Design and Development of an eHealth Service for Collaborative Self-Management among Older Adults with Chronic Diseases: A Theory-Driven User-Centered Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Theoretical Underpinnings
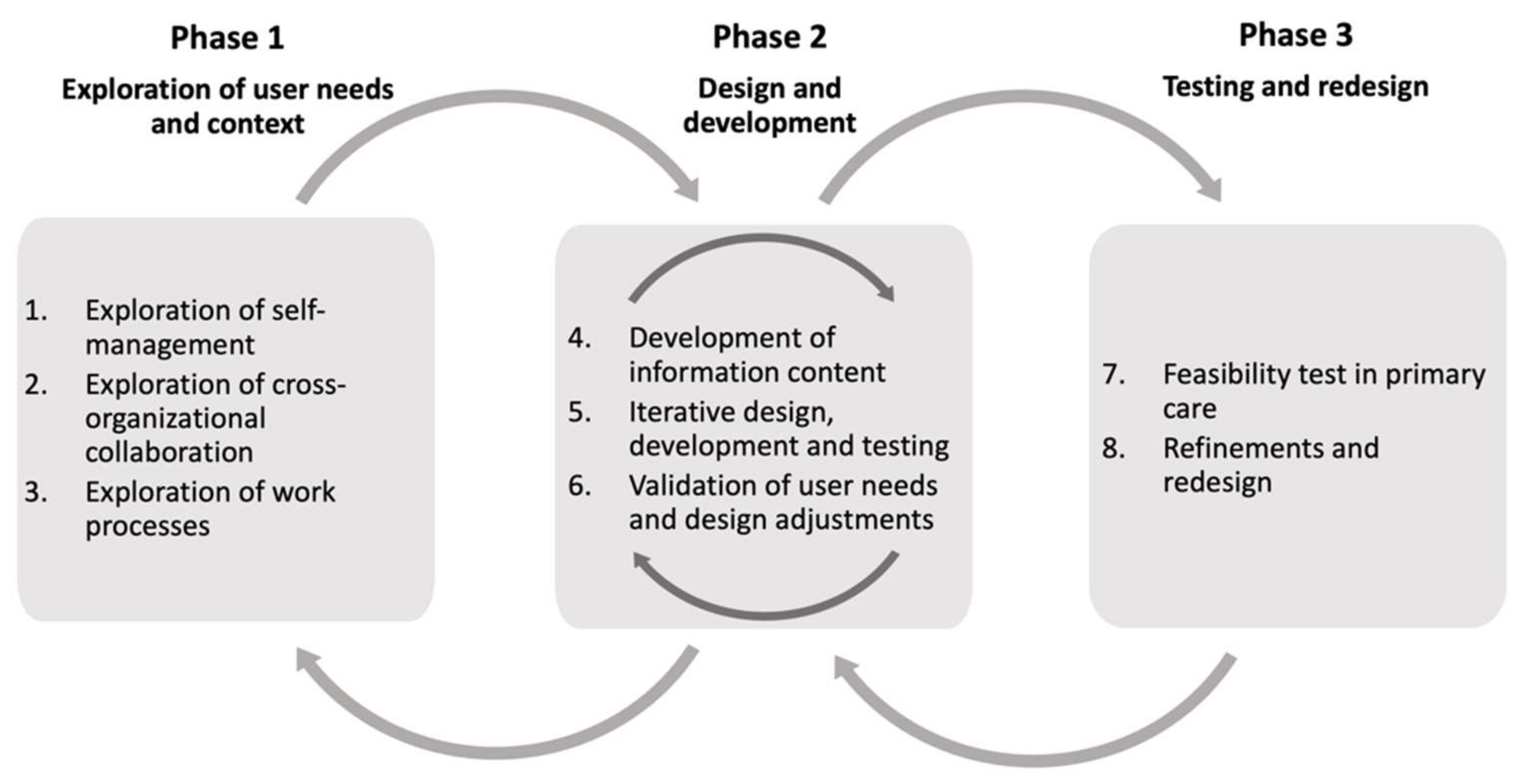
2.3. Overview of the User-Centered Design Process
2.4. Setting and Participants
2.5. Phase 1—Exploration of User Needs
2.5.1. Step 1—Exploration of Self-Management
2.5.2. Step 2—Exploration of Cross-Organizational Collaboration
2.5.3. Step 3—Exploration of Work Processes
2.6. Phase 2—Design and Development
2.6.1. Step 4—Development of Information Content
2.6.2. Step 5—Iterative Software Design, Development, and Testing
2.6.3. Step 6—Validation of User Needs and Design Adjustments
2.7. Phase 3—Testing and Redesign
2.7.1. Step 7—Feasibility Test in Primary Care
2.7.2. Step 8—Refinements and Redesign
3. Results
3.1. Phase 1—Exploration of User Needs
3.1.1. Theme 1—Diagnosis-Specific Information
In the best of worlds, there would be systems for carrying information around and not having to have it in your head or on paper slips or something like that … a good anamnesis too, so you’re not, like, starting from scratch.(Staff)
But, like, I think this is really exciting, because I … I think that, like, this technology with gathering information, and then it can really be facilitated by so much being gathered there, and I know what it says there, I have access to it.(Patient)
3.1.2. Theme 2—Medication Management Support
A lot of people have misunderstood the medication list, I think. … or they’ve taken their medications like they should, but haven’t really, like, understood what it’s all about.(Staff)
The last time I was admitted to hospital, there was a lady (doctor) there who took care of all that and she had rewritten the medication list in a very clear and simply way, with reasons and causes for the tablets and what they were for. And a list like that, where you get both the regular support for filling up the pill organizers and because you can see that this tablet is for that particular thing.(Patient)
3.1.3. Theme 3—Self-Management Support
So maybe they don’t weigh themselves every day either, so they don’t, like, notice right away when they start to gain weight, it’s just suddenly: “Oh, but now I weigh ten kilos more than I did two weeks ago.” But if they had weighed themselves every day, then maybe they would have noticed that already on day two, maybe. And then they could have gone to the care center and just: “I’ve started to gain weight.”(Staff)
So it feels like it kind of depends on when they got the diagnosis. If they got, let’s say heart failure or COPD when they were maybe fifty to sixty, then they know more about it, they’ve had it for a few years and are more familiar with it. But if they get heart failure or COPD when they’re like eighty-two, then it feels like they can’t be bothered to take in that information, it feels like they think like: “But you can solve that.”(Staff)
