. Public Health 2022, 19, 455. https://doi.org/10.3390/ijerph19010455
Zielińska-Dawidziak M, Czlapka-Matyasik M, Wojciechowska Z, Proch J, Kowalski R, Niedzielski P. Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status. International Journal of Environmental Research and Public Health. 2022; 19(1):455. https://doi.org/10.3390/ijerph19010455
Chicago/Turabian StyleZielińska-Dawidziak, Magdalena, Magdalena Czlapka-Matyasik, Zofia Wojciechowska, Jędrzej Proch, Ryszard Kowalski, and Przemysław Niedzielski. 2022. "Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status" International Journal of Environmental Research and Public Health 19, no. 1: 455. https://doi.org/10.3390/ijerph19010455
APA StyleZielińska-Dawidziak, M., Czlapka-Matyasik, M., Wojciechowska, Z., Proch, J., Kowalski, R., & Niedzielski, P. (2022). Rare Earth Elements Accumulation in the Hair of Malagasy Children and Adolescents in Relation to Their Age and Nutritional Status. International Journal of Environmental Research and Public Health, 19(1), 455. https://doi.org/10.3390/ijerph19010455











