The Effect of Extreme Cold on Complete Blood Count and Biochemical Indicators: A Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject’s Characteristics
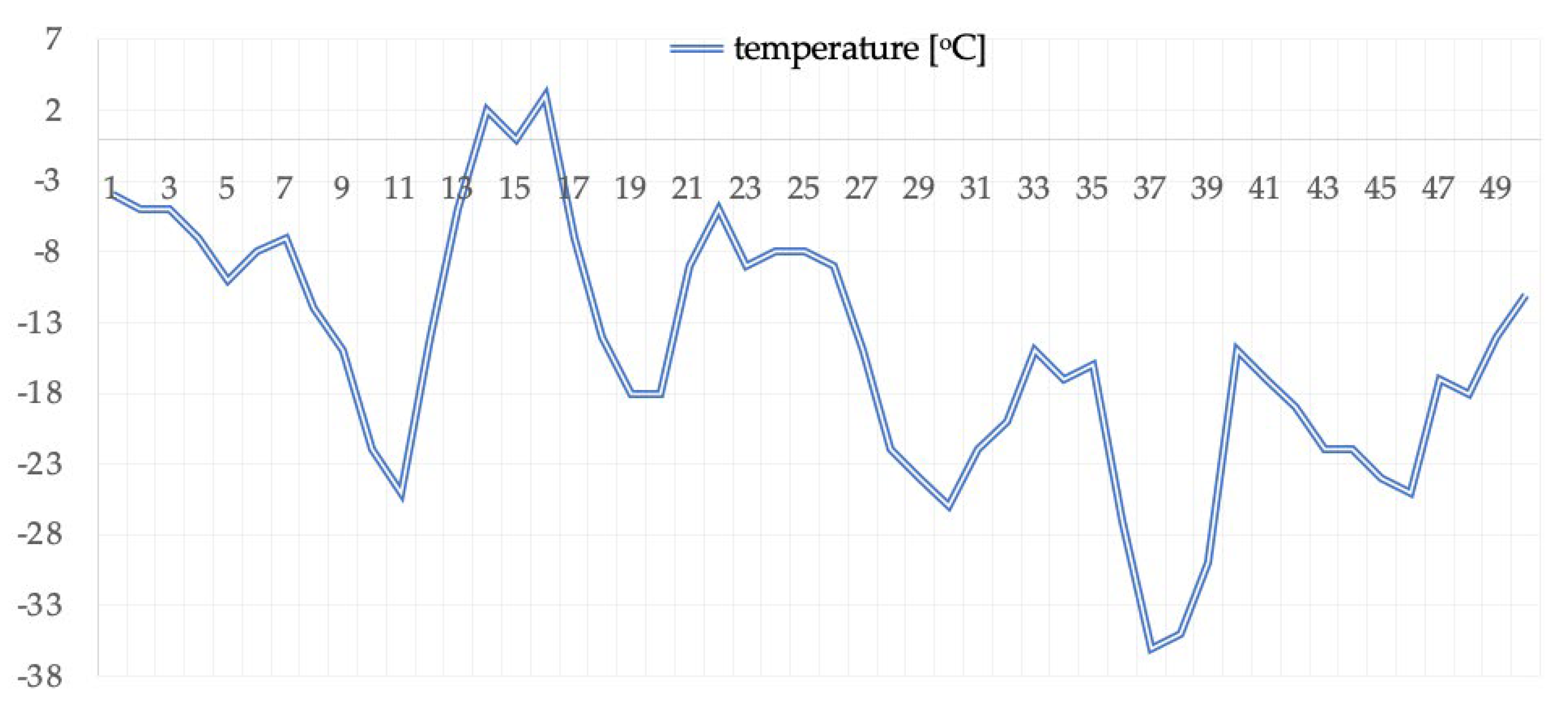
2.2. Scheme of Study Organization
2.3. Somatic Measurements
2.4. Aerobic Capacity Measurements: Graded Test on a Cycle Ergometer
2.5. Complete Blood Count and Biochemical Measurements
2.6. Ethical Considerations
2.7. Calculation Methods
3. Results
3.1. Somatic Parameters
3.2. Complete Blood Count
3.3. Coagulology
3.4. Blood Biochemical Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knechtle, B.; Waśkiewicz, Z.; Sousa, C.V.; Hill, L.; Nikolaidis, P.T. Cold Water Swimming—Benefits and Risks: A Narrative Review. Int. J. Environ. Res. Public. Health 2020, 17, 8984. [Google Scholar] [CrossRef] [PubMed]
- Teległów, A.; Dąbrowski, Z.; Marchewka, A.; Głodzik, J.; Rembiasz, K.; Krawczyk, M.; Marchewka, J.; Wcisło, M.; Ostrowski, A. Effects of Winter Swimming and Whole-Body Cryotherapy on the Hematological and Rheological Properties of Blood in Regular Winter Swimmers and Individuals Exposed to Whole-Body Cryotherapy. Med. Sport. 2014, 18, 52–57. [Google Scholar] [CrossRef]
- Teległów, A.; Marchewka, J.; Marchewka, A.; Kulpa, J. Changes in Biochemical Properties of the Blood in Winter Swimmers. Folia Biol. 2016, 64, 285–290. [Google Scholar] [CrossRef]
- Kolettis, T.M.; Kolettis, M.T. Winter Swimming: Healthy or Hazardous? Evidence and Hypotheses. Med. Hypotheses 2003, 61, 654–656. [Google Scholar] [CrossRef]
- Huttunen, P.; Kokko, L.; Ylijukuri, V. Winter Swimming Improves General Well-Being. Int. J. Circumpolar Health 2004, 63, 140–144. [Google Scholar] [CrossRef]
- van Tulleken, C.; Tipton, M.; Massey, H.; Harper, C.M. Open Water Swimming as a Treatment for Major Depressive Disorder. BMJ Case Rep. 2018, 2018, bcr-2018225007. [Google Scholar] [CrossRef]
- Teległów, A.; Dąbrowski, Z.; Marchewka, A.; Tyka, A.; Krawczyk, M.; Głodzik, J.; Szyguła, Z.; Mleczko, E.; Bilski, J.; Tyka, A.; et al. The Influence of Winter Swimming on the Rheological Properties of Blood. Clin. Hemorheol. Microcirc. 2014, 57, 119–127. [Google Scholar] [CrossRef]
- Pugh, L.G.; Edholm, O.G. The Physiology of Channel Swimmers. Lancet Lond. Engl. 1955, 269, 761–768. [Google Scholar] [CrossRef]
- Biswas, R.; Shibu, P.K.; James, C.M. Pulmonary Oedema Precipitated by Cold Water Swimming. Br. J. Sports Med. 2004, 38, e36. [Google Scholar] [CrossRef]
- Lund, K.L.; Mahon, R.T.; Tanen, D.A.; Bakhda, S. Swimming-Induced Pulmonary Edema. Ann. Emerg. Med. 2003, 41, 251–256. [Google Scholar] [CrossRef]