It really has to be easy to get started, to get into it, of course … I mean, of course, it can’t be anything childish, but I mean, like, something like … “This week you’ve exercised every single day or … seven times, well done!” I think you would see that as something positive, like feedback … I think maybe you could have one of those simple, that you just have like a smiley, instead of having to write.(Staff)
3.1.4. Theme 4—Care Coordination Support
That kind of information could be there, that if the home care staff doesn’t show up, you call such and such and …? Yeah, or if you feel uncertain or anything like that, and that there is contact information.(Staff)
3.1.5. Theme 5—Psychosocial Support
The thing you think about most is the enormous difference between living in a home with your wife, active and strong, so strong that she does all the day-to-day stuff. You think about that, that is an enormous difference. All those people who live alone and only have an alarm button. I have a, a safe surrounding, without having to use, use an alarm button. Now that is the big difference.(Patient)
If you think like this, that I know … there were next-of-kin, if there had been written information, then maybe that could have been shared with them … by e-mail or like … or through some other system … that they are included in this too and can support her in it.(Staff)
3.2. Phase 2—Design and Development
3.2.1. Information Content
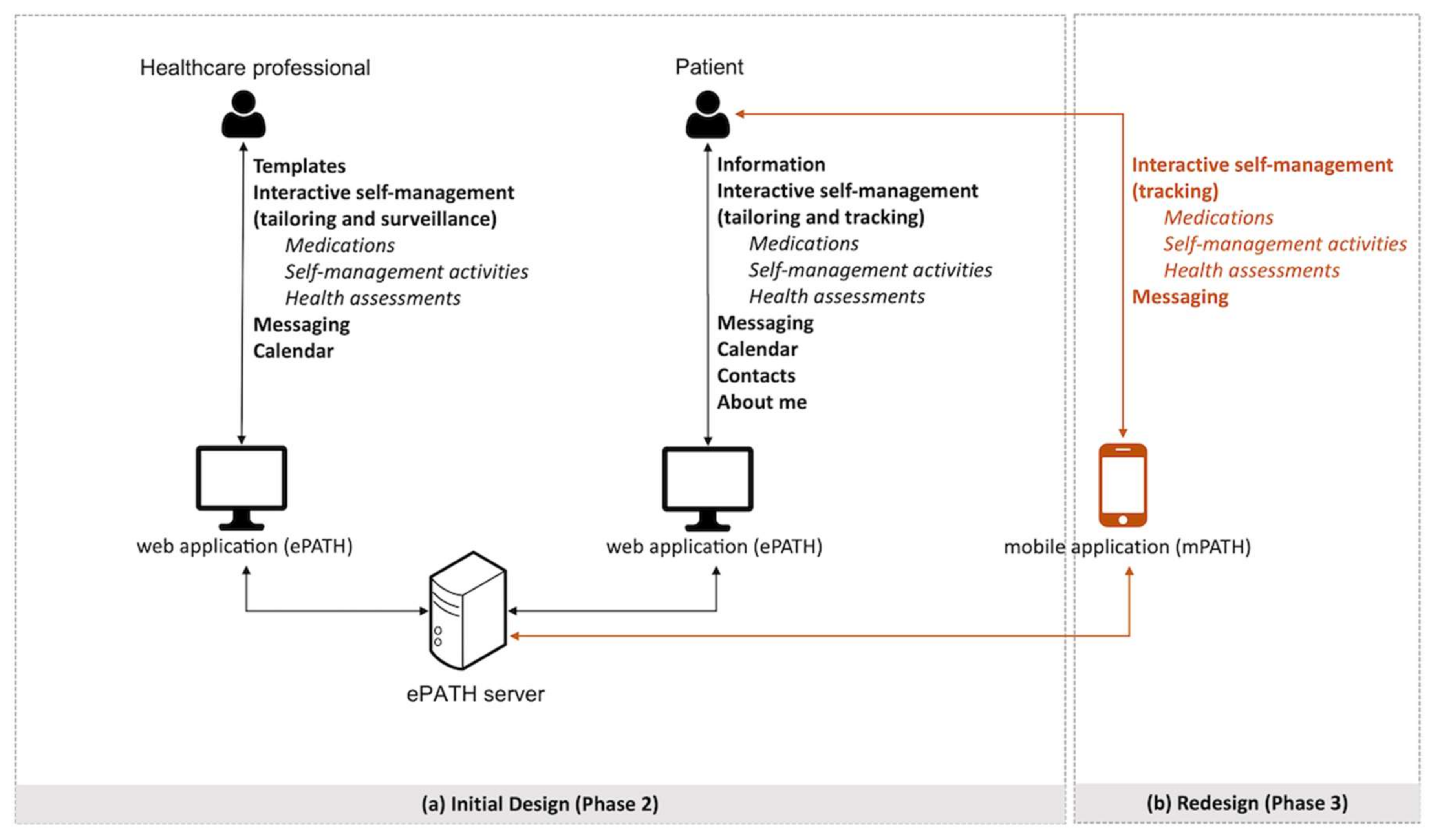
3.2.2. eHealth Service
3.2.3. Templates Module
3.2.4. Information Module
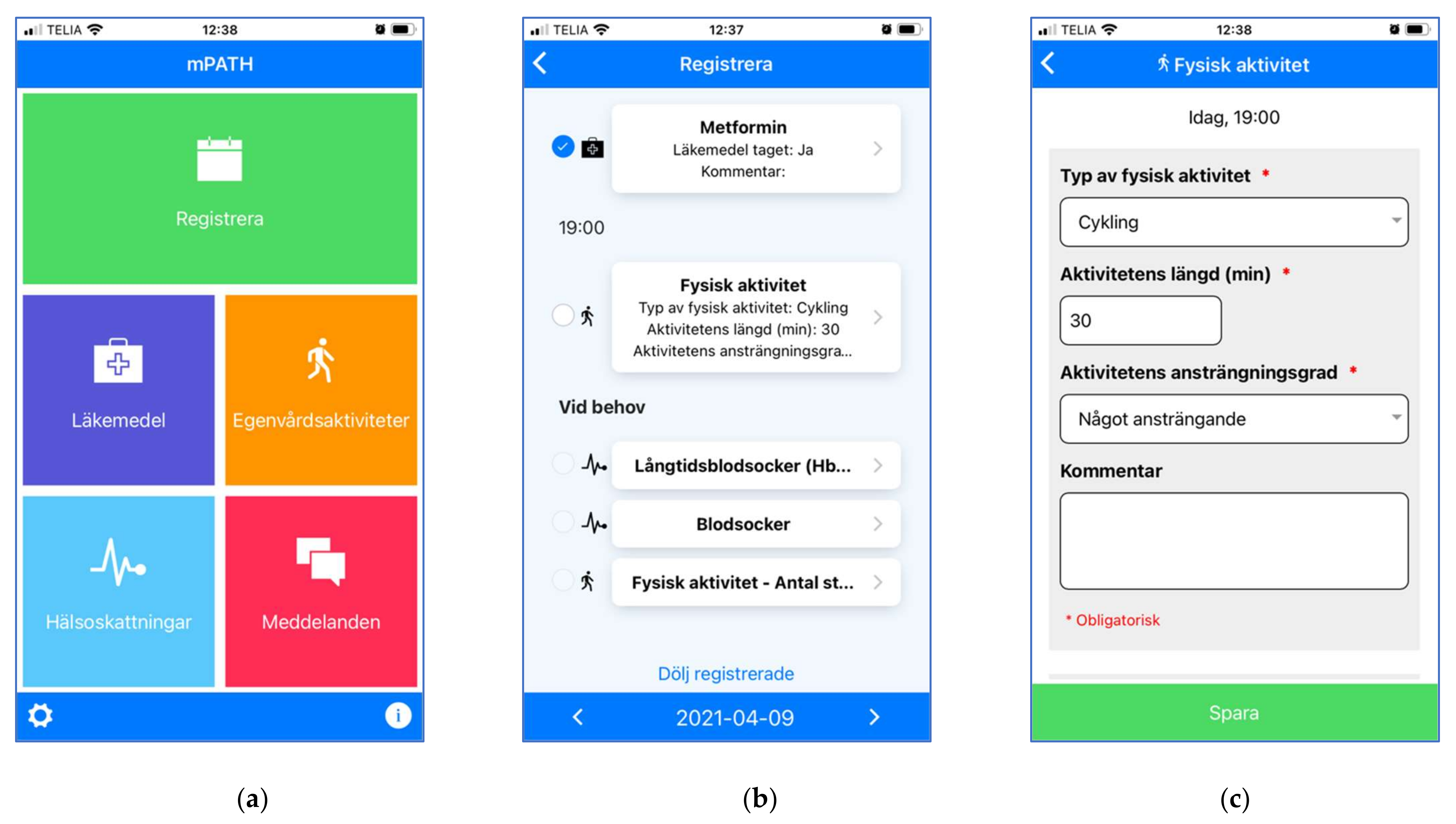
3.2.5. Interactive Self-Management Modules
3.2.6. Messaging Module
3.2.7. Care Planning and Coordination Modules
3.3. Phase 3—Testing and Redesign
4. Discussion
4.1. User-Centered Design Process
4.2. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; ISBN 9789241564229. [Google Scholar]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Wallace, E.; Salisbury, C.; Guthrie, B.; Lewis, C.; Fahey, T.; Smith, S.M. Managing patients with multimorbidity in primary care. BMJ 2015, 350, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Bodenheimer, T.; Wagner, E.H.; Grumbach, K. Improving primary care for patients with chronic illness: The chronic care model, Part 2. J. Am. Med. Assoc. 2002, 288, 1909–1914. [Google Scholar] [CrossRef]
- Lang, A.; Macdonald, M.; Marck, P.; Toon, L.; Griffin, M.; Easty, T.; Fraser, K.; MacKinnon, N.; Mitchell, J.; Lang, E.; et al. Seniors managing multiple medications: Using mixed methods to view the home care safety lens. BMC Health Serv. Res. 2015, 15, 548. [Google Scholar] [CrossRef] [Green Version]
- Rijken, M.; van der Heide, I. Identifying subgroups of persons with multimorbidity based on their needs for care and support. BMC Fam. Pract. 2019, 20, 179. [Google Scholar] [CrossRef]
- Boehmer, K.R.; Gionfriddo, M.R.; Rodriguez-Gutierrez, R.; Dabrh, A.M.A.; Leppin, A.L.; Hargraves, I.; May, C.R.; Shippee, N.D.; Castaneda-Guarderas, A.; Palacios, C.Z.; et al. Patient capacity and constraints in the experience of chronic disease: A qualitative systematic review and thematic synthesis. BMC Fam. Pract. 2016, 17, 127. [Google Scholar] [CrossRef] [Green Version]
