Cadmium Removal from Giant Squid (Dosidicus gigas) Hydrolysate in Fixed-Bed Columns Packed with Iminodiacetic Resin: Tools for Scaling up the Process
Abstract
:1. Introduction
2. Materials and Methods
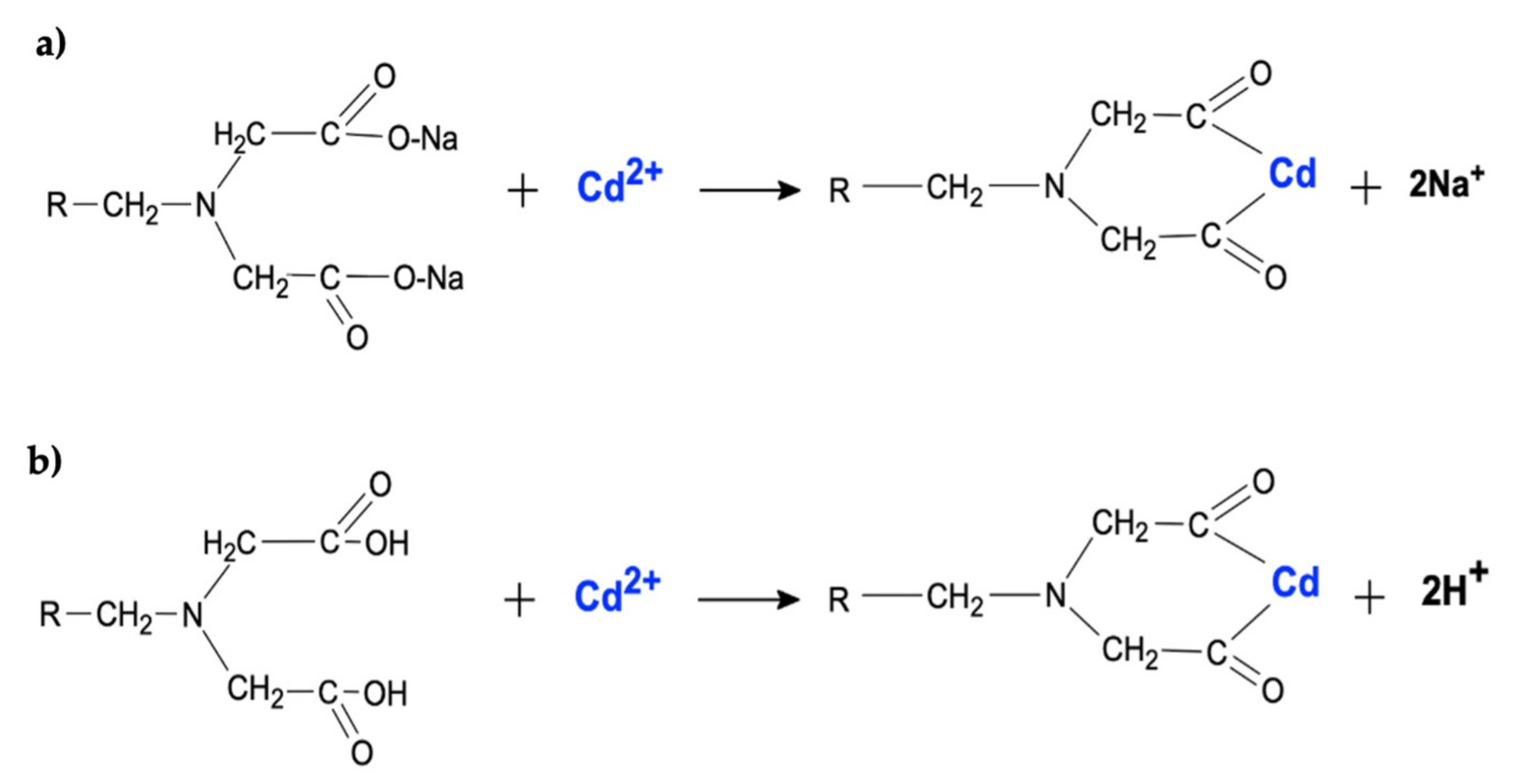
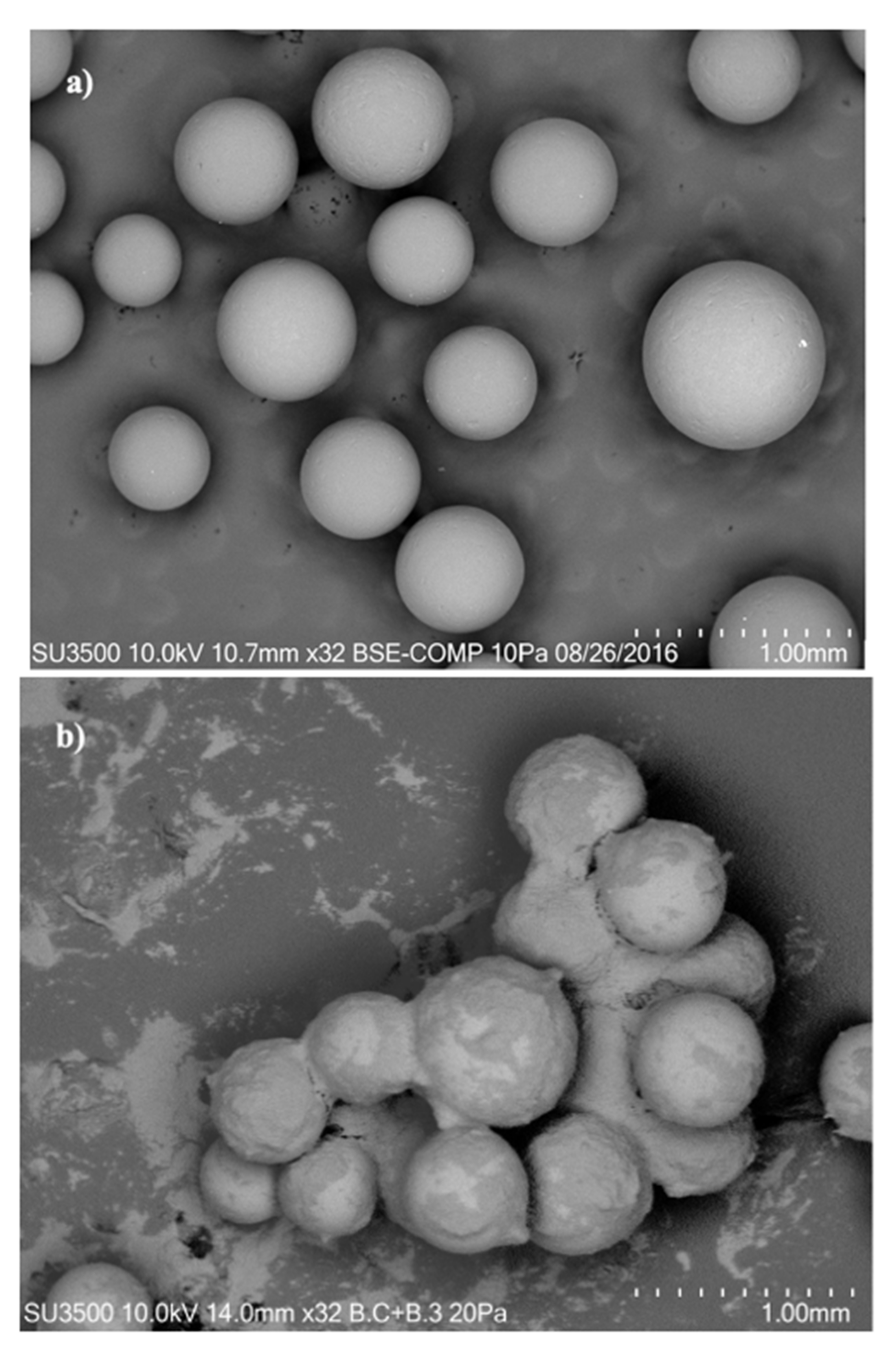
2.1. Adsorbent
2.2. Giant Squid Hydrolysate
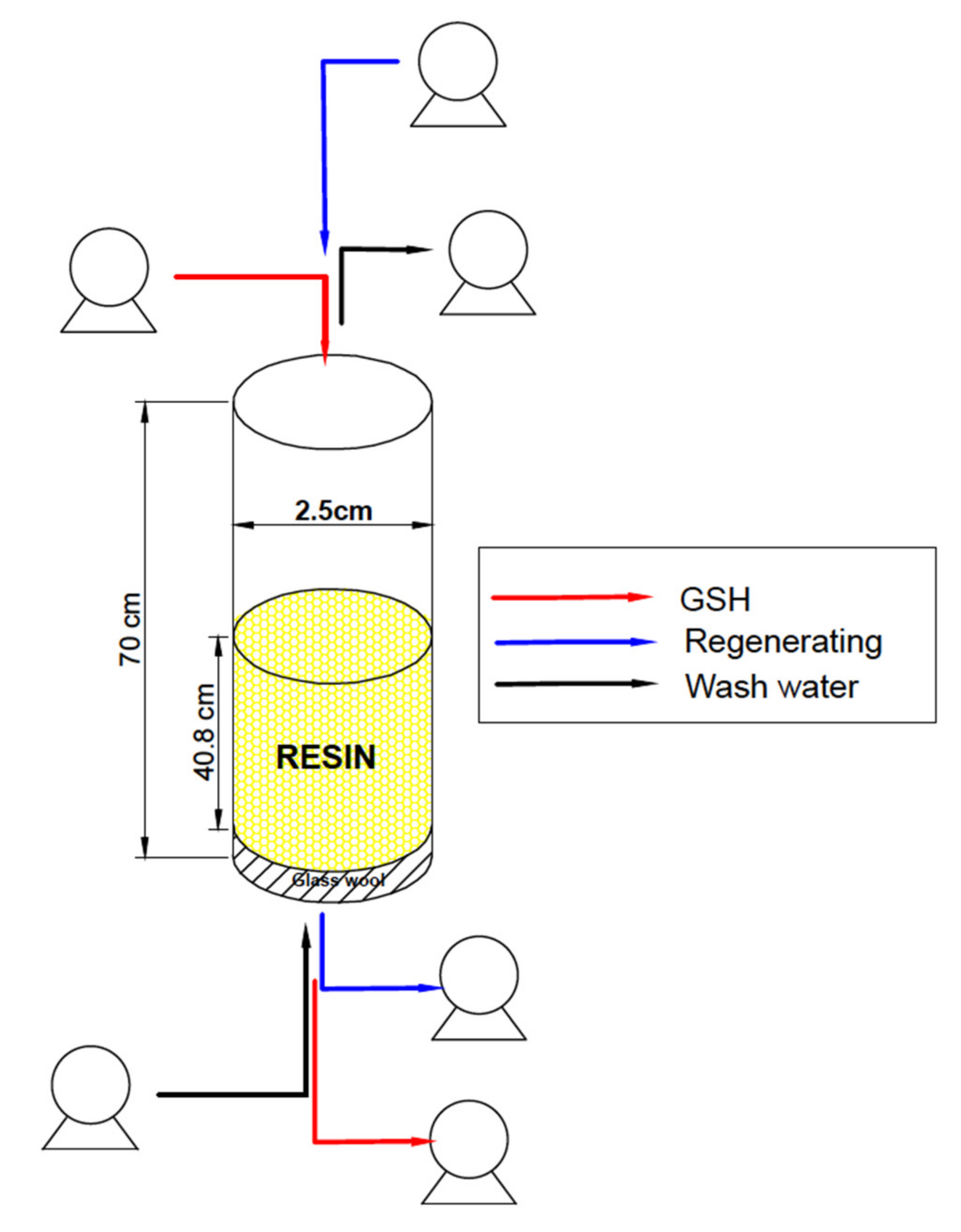
2.3. Column Design at Lab Scale
2.3.1. Operation of the Fixed-Bed Column
2.3.2. Modeling of Cadmium Adsorption in the Fixed-Bed Column
2.4. Column Desorption, Regeneration, and Reusability Assay
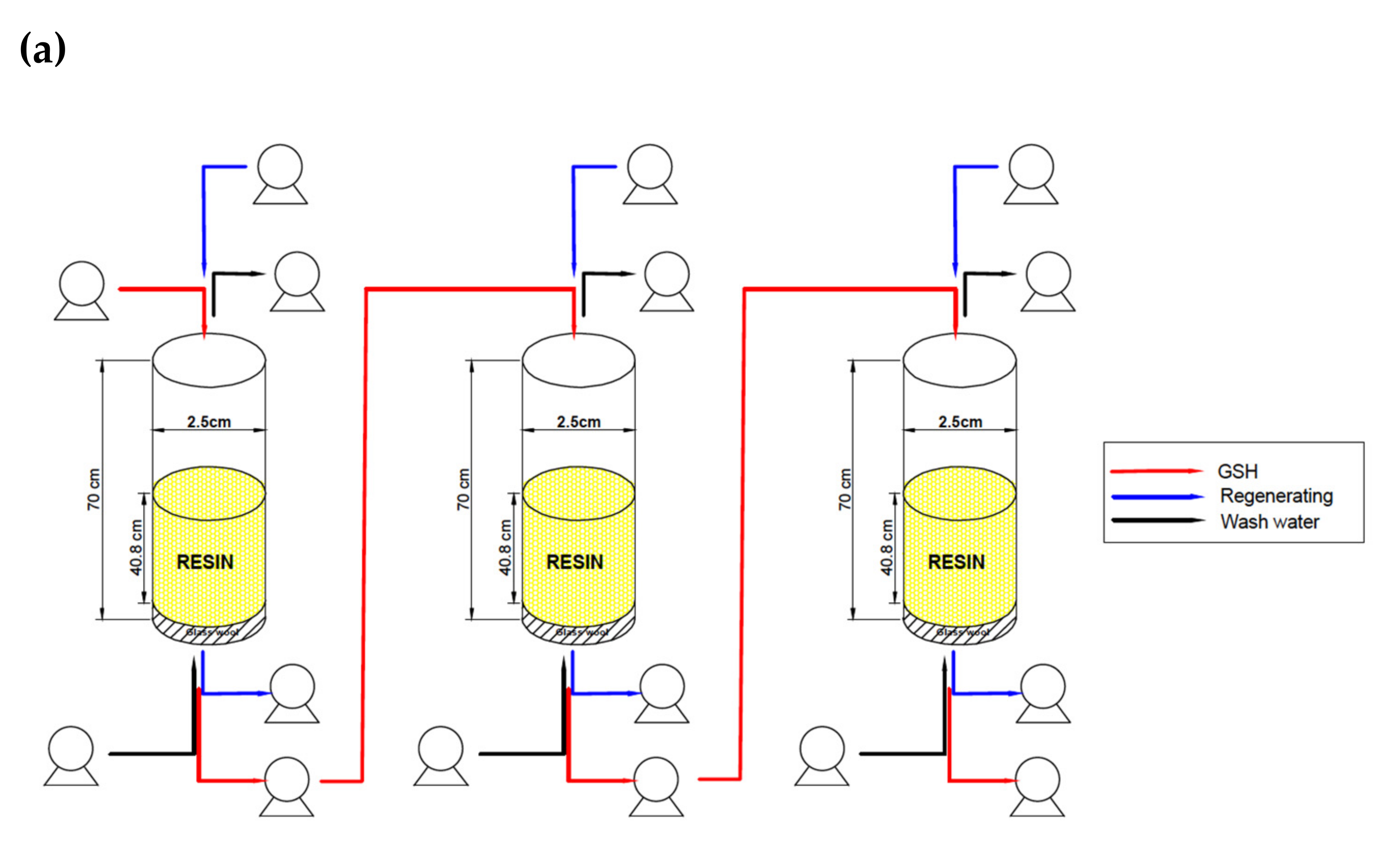
2.5. In-Series Columns System Evaluation
2.6. Statistical Analysis
2.7. GSH Characterization
3. Results and Discussion
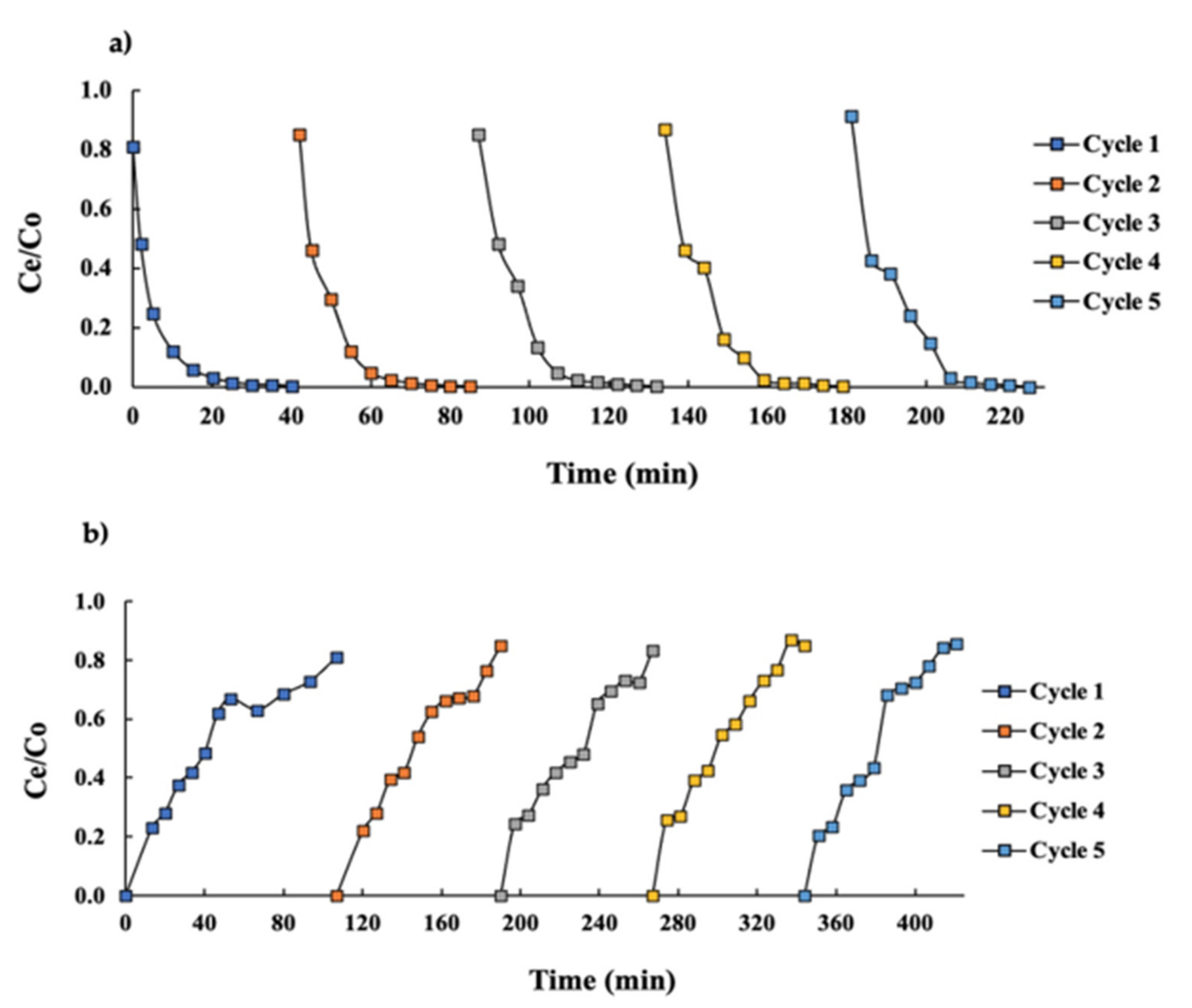
3.1. Breakthrough Curves
3.2. Thomas Modeling in Columns Assays
3.3. Desorption and Reusability System
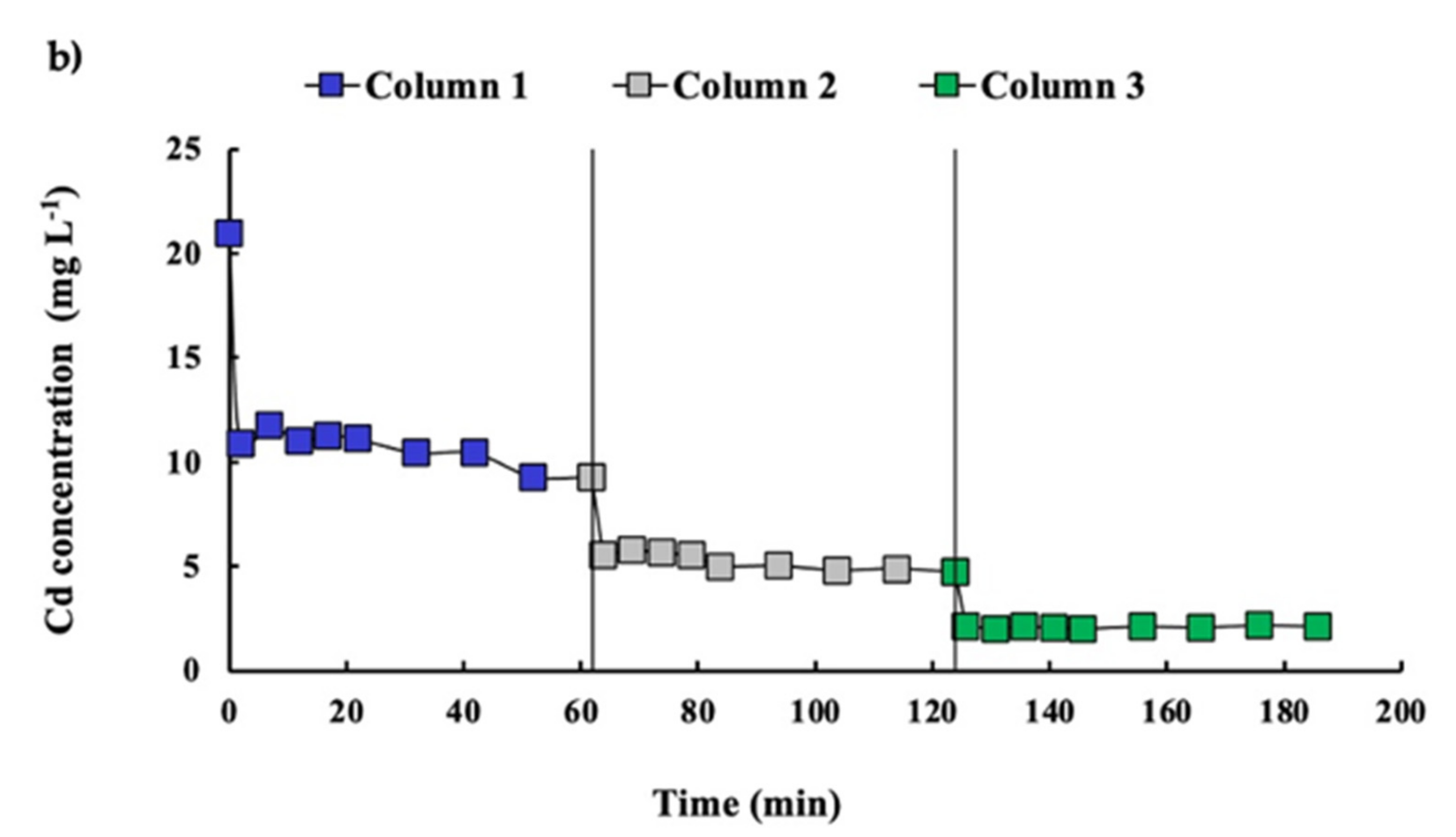
3.4. In-Series Columns System Evaluation
3.5. GSH Characterization
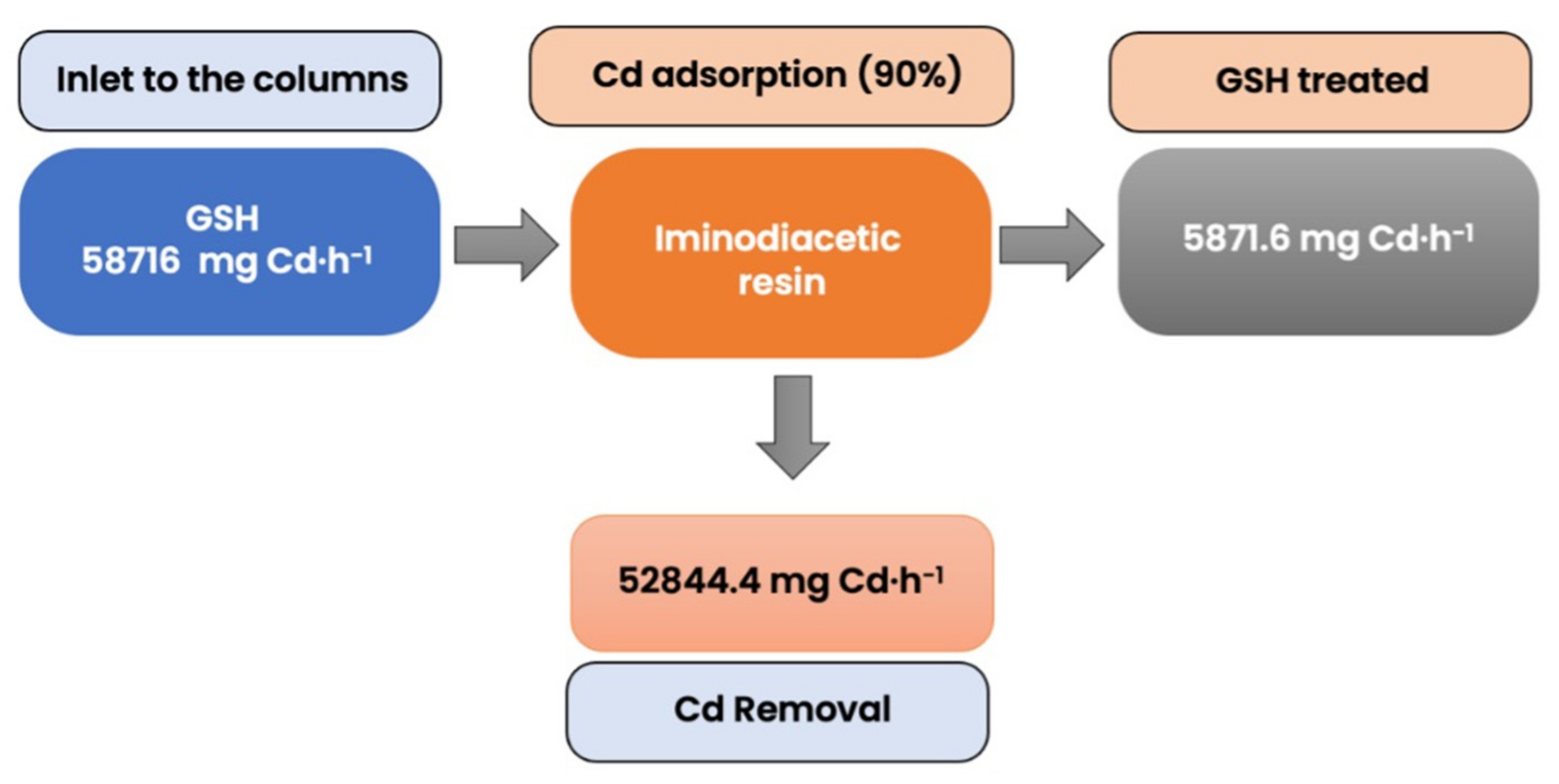
3.6. Mass Balance and Analysis Data for Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Wang, W.X.; Fisher, N.S. Accumulation of trace elements in a marine copepod. Limnol. Oceanogr. 1998, 43, 273–283. [Google Scholar] [CrossRef]