- Ganta, C.K.; Helwig, B.G.; Blecha, F.; Ganta, R.R.; Cober, R.; Parimi, S.; Musch, T.I.; Fels, R.J.; Kenney, M.J. Hypothermia-Enhanced Splenic Cytokine Gene Expression Is Independent of the Sympathetic Nervous System. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R558–R565. [Google Scholar] [CrossRef]
- Leppäluoto, J.; Westerlund, T.; Huttunen, P.; Oksa, J.; Smolander, J.; Dugué, B.; Mikkelsson, M. Effects of Long-Term Whole-Body Cold Exposures on Plasma Concentrations of ACTH, Beta-Endorphin, Cortisol, Catecholamines and Cytokines in Healthy Females. Scand. J. Clin. Lab. Invest. 2008, 68, 145–153. [Google Scholar] [CrossRef]
- Janský, P.; Janský, L. Sites and Cellular Mechanisms of Human Adrenergic Thermogenesis-a Review. J. Therm. Biol. 2002, 4, 269–277. [Google Scholar] [CrossRef]
- Griffin, J.; Reddin, G. Shoulder Pain in Patients with Hemiplegia. A Literature Review. Phys. Ther. 1981, 61, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Sockler, J.M.; Fry, J.M. Thermoregulatory, Metabolic and Sympathoadrenal Responses to Repeated Brief Exposure to Cold. Scand. J. Clin. Lab. Invest. 1998, 58, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Nadler, S.F.; Weingand, K.; Kruse, R.J. The Physiologic Basis and Clinical Applications of Cryotherapy and Thermotherapy for the Pain Practitioner. Pain Physician 2004, 7, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu I Wartości Odżywczej Żywności; PZWL: Warszawa, Poland, 2020. [Google Scholar]
- Vogelaere, P.; Brasseur, M.; Quirion, A.; Leclercq, R.; Laurencelle, L.; Bekaert, S. Hematological Variations at Rest and during Maximal and Submaximal Exercise in a Cold (0 °C) Environment. Int. J. Biometeorol. 1990, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Banfi, G.; Dołegowska, B.; d’Eril, G.V.M.; Łuczak, J.; Barassi, A. Changes in Lipid Profile in Response to Three Different Protocols of Whole-Body Cryostimulation Treatments. Cryobiology 2010, 61, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Brenner, I.K.; Castellani, J.W.; Gabaree, C.; Young, A.J.; Zamecnik, J.; Shephard, R.J.; Shek, P.N. Immune Changes in Humans during Cold Exposure: Effects of Prior Heating and Exercise. J. Appl. Physiol. 1999, 87, 699–710. [Google Scholar] [CrossRef]
- Lombardi, G.; Ricci, C.; Banfi, G. Effect of Winter Swimming on Haematological Parameters. Biochem. Med. 2011, 21, 71–78. [Google Scholar] [CrossRef]