- Jonkman, N.H.; Schuurmans, M.J.; Groenwold, R.H.H.; Hoes, A.W.; Trappenburg, J.C.A. Identifying components of self-management interventions that improve health-related quality of life in chronically ill patients: Systematic review and meta-regression analysis. Patient Educ. Couns. 2016, 99, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Eysenbach, G. What is e-health? J. Med. Internet Res. 2001, 3, e20. [Google Scholar] [CrossRef] [PubMed]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J. Med. Internet Res. 2015, 17, e3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, P.; Daines, L.; Campbell, C.; Mckinstry, B.; Weller, D.; Pinnock, H. Telehealth interventions to support self-management of long-term conditions: A systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J. Med. Internet Res. 2017, 19, e6688. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, M.G.; Lamura, G.; Barbabella, F. EHealth for people with multimorbidity: Results from the ICARE4EU project and insights from the “10 e’s” by Gunther Eysenbach. PLoS ONE 2018, 13, e0207292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbert, N.J.; van Os-Medendorp, H.; van Renselaar, W.; Ekeland, A.G.; Hakkaart-van Roijen, L.; Raat, H.; Nijsten, T.E.E.C.; Pasmans, S.G.M.A. Effectiveness and cost-effectiveness of ehealth interventions in somatic diseases: A systematic review of systematic reviews and meta-analyses. J. Med. Internet Res. 2014, 16, e110. [Google Scholar] [CrossRef]
- Morton, K.; Dennison, L.; May, C.; Murray, E.; Little, P.; McManus, R.J.; Yardley, L. Using digital interventions for self-management of chronic physical health conditions: A meta-ethnography review of published studies. Patient Educ. Couns. 2017, 100, 616–635. [Google Scholar] [CrossRef] [Green Version]
- Petersen, C.L.; Weeks, W.B.; Norin, O.; Weinstein, J.N. Development and implementation of a person-centered, technology-enhanced care model for managing chronic conditions: Cohort study. J. Med. Internet Res. 2019, 21, e11082. [Google Scholar] [CrossRef] [PubMed]
- Wildevuur, S.E.; Simonse, L.W.L.; Wildevuur, S.E.; Simonse, L.W.L. Information and Communication Technology-Enabled Person-Centered Care for the “Big Five” Chronic Conditions: Scoping Review. J. Med. Internet Res. 2015, 17, e77. [Google Scholar] [CrossRef]
- Tran, V.-T.; Riveros, C.; Ravaud, P. Patients’ views of wearable devices and AI in healthcare: Findings from the ComPaRe e-cohort. NPJ Digit. Med. 2019, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Gordon, N.P.; Hornbrook, M.C. Older adults’ readiness to engage with eHealth patient education and self-care resources: A cross-sectional survey. BMC Health Serv. Res. 2018, 18, 220. [Google Scholar] [CrossRef]