- Raimundo, J.; Vale, C.; Rosa, R. Trace element concentrations in the top predator jumbo squid (Dosidicus gigas) from the Gulf of California. Ecotoxicol. Environ. Saf. 2014, 102, 179–186. [Google Scholar] [CrossRef]
- Sasaki, T.; Araki, R.; Michihata, T.; Kozawa, M.; Tokuda, K.; Koyanagi, T.; Enomoto, T. Removal of cadmium from fish sauce using chelate resin. Food Chem. 2015, 173, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.; Benianno, M.J.; Porte, C. Hepatic levels of metal and metallothioneins in two commercial fish species of the Northern Iberian shelf. Sci. Total Environ. 2008, 391, 159–167. [Google Scholar] [CrossRef]
- Xiong, C.H.; Yao, C.P. Study on the adsorption of cadmium(II) from aqueous solution by D152 resin. J. Hazard. Mater. 2009, 166, 815–820. [Google Scholar] [CrossRef]
- Yelebe, Z.R.; Yelebe, B.Z.; Samuel, R.J. Design of fixed bed column for the removal of metal contaminants from industrial wastewater. J. Eng. Appl. Sci. 2013, 5, 57–66. [Google Scholar]
- Purkayastha, D.; Mishra, U.; Biswas, S. A comprehensive review on Cd(II) removal from aqueous solution. J. Water Process. Eng. 2014, 2, 105–128. [Google Scholar] [CrossRef]