- Sen, U.; Tyagi, N.; Patibandla, P.K.; Dean, W.L.; Tyagi, S.C.; Roberts, A.M.; Lominadze, D. Fibrinogen-Induced Endothelin-1 Production from Endothelial Cells. Am. J. Physiol. Cell Physiol. 2009, 296, C840–C847. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, C.; Tran, N.; Paulus, P.; Ellinghaus, P.; Eble, J.A.; Zacharowski, K. Novel Aspects of Fibrin(Ogen) Fragments during Inflammation. Mol. Med. 2011, 17, 568–573. [Google Scholar] [CrossRef]
- Szaba, F.M.; Smiley, S.T. Roles for Thrombin and Fibrin(Ogen) in Cytokine/Chemokine Production and Macrophage Adhesion In Vivo. Blood 2002, 99, 1053–1059. [Google Scholar] [CrossRef]
- Gauldie, J.; Richards, C.; Baumann, H. IL6 and the Acute Phase Reaction. Res. Immunol. 1992, 143, 755–759. [Google Scholar] [CrossRef]
- Dugué, B.; Leppänen, E. Adaptation Related to Cytokines in Man: Effects of Regular Swimming in Ice-Cold Water. Clin. Physiol. Oxf. Engl. 2000, 20, 114–121. [Google Scholar] [CrossRef]
- Castellani, J.W.; Degroot, D.W. Human Endocrine Responses to Exercise-Cold Stress; US Army Research Institute of Environmental Medicine (USARIEM), Thermal and Mountain Medicine Division: Natick, MA, USA, 2005. [Google Scholar]
- Dinarello, C.A. Historical Review of Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef]
- Favrot, M.; Negrier, S.; Combaret, V.; Philip, T. Cytokines and their receptors networks: Biology and therapeutic possibilities. Pediatrie 1989, 44, 531–538. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Janský, L.; Pospísilová, D.; Honzová, S.; Ulicný, B.; Srámek, P.; Zeman, V.; Kamínková, J. Immune System of Cold-Exposed and Cold-Adapted Humans. Eur. J. Appl. Physiol. 1996, 72, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Melegati, G.; Barassi, A.; Dogliotti, G.; Melzi d’Eril, G.; Dugué, B.; Corsi, M.M. Effects of Whole-Body Cryotherapy on Serum Mediators of Inflammation and Serum Muscle Enzymes in Athletes. J. Therm. Biol. 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Banfi, G.; Lombardi, G.; Colombini, A.; Melegati, G. Whole-Body Cryotherapy in Athletes. Sports Med. Auckl. NZ 2010, 40, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Kaczor, J.J.; Antosiewicz, J.; Skrobot, W.; Kujach, S.; Laskowski, R. Whole-Body Cryostimulation as an Effective Way of Reducing Exercise-Induced Inflammation and Blood Cholesterol in Young Men. Eur. Cytokine Netw. 2014, 25, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Chao, T.; Porter, C.; Annamalai, P.; Yfanti, C.; Labbe, S.M.; Hurren, N.M.; Malagaris, I.; et al. Brown adipose tissue is linked to a distinct thermoregulatory response to mild cold in people. Front. Physiol. 2016, 7, 129. [Google Scholar] [CrossRef]