- Van Gemert-Pijnen, J.E.W.C.; Nijland, N.; van Limburg, M.; Ossebaard, H.C.; Kelders, S.M.; Eysenbach, G.; Seydel, E.R. A holistic framework to improve the uptake and impact of ehealth technologies. J. Med. Internet Res. 2011, 13, e111. [Google Scholar] [CrossRef]
- Greenhalgh, T.; A’Court, C.; Shaw, S. Understanding heart failure; explaining telehealth—A hermeneutic systematic review. BMC Cardiovasc. Disord. 2017, 17, 156. [Google Scholar] [CrossRef]
- Perlmutter, A.; Benchoufi, M.; Ravaud, P.; Tran, V.T. Identification of patient perceptions that can affect the uptake of interventions using biometric monitoring devices: Systematic review of randomized controlled trials. J. Med. Internet Res. 2020, 22, e18986. [Google Scholar] [CrossRef]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Medical Research Council Guidance Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.; et al. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- Jagosh, J.; MacAulay, A.C.; Pluye, P.; Salsberg, J.; Bush, P.L.; Henderson, J.; Sirett, E.; Wong, G.; Cargo, M.; Herbert, C.P.; et al. Uncovering the benefits of participatory research: Implications of a realist review for health research and practice. Milbank Q. 2012, 90, 311–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonsen, J.; Robertson, T. Routledge International Handbook of Participatory Design; Routledge: New York, NY, USA, 2012; ISBN 9780415694407. [Google Scholar]
- Wagner, E.H. Chronic disease management: What will it take to improve care for chronic illness? Eff. Clin. Pract. 1998, 1, 2–4. [Google Scholar] [PubMed]
- Wagner, E.H.; Austin, B.T.; von Korff, M. Organizing care for patients with chronic illness. Milbank Q. 1996, 74, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness; Ryan, R.M., Deci, E.L., Eds.; Guilford Press: New York, NY, USA, 2017; ISBN 9781462538966. [Google Scholar]
- Coleman, E.A.; Parry, C.; Chalmers, S.; Min, S.J. The care transitions intervention: Results of a randomized controlled trial. Arch. Intern. Med. 2006, 166, 1822–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, P.M.; Greenwood, D.A.; Paterniti, D.A.; Ward, D.; Miller, L.M.S. The eHealth enhanced chronic care model: A theory derivation approach. J. Med. Internet Res. 2015, 17, e86. [Google Scholar] [CrossRef]
- Ntoumanis, N.; Ng, J.Y.Y.; Prestwich, A.; Quested, E.; Hancox, J.E.; Thøgersen-Ntoumani, C.; Deci, E.L.; Ryan, R.M.; Lonsdale, C.; Williams, G.C. A meta-analysis of self-determination theory-informed intervention studies in the health domain: Effects on motivation, health behavior, physical, and psychological health. Health Psychol. Rev. 2020, 15, 214–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.Y.Y.; Ntoumanis, N.; Thøgersen-Ntoumani, C.; Deci, E.L.; Ryan, R.M.; Duda, J.L.; Williams, G.C. Self-Determination Theory Applied to Health Contexts: A Meta-Analysis. Perspect. Psychol. Sci. 2012, 7, 325–340. [Google Scholar] [CrossRef]
- Williams, G.C.; Freedman, Z.R.; Deci, E.L. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care 1998, 21, 1644–1651. [Google Scholar] [CrossRef]
