Transient and Recurrent Vision Loss in a High-Altitude Porter from Pakistan on a Polish Winter Karakoram Expedition
Abstract
1. Introduction
2. Materials and Methods
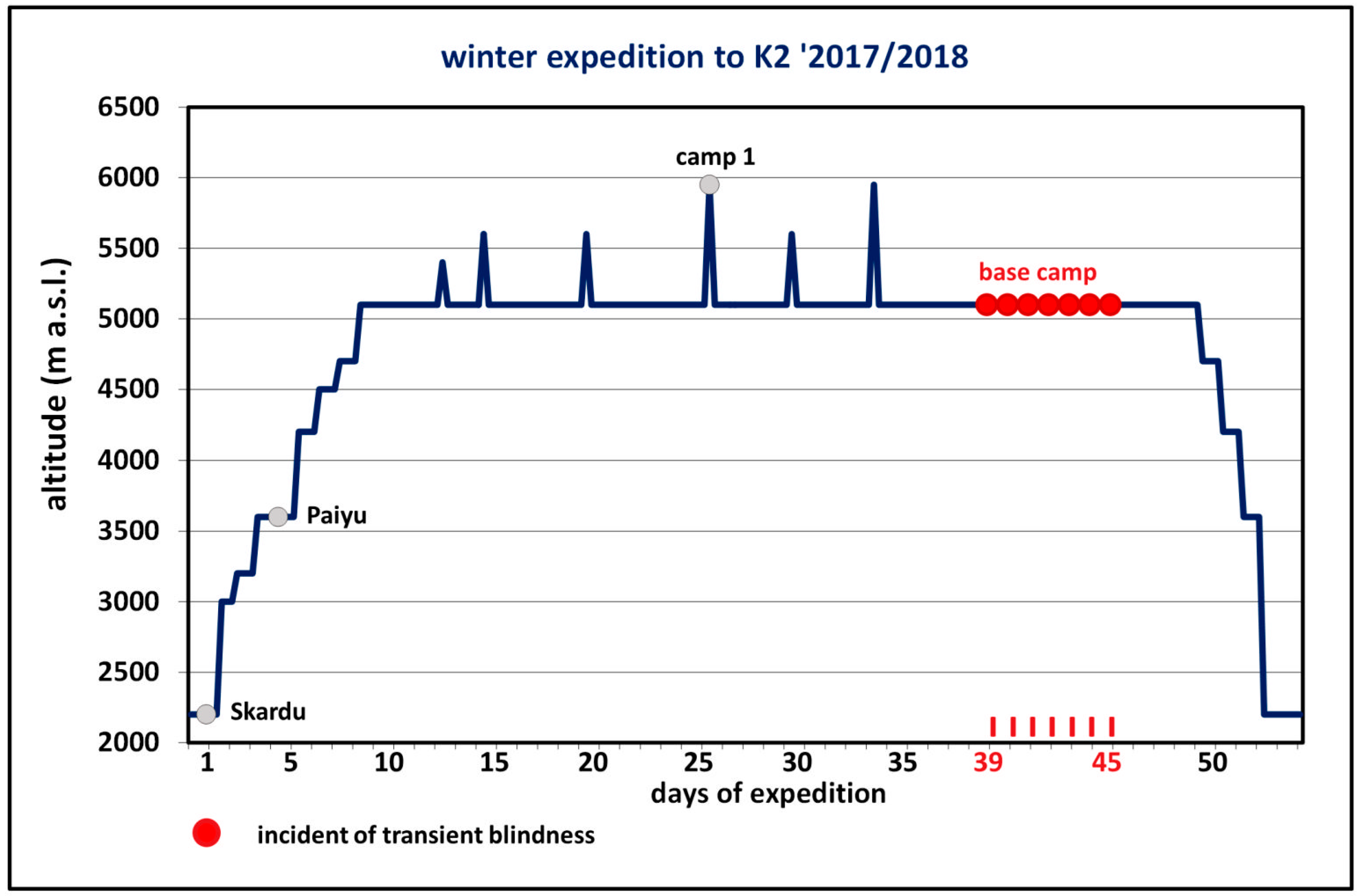
Case Description
3. Results
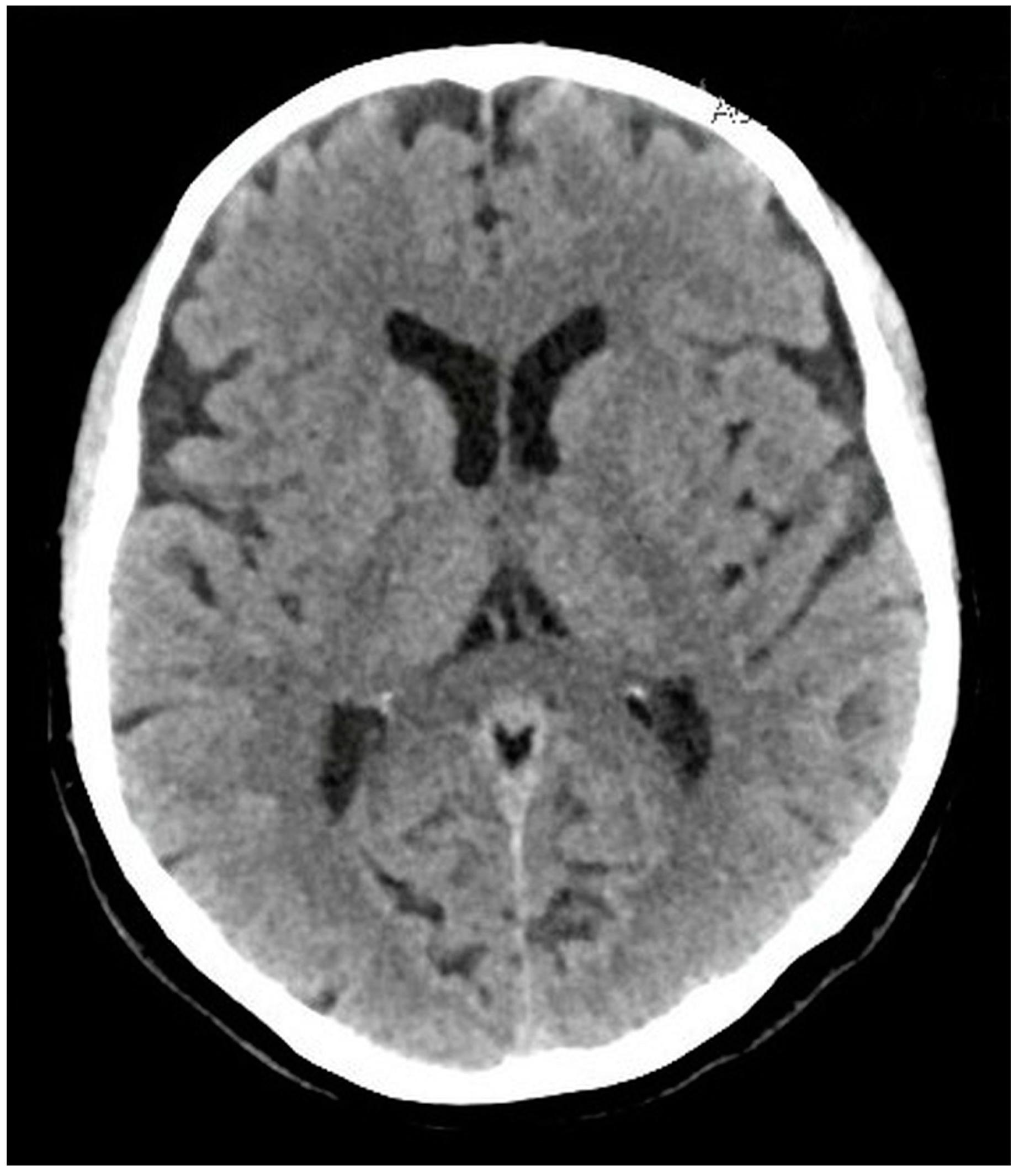
Diagnostic Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, C. Migraine with aura. Rev. Neurol. 2021, 177, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Dilwali, S.; Friedman, D.I. Migraine Aura Without Headache [corrected]. Curr. Pain Headache Rep. 2018, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Faulhaber, M.; Messlinger, K.; Accinelli, C.; Peball, M.; Schiefecker, A.; Kaltseis, K.; Burtscher, M.; Broessner, G. Migraine and aura triggered by normobaric hypoxia. Cephalalgia 2020, 40, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Nie, Z. Typical aura without headache: A case report and review of the literature. J. Med. Case Rep. 2015, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Pescador Ruschel, M.A.; De Jesus, O. Migraine Headache; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar] [PubMed]
- Rozen, T.D. Migraine with binocular blindness: A clinic-based study. Headache 2011, 51, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Hadjikhani, N.; Vincent, M. Visual Perception in Migraine: A Narrative Review. Vision 2021, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ashraf, M.Z. Exposure to High Altitude: A Risk Factor for Venous Thromboembolism? Semin. Thromb. Hemost. 2012, 38, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, M.; Szymczak, R.K. Paradoxical embolism to the central nervous system in a young Polish woman on a trek in the Himalayas. J. Travel Med. 2021, taab110. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Tronvik, E.A.; Do, T.P.; Zecca, C.; Anders Hougaard, A. Clinical features of visual migraine aura: A systematic review. J. Headache Pain. 2019, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Shlim, D.R.; Nepal, K.; Meijer, H.J. Suddenly symptomatic brain tumors at altitude. Ann. Emerg. Med. 1991, 20, 315–316. [Google Scholar] [CrossRef]
- Speidel, V.; Purrucker, J.C.; Klobučníková, K. Manifestation of Intracranial Lesions at High Altitude: Case Report and Review of the Literature. High Alt. Med. Biol. 2021, 22, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, J. Hypoxia, a turning point in migraine pathogenesis? Brain 2016, 139, 644–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Britze, J.; Arngrim, N.; Schytz, H.W.; Ashina, M. Hypoxic mechanisms in primary headaches. Cephalalgia 2017, 37, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Broessner, G.; Rohregger, J.; Wille, M.; Lackner, P.; Ndayisaba, J.P.; Burtscher, M. Hypoxia triggers high-altitude headache with migraine features: A prospective trial. Cephalalgia 2016, 36, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J. Triggers, Protectors, and Predictors in Episodic Migraine. Curr. Pain Headache Rep. 2018, 22, 81. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, R.K.; Sawicka, M. Transient and Recurrent Vision Loss in a High-Altitude Porter from Pakistan on a Polish Winter Karakoram Expedition. Int. J. Environ. Res. Public Health 2021, 18, 12204. https://doi.org/10.3390/ijerph182212204
Szymczak RK, Sawicka M. Transient and Recurrent Vision Loss in a High-Altitude Porter from Pakistan on a Polish Winter Karakoram Expedition. International Journal of Environmental Research and Public Health. 2021; 18(22):12204. https://doi.org/10.3390/ijerph182212204
Chicago/Turabian StyleSzymczak, Robert K., and Magdalena Sawicka. 2021. "Transient and Recurrent Vision Loss in a High-Altitude Porter from Pakistan on a Polish Winter Karakoram Expedition" International Journal of Environmental Research and Public Health 18, no. 22: 12204. https://doi.org/10.3390/ijerph182212204
APA StyleSzymczak, R. K., & Sawicka, M. (2021). Transient and Recurrent Vision Loss in a High-Altitude Porter from Pakistan on a Polish Winter Karakoram Expedition. International Journal of Environmental Research and Public Health, 18(22), 12204. https://doi.org/10.3390/ijerph182212204