- Stangier, C.; Abel, T.; Mierau, J.; Hollmann, W.; Strüder, H.K. Effects of cycling versus running training on sprint and endurance capacity in inline speed skating. J. Sports Sci. Med. 2016, 15, 41–49. [Google Scholar]
- Hottenrott, K.; Ludyga, S.; Schulze, S. Effects of high intensity training and continuous endurance training on aerobic capacity and body composition in recreationally active runners. J. Sports Sci. Med. 2012, 11, 483–488. [Google Scholar]
- Paoli, A.; Pacelli, Q.F.; Moro, T.; Marcolin, G.; Neri, M.; Battaglia, G.; Sergi, G.; Bolzetta, F.; Bianco, A. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis. 2013, 12, 131. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Teległów, A.; Simoniuk, A.; Ryś, B.; Marchewka, J.; Marchewka, A. Effect of Winter Swimming and Taking in a Sauna on the Biochemical Properties of the Blood in “Walruses”. J. Kinesiol. Exerc. Sci. 2014, 68, 11–17. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Nonshivering Thermogenesis and Its Adequate Measurement in Metabolic Studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef]
- Hildebrand, F.; Giannoudis, P.V.; van Griensven, M.; Chawda, M.; Pape, H.-C. Pathophysiologic Changes and Effects of Hypothermia on Outcome in Elective Surgery and Trauma Patients. Am. J. Surg. 2004, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Suganuma, K.; Funase, Y.; Yamauchi, K.; Aizawa, T. Elevation of Thyrotropin upon Accidental Hypothermia in an Elderly Man. Thyroid 2012, 22, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Tipton, K.D.; Doyle, D.; Phillips, S.M.; Cortiella, J.; Wolfe, R.R. Testosterone Injection Stimulates Net Protein Synthesis but Not Tissue Amino Acid Transport. Am. J. Physiol. 1998, 275, E864–E871. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Wakabayashi, I.; Yoshimoto, S.; Masui, H.; Katsuno, S. Effects of Physical Exercise and Cold Stimulation on Serum Testosterone Level in Men. Nihon Eiseigaku Zasshi Jpn. J. Hyg. 1991, 46, 635–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujibayashi, S.; Yoshida, K. Effect of Sojourn at High Altitude (4250 m) in Peru on Plasma Testosterone Level of Peruvian Lowlanders. Jpn. J. Biometeorol. 1981, 18, 80–85. [Google Scholar]
- Ismail, A.H.; Young, R.J. Effect of Chronic Exercise on the Personality of Adults. Ann. N. Y. Acad. Sci. 1977, 301, 958–969. [Google Scholar] [CrossRef]
| Index → Measurement ↓ | BM [kg] | BCM [kg] | FFM [kg] | FM [kg] | F% [%] | TBW [L] | ECW [L] |
|---|---|---|---|---|---|---|---|
| Measurement I | 85.5 | 41.2 | 65.9 | 19.6 | 23.0 | 51.4 | 22.3 |
| Measurement II | 76.3 | 36.0 | 62.4 | 13.9 | 17.8 | 44.1 | 17.2 |
| Parameters | t [min] | P [W] | HR [Beats/min] | VO2 (L∙min−1) | VO2/kg (mL·min−1·kg−1) | Ve [L/min] |
|---|---|---|---|---|---|---|
| VT2 | 14.00 | 225 | 138 | 3.24 | 37.9 | 78.4 |
| max | 21.00 | 330 | 159 | 4.59 | 53.7 | 132.3 |
| Analyzed Parameter (Unit of Measure) | Results before Exposure to Extreme Cold | Results after Exposure to Extreme Cold | Change Compared to the Result before (% of Change) | Standard |
|---|---|---|---|---|
| RBC [×10^12/L] | 4.74 | 4.55 | −4.01 | 4.6–6.2 |
| HGB [g/dL] | 14.4 | 13.9 | −3.47 | 14–18 |
| HCT [%] | 44.1 | 42.6 | −3.40 | 42–52 |
| MCV [fl] | 93.1 | 93.7 | 0.64 | 82–94 |
| MCH [pg] | 30.5 | 30.6 | 0.33 | 27–32 |
| MCHC [g/dL] | 32.7 | 32.7 | no change | 30–38 |
| CHCM [g/dL] | 33.3 | 32.6 | −2.10 | 31–37 |
| RDW [%] | 13 | 12.4 | −4.62 | 11.5–14.5 |
| HDW [g/dL] | 2.48 | 2.51 | 1.21 | 2.2–3.2 |
| WBC [×10^9/L] | 4.18 | 3.28 | −21.53 | 4–10 |
| Neutrocytes [×10^9/L] | 2.08 | 1.72 | −17.31 | 2.2–7.0 |
| Eosinocytes [×10^9/L] | 0.19 | 0.1 | −47.37 | 0.0–0.5 |
| Basophils [×10^9/L] | 0.05 | 0.04 | −20.00 | 0.0–0.1 |
| Lymphocytes [×10^9/L] | 1.49 | 1.09 | −26.85 | 1.0–3.5 |
| Monocytes [×10^9/L] | 0.29 | 0.27 | −6.90 | 0.0–0.9 |
| PLT [×10^9/L] | 200 | 185 | −7.50 | 150–400 |
| MPV [fl] | 10.3 | 9.6 | −6.80 | 7.2–11.1 |
| PCT [%] | 0.21 | 0.18 | −14.29 | 0.120–0.380 |
| PDW [%] | 65.9 | 69.4 | 5.31 | 28.0–60.0 |
| Coagulology | ||||
| Prothrombin activity [%] | 92.17 | 110 | 19.34 | 80–130 |
| INR | 1.04 | 0.9 | −13.46 | 0.85–1.15 |
| aPTT [s] | 25.7 | 26.9 | 4.67 | 20.0–36.0 |
| Fibrinogen [g/L] | 2.13 | 2.65 | 24.41 | 1.8–3.5 |
| D-dimer [mg/L] | 0.17 | 0.102 | −40.00 | <0.5 |
| Biochemistry | ||||
| Iron [umol/L] | 23.3 | 17.2 | −26.18 | 14.0–32.0 |
| B12 [pg/mL] | 406 | 365 | −10.10 | 200–900 |
| Total bilirubin [umol/L] | 9.4 | 5.6 | −40.43 | 2.0–21.0 |
| ALP [U/L] | 44.4 | 56.1 | 26.35 | |
| AspAT [U/L] | 26.7 | 40.8 | 52.81 | 5–38 |
| AlAT [U/L] | 30.4 | 51.3 | 68.75 | 5–41 |
| GGT [U/L] | 13 | 12 | −7.69 | 5–55 |
| LDH [U/L] | 179.6 | 171.3 | −4.62 | 100–225 |
| Total protein [g/L] | 76.8 | 71.4 | −7.03 | 64–83 |
| Albumin [g/L] | 48.2 | 44.2 | −8.30 | 38.0–49.0 |
| Alpha-1-globulins [g/L] | 2.4 | 2.6 | 8.33 | 2.4–4.0 |
| Alpha-2-globulins [g/L] | 5.3 | 5.7 | 7.55 | 4.8–9.0 |
| Beta-1-globulins [g/L] | 5 | 4.8 | −4.00 | 3.2–6.3 |
| Beta-2-globulins [g/L] | 3.8 | 3.4 | −10.53 | 2.2–5.2 |