- Peters, D.; Calvo, R.A.; Ryan, R.M. Designing for motivation, engagement and wellbeing in digital experience. Front. Psychol. 2018, 9, 797. [Google Scholar] [CrossRef] [Green Version]
- Coleman, E.A.; Smith, J.D.; Frank, J.C.; Min, S.J.; Parry, C.; Kramer, A.M. Preparing patients and caregivers to participate in care delivered across settings: The care transitions intervention. J. Am. Geriatr. Soc. 2004, 52, 1817–1825. [Google Scholar] [CrossRef]
- Coleman, E.A.; Smith, J.D.; Frank, J.C.; Eilertsen, T.B.; Thiare, J.N.; Kramer, A.M. Development and testing of a measure designed to assess the quality of care transitions. Int. J. Integr. Care 2002, 2, e02. [Google Scholar] [CrossRef]
- ISO 9241-210:2010. Ergonomics of Human-System Interaction—Part 210: Human-Centred Design for Interactive Systems; ISO: Geneva, Switzerland, 2010; pp. 1–21. [Google Scholar]
- Øvretveit, J.; Hansson, J.; Brommels, M. An integrated health and social care organisation in Sweden: Creation and structure of a unique local public health and social care system. Health Policy 2010, 97, 113–121. [Google Scholar] [CrossRef]
- Krueger, R.A.; Casey, M.A. Focus Groups: A Practical Guide for Applied Research; Sage Publications: Thousand Oaks, CA, USA, 2015; ISBN 9781483365244. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]
- Gulliksen, J.; Göransson, B.; Boivie, I.; Blomkvist, S.; Persson, J.; Cajander, Å. Key principles for user-centred systems design. Behav. Inf. Technol. 2003, 22, 397–409. [Google Scholar] [CrossRef]
- Flink, M.; Ekstedt, M. Planning for the discharge, not for patient self-management at home—An observational and interview study of hospital discharge. Int. J. Integr. Care 2017, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- Kneck, Å.; Flink, M.; Frykholm, O.; Kirsebom, M.; Ekstedt, M. The information flow in a healthcare organisation with integrated units. Int. J. Integr. Care 2019, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- Wagner, E.H. Organizing Care for Patients With Chronic Illness Revisited. Milbank Q. 2019, 97, 659–664. [Google Scholar] [CrossRef]
- Ayat, M.; Imran, M.; Ullah, A.; Kang, C.W. Current trends analysis and prioritization of success factors: A systematic literature review of ICT projects. Int. J. Manag. Proj. Bus. 2021, 14, 652–679. [Google Scholar] [CrossRef]
- He, J.; King, W.R. The Role of User Participation in Information Systems Development: Implications from a Meta-Analysis. J. Manag. Inf. Syst. Summer 2008, 25, 301–331. [Google Scholar] [CrossRef]
- Bano, M.; Zowghi, D. A systematic review on the relationship between user involvement and system success. Inf. Softw. Technol. 2015, 58, 148–169. [Google Scholar] [CrossRef]
- Fischer, B.; Peine, A.; Östlund, B.; Heyn, P.C. The Importance of User Involvement: A Systematic Review of Involving Older Users in Technology Design. Gerontologist 2020, 60, E513–E523. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, S.J.; Nieboer, A.P.; Cramm, J.M. Views of patients with multi-morbidity on what is important for patient-centered care in the primary care setting. BMC Fam. Pract. 2020, 21, 71. [Google Scholar] [CrossRef] [Green Version]