- Seki, H.; Okada, I.; Maruyama, H.; Kawabe, M.; Nakade, A. Removal of Cadmium from Squid Liver Method by Competitive Adsorption Method. Aquac. Sci. 2006, 54, 449–453. [Google Scholar]
- Volesky, B. Sorption and Biosorption; BV Sorbex, Inc.: Saint-Lambert, QC, Canada, 2003. [Google Scholar]
- Calderón, C.; Levio-Raiman, M.; Diez, M.C. Cadmium removal for marine food application: Comparative study of different adsorbents. Int. J. Environ. Sci. Technol. 2021, 1–14. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Sangeetha, K.; Sudha, P.N. Analysis of packed bed adsorption column with nanochitosan/sodium alginate/microcrystalline cellulose bead for copper (II) removal from aqueous solution. IOSR J. Pharm. 2017, 2, 69–78. [Google Scholar]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M.; Sadeghi Pouya, E. Aqueous phase adsorption of cephalexin by walnut shell-based activated carbon: A fixed-bed column study. Appl. Surf. Sci. 2016, 375, 144–153. [Google Scholar] [CrossRef]
- Saleh, A.D.; Sirhan, M.M.; Ismail, A.S. Study sorption and desorption of Cd+2, Pb+2 ions by selected chelating resin to removal them from industrial and environmental wastes. Energy Rep. 2020, 6, 243–249. [Google Scholar] [CrossRef]
- Taha, M.H.; Masoud, A.M.; Khawassek, Y.M.; Hussein, A.E.M.; Aly, H.F.; Guibal, E. Cadmium and iron removal from phosphoric acid using commercial resins for purification purpose. Environ. Sci. Pollut. Res. 2020, 27, 31278–31288. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Vukojević Medvidović, N. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, H.A.; Abdel-Salam, A.H.; Khalil, T.E. The impact of an Amberlite XAD-16-based chelating resin for the removal of aqueous Cd(II) and Pb(II)ions. Microchem. J. 2021, 165, 106097. [Google Scholar] [CrossRef]
- Lázaro, N.; López Sevilla, A.; Morales, S.; Marqués, A.M. Heavy metal biosorption by gellan gum gel beads. Water Res. 2003, 37, 2118–2126. [Google Scholar] [CrossRef]
- Corami, A.; Mignardi, S.; Ferrini, V. Cadmium removal from single- and multi-metal (Cd + Pb + Zn + Cu) solutions by sorption on hydroxyapatite. J. Colloid Interface Sci. 2008, 317, 402–408. [Google Scholar] [CrossRef]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Levio-Raiman, M.; Briceño, G.; Schalchli, H.; Bornhardt, C.; Diez, M.C. Alternative