| Gamma globulins [g/L] | 12.2 | 10.7 | −12.30 | 6.8–15.0 |
| AG index | 1.68 | 1.62 | −3.57 | |
| IgG [g/L] | 12.2 | 11.3 | −7.38 | 6.9–14.0 |
| IgA [g/L] | 3.2 | 2.9 | −9.38 | 0.9–4.1 |
| IgM [g/L] | 1.5 | 1.2 | −20.00 | 0.3–2.1 |
| CRP [mg/L] | 0.21 | 0.26 | 23.81 | <5.0 |
| Prealbumins [g/L] | 0.377 | 0.319 | −15.38 | 0.210–0.410 |
| CK [U/L] | 239.3 | 259.9 | 8.61 | 24–198 |
| CK-MB [U/L] | 12.9 | 13.3 | 3.10 | 4–24 |
| Total cholesterol [mmol/L] | 6.29 | 5.92 | −5.88 | 3.0–5.0 |
| HDL [mmol/L] | 1.1 | 1.41 | 28.18 | >1.0 |
| LDL [mmol/L] | 4.9 | 4.31 | −12.04 | <3.0 |
| TG [mmol/L] | 1.82 | 1.46 | −19.78 | <1.7 |
| Alpha-amylase [U/L] | 30.7 | 30.3 | −1.30 | 28–100 |
| Lipase [U/L] | 25 | 30.8 | 23.20 | 13–60 |
| Glucose [mmol/L] | 5.89 | 5.21 | −11.54 | 3.9–5.5 |
| HBA1C [%] | 5.2 | 4.7 | −9.62 | 4.0–6.5 |
| Immunochemistry | ||||
| Testosterone [nmol/L] | 23.33 | 37.36 | 60.14 | 4.94–32.01 |
| NTproBNP [pg/mL] | 33.5 | 22.5 | −32.84 | ≤125 |
| Troponin I [pg/mL] | 10 | 10 | no change | 0.26.2 |
| TSH [microIU/mL] | 0.72 | 0.78 | 8.33 | 0.35–4.94 |
| FT3 [pmol/L] | 5.76 | 5.31 | −7.81 | 2.63–5.70 |
| FT4 [pmol/L] | 15.58 | 11.44 | −26.57 | 9.0–19.0 |
| IL6 [pg/mL] | 1.5 | 2.4 | 60.00 | ≤7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teległów, A.; Romanovski, V.; Skowron, B.; Mucha, D.; Tota, Ł.; Rosińczuk, J.; Mucha, D. The Effect of Extreme Cold on Complete Blood Count and Biochemical Indicators: A Case Study. Int. J. Environ. Res. Public Health 2022, 19, 424. https://doi.org/10.3390/ijerph19010424
Teległów A, Romanovski V, Skowron B, Mucha D, Tota Ł, Rosińczuk J, Mucha D. The Effect of Extreme Cold on Complete Blood Count and Biochemical Indicators: A Case Study. International Journal of Environmental Research and Public Health. 2022; 19(1):424. https://doi.org/10.3390/ijerph19010424
Chicago/Turabian StyleTeległów, Aneta, Valerjan Romanovski, Beata Skowron, Dawid Mucha, Łukasz Tota, Joanna Rosińczuk, and Dariusz Mucha. 2022. "The Effect of Extreme Cold on Complete Blood Count and Biochemical Indicators: A Case Study" International Journal of Environmental Research and Public Health 19, no. 1: 424. https://doi.org/10.3390/ijerph19010424
APA StyleTeległów, A., Romanovski, V., Skowron, B., Mucha, D., Tota, Ł., Rosińczuk, J., & Mucha, D. (2022). The Effect of Extreme Cold on Complete Blood Count and Biochemical Indicators: A Case Study. International Journal of Environmental Research and Public Health, 19(1), 424. https://doi.org/10.3390/ijerph19010424