- Schulman-Green, D.; Jaser, S.S.; Park, C.; Whittemore, R. A metasynthesis of factors affecting self-management of chronic illness. J. Adv. Nurs. 2016, 72, 1469–1489. [Google Scholar] [CrossRef] [PubMed]
- May, C.R.; Eton, D.T.; Boehmer, K.; Gallacher, K.; Hunt, K.; MacDonald, S.; Mair, F.S.; May, C.M.; Montori, V.M.; Richardson, A.; et al. Rethinking the patient: Using Burden of Treatment Theory to understand the changing dynamics of illness. BMC Health Serv. Res. 2014, 14, 281. [Google Scholar] [CrossRef]
- May, C.; Montori, V.M.; Mair, F.S. We need minimally disruptive medicine. BMJ 2009, 339, b2803. [Google Scholar] [CrossRef] [PubMed]
- Leppin, A.; Montori, V.; Gionfriddo, M. Minimally Disruptive Medicine: A Pragmatically Comprehensive Model for Delivering Care to Patients with Multiple Chronic Conditions. Healthcare 2015, 3, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Pearce, G.; Parke, H.L.; Pinnock, H.; Epiphaniou, E.; Bourne, C.L.A.; Sheikh, A.; Taylor, S.J.C. The PRISMS taxonomy of self-management support: Derivation of a novel taxonomy and initial testing of its utility. J. Health Serv. Res. Policy 2016, 21, 73–82. [Google Scholar] [CrossRef]
- Irfan Khan, A.; Gill, A.; Cott, C.; Hans, P.K.; Steele Gray, C. mHealth tools for the self-management of patients with multimorbidity in primary care settings: Pilot study to explore user experience. JMIR mHealth uHealth 2018, 6, e8593. [Google Scholar] [CrossRef] [Green Version]
- Van Velsen, L.; Broekhuis, M.; Jansen-Kosterink, S.; op den Akker, H. Tailoring persuasive electronic health strategies for older adults on the basis of personal motivation: Web-based survey study. J. Med. Internet Res. 2019, 21, e11759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalfont, G.; Mateus, C.; Varey, S.; Milligan, C. Self-Efficacy of Older People Using Technology to Self-Manage COPD, Hypertension, Heart Failure, or Dementia at Home: An Overview of Systematic Reviews. Gerontologist 2021, 61, e318–e334. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.; Prince, A.; Thomas, L.; Song, H.; Milosevic, D.; Harris, M.F. Electronic, mobile and telehealth tools for vulnerable patients with chronic disease: A systematic review and realist synthesis. BMJ Open 2018, 8, e019192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oinas-Kukkonen, H.; Harjumaa, M. Persuasive systems design: Key issues, process model, and system features. Commun. Assoc. Inf. Syst. 2009, 24, 485–500. [Google Scholar] [CrossRef]
- Lentferink, A.J.; Oldenhuis, H.K.E.; de Groot, M.; Polstra, L.; Velthuijsen, H.; van Gemert-Pijnen, J.E.W.C. Key components in ehealth interventions combining self-tracking and persuasive eCoaching to promote a healthier lifestyle: A scoping review. J. Med. Internet Res. 2017, 19, e7288. [Google Scholar] [CrossRef]