treatment for metal ions removal from acid mine drainage using an organic biomixture as a low cost adsorbent. Environ. Technol. Innov. 2021, 24, 101853. [Google Scholar] [CrossRef]

| Batch N° | Digestive Glands (%) | Cadmium Concentration (mg L−1) | pH |
|---|---|---|---|
| 1 | 70 | 48.27 | 6.16 |
| 2 | 40 | 20.97 | 6.20 |
| 3 | 20 | 12.13 | 6.15 |
| 4 | 5 | 3.26 | 6.11 |
| Initial Cd Concentration (mg L−1) | qmax (mg g−1) | KT (mL min−1 mg−1) | R2 | qtotal (mg g−1) |
|---|---|---|---|---|
| 48.37 | 1137.4 | 0.0014 | 0.972 | 1015.3 |
| 20.97 | 860.4 | 0.0014 | 0.978 | 827.1 |
| 12.13 | 557.4 | 0.0020 | 0.979 | 519.8 |
| 3.26 | 203.1 | 0.0055 | 0.908 | 200.3 |
| Parameter | GSH before Adsorption | GSH after Adsorption |
|---|---|---|
| (% d.w) | (% d.w) | |
| Protein | 83.9 ± 0.9 a | 86.3 ± 0.9 a |
| Fat | 7.8 ± 0.6 b | 5.9 ± 0.1 b |
| Ash | 8.0 ± 0.4 a | 7.6 ± 0.1 a |
| Salt (NaCl) | 4.8 ± 0.2 ab | 3.9 ± 0.1 ab |
| Soluble protein | 63.5 ± 2.5 a | 62.6 ± 0.5 a |
| Digestibility (pepsin) | 99.9 ± 0.0 a | 99.9 ± 0.0 a |
| Metal | GSH before Adsorption (mg kg−1) | GSH after Adsorption (mg kg−1) | Removal (%) |