- Cheng, C.; Beauchamp, A.; Elsworth, G.R.; Osborne, R.H. Applying the electronic health literacy lens: Systematic review of electronic health interventions targeted at socially disadvantaged groups. J. Med. Internet Res. 2020, 22, e18476. [Google Scholar] [CrossRef]
- Keränen, N.S.; Kangas, M.; Immonen, M.; Similä, H.; Enwald, H.; Korpelainen, R.; Jämsä, T. Use of information and communication technologies among older people with and without frailty: A population-based survey. J. Med. Internet Res. 2017, 19, e5507. [Google Scholar] [CrossRef] [PubMed]
- Wannheden, C.; Stenfors, T.; Stenling, A.; von Thiele Schwarz, U. Satisfied or Frustrated? A Qualitative Analysis of Need Satisfying and Need Frustrating Experiences of Engaging With Digital Health Technology in Chronic Care. Front. Public Health 2021, 8, 623773. [Google Scholar] [CrossRef]
- Scott Kruse, C.; Karem, P.; Shifflett, K.; Vegi, L.; Ravi, K.; Brooks, M. Evaluating barriers to adopting telemedicine worldwide: A systematic review. J. Telemed. Telecare 2018, 24, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varsi, C.; Nes, L.S.; Kristjansdottir, O.B.; Kelders, S.M.; Stenberg, U.; Zangi, H.A.; Børøsund, E.; Weiss, K.E.; Stubhaug, A.; Asbjørnsen, R.A.; et al. Implementation strategies to enhance the implementation of eHealth programs for patients with chronic illnesses: Realist systematic review. J. Med. Internet Res. 2019, 21, e14255. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Wherton, J.; Papoutsi, C.; Lynch, J.; Hughes, G.; A’Court, C.; Hinder, S.; Fahy, N.; Procter, R.; Shaw, S. Beyond adoption: A new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J. Med. Internet Res. 2017, 19, e367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plsek, P.E.; Greenhalgh, T. The challenge of complexity in health care. Br. Med. J. 2001, 323, 625–628. [Google Scholar] [CrossRef]
- Kujala, S.; Ammenwerth, E.; Kolanen, H.; Ervast, M. Applying and extending the FITT framework to identify the challenges and opportunities of successful ehealth services for patient self-management: Qualitative interview study. J. Med. Internet Res. 2020, 22, e17696. [Google Scholar] [CrossRef]
- Grates, M.G.; Heming, A.C.; Vukoman, M.; Schabsky, P.; Sorgalla, J. New Perspectives on User Participation in Technology Design Processes: An Interdisciplinary Approach. Gerontologist 2019, 59, 45–57. [Google Scholar] [CrossRef]

| Design Steps | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Participant Role | Organization and Level of Care | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Healthcare management | ||||||||||
| 1 | Occupational therapist, Manager | ICO, home care | • | • | • | |||||
| 2 | Registered nurse, Manager | ICO, hospital care | • | • | • | |||||
| 3 | Physician, Manager | ICO, hospital care | • | • | ||||||
| 4 | Pharmacist, Manager | ICO, hospital care | • | • | ||||||
| 5 | Registered nurse, Manager | ICO, primary care | • | • | ||||||
| Healthcare administration & quality development | ||||||||||
| 6 | Administrator, Social worker | ICC, social service | • | • | • | |||||