|---|---|---|---|
| Cadmium (Cd) | 139 ± 3.8 | 14 ± 0.4 | 90.0 |
| Calcium (Ca) | 643 ± 5.3 | 368 ± 17.7 | 34.3 |
| Magnesium (Mg) | 600 ± 15.9 | 190 ± 22.5 | 68.4 |
| Iron (Fe) | 40 ± 1.5 | 9 ± 0.3 | 81.9 |
| Copper (Cu) | 219 ± 8.0 | 23 ± 0.5 | 89.7 |
| Zinc (Zn) | 77 ± 2.8 | 15 ± 0.8 | 78.6 |
| Sodium (Na) | 16,000 ± 870.0 | 12,900 ± 451.5 | 19.4 |
| Aminoacidic Composition | GSH before Adsorption (g (100 g)−1) | GSH after Adsorption (g (100 g)−1) |
|---|---|---|
| Aspartic acid | 3.13 ± 0.130 a | 3.09 ± 0.121 a |
| Serine | 8.03 ± 0.310 b | 5.59 ± 0.123 b |
| Glutamic acid | 12.23 ± 0.220 a | 10.32 ± 0.187 a |
| Glycine | 0.23 ± 0.010 a | 0.38 ± 0.009 a |
| Histidine | 9.86 ± 0.150 b | 12.35 ± 0.121 b |
| Arginine | 8.68 ± 0.230 a | 8.31 ± 0.121 a |
| Threonine | 2.48 ± 0.080 b | 1.98 ± 0.021 b |
| Alanine | 3.42 ± 0.070 b | 2.71 ± 0.052 b |
| Proline | 2.72 ± 0.050 a | 2.23 ± 0.041 a |
| Cysteine | 0.12 ± 0.003 a | 0.13 ± 0.002 a |
| Tyrosine | 1.77 ± 0.020 a | 2.10 ± 0.027 a |
| Valine | 2.71 ± 0.031 a | 2.16 ± 0.028 a |
| Methionine | 1.38 ± 0.040 a | 1.42 ± 0.029 a |
| Lysine | 4.39 ± 0.081 b | 3.06 ± 0.072 b |
| Isoleucine | 2.42 ± 0.056 a | 1.87 ± 0.034 a |
| Leucine | 5.37 ± 0.123 b | 3.48 ± 0.071 b |
| Phenylalanine | 2.40 ± 0.054 a | 1.60 ± 0.036 a |
| Parameter | Value | Unit |
|---|---|---|
| Data for calculations | ||
| Resin packaged per column | 140 | kg |
| N° of columns | 3 | kg |
| Total resin | 420 | kg |
| Flow rate (*) | 2800 | L h−1 |
| Total solids of liquid of GSH (*) | 15.0 | % |
| Cd initial concentration (w.w) | 21.0 | mg L−1 |
| In series total Cd removal (**) | 90 | % |
| Final conc. of Cd (w.w) (**) | 2.1 | mg L−1 |
| Density of liquid GSH (*) | 1.1 | g mL−1 |
| Total solids of dried GSH (*) | 95 | % |
| Cation exchange capacity of resin (***) | 30 | g cation L−1 |
| Parameter calculated | ||
| Cd final concentration (d.w) | 13.9 | mg kg−1 |
| Cd final conc. in powder (5% of moisture) | 13.3 | mg kg−1 |
| Mass of Cd inlet | 58,716.0 | mg Cd h−1 |
| Mass of Cd removed by the resin | 52,844.4 | mg Cd h−1 |
| Mass of Cd outlet the column | 5871.6 | mg Cd h−1 |
| Level of resin exhaustion by Cd | 0.589 | % |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón, C.; Levío-Raimán, M.; Diez, M.C. Cadmium Removal from Giant Squid (Dosidicus gigas) Hydrolysate in Fixed-Bed Columns Packed with Iminodiacetic Resin: Tools for Scaling up the Process. Int. J. Environ. Res. Public Health 2022, 19, 442. https://doi.org/10.3390/ijerph19010442
Calderón C, Levío-Raimán M, Diez MC. Cadmium Removal from Giant Squid (Dosidicus gigas) Hydrolysate in Fixed-Bed Columns Packed with Iminodiacetic Resin: Tools for Scaling up the Process. International Journal of Environmental Research and Public Health. 2022; 19(1):442. https://doi.org/10.3390/ijerph19010442
Chicago/Turabian StyleCalderón, Carolina, Marcela Levío-Raimán, and M. Cristina Diez. 2022. "Cadmium Removal from Giant Squid (Dosidicus gigas) Hydrolysate in Fixed-Bed Columns Packed with Iminodiacetic Resin: Tools for Scaling up the Process" International Journal of Environmental Research and Public Health 19, no. 1: 442. https://doi.org/10.3390/ijerph19010442
APA StyleCalderón, C., Levío-Raimán, M., & Diez, M. C. (2022). Cadmium Removal from Giant Squid (Dosidicus gigas) Hydrolysate in Fixed-Bed Columns Packed with Iminodiacetic Resin: Tools for Scaling up the Process. International Journal of Environmental Research and Public Health, 19(1), 442. https://doi.org/10.3390/ijerph19010442