| 7 | Quality developer, Registered nurse | ICO, hospital care | • | • | • | |||||
| 8 | Quality developer, Social worker | ICC, social service | • | • | ||||||
| 9 | Administrator/Coordinator | ICO, hospital care | • | |||||||
| Healthcare staff, frontline | ||||||||||
| 10 | Specialist nurse, cardiology | ICO, hospital care | • | • | • | • | ||||
| 11 | Physician, internal medicine | ICO, hospital care | • | |||||||
| 12 | Specialist nurse, oncology | ICO, hospital care | • | • | ||||||
| 13 | Registered nurse | ICO, hospital acute care | • | |||||||
| 14–15 | Registered nurses (n = 2) | HDC | • | |||||||
| 16–17 | Assistant nurses (n = 2) | HDC | • | |||||||
| 18–19 | District nurses (n = 2) | ICO, primary care | • | • | • | |||||
| 20–22 | District nurses (n = 3) | ICO, primary care | • | • | ||||||
| Patients & family carers | ||||||||||
| 23–24 | Family carers (n = 2) | HDC | • | |||||||
| 25–32 | Patients, HF/T2D/COPD (n = 8) | HDC | • | |||||||
| 33 | Patient, T2D | Personal contact | • | |||||||
| 34–35 | Patients, T2D (n = 2) | ICO, primary care | • | |||||||
| 36–38 | Patients, HF/COPD (n = 3) | ICO, primary care | • | |||||||
| 39 | Patient, prostate cancer | Personal contact | • | |||||||
| Step | ||||
|---|---|---|---|---|
| Theme | User Need | 1 | 2 | 3 |
| 1. Diagnosis-specific information | 1.1 Easily accessible information | • | • | |
| 1.2 Trustworthy (evidence-based) information | • | • | • | |
| 1.3 Comprehensive information | • | • | • | |
| 1.4 Understandable information | • | • | ||
| 1.5 Information tailored to individual needs | • | • | ||
| 2. Medication management support | 2.1 Individualized medication management instructions | • | • | |
| 2.2 Medication reminders | • | • | ||
| 2.3 Access to updated medication lists | • | • | ||
| 2.4 Medication adherence and reasons for non-adherence | • | • | ||
| 2.5 Monitoring of intended and unintended effects | • | |||
| 3. Self-management support | 3.1 Monitoring of symptoms and wellbeing | • | • | • |
| 3.2 Support for providing tailored guidance | • | • | • | |
| 3.3 Reminders and motivational support | • | • | ||
| 3.4 Information exchange between patients and HCPs | • | • | ||
| 4. Care coordination support | 4.1 Clarification of roles, responsibilities and contact details | • | • | |
| 4.2 Appointment reminders for patients | • | |||
| 4.3 Overview of patients’ care plan and trajectory | • | • | • | |
| 4.4 Support for collecting patient preferences | • | |||
| * 4.5 Information exchange between providers | • | |||
| 5. Psychosocial support | 5.1 Assurance of available support | • | ||
| * 5.2 Support for connecting with other patients | • | |||
| * 5.3 Support for inviting family caregivers as users | • | • | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekstedt, M.; Kirsebom, M.; Lindqvist, G.; Kneck, Å.; Frykholm, O.; Flink, M.; Wannheden, C. Design and Development of an eHealth Service for Collaborative Self-Management among Older Adults with Chronic Diseases: A Theory-Driven User-Centered Approach. Int. J. Environ. Res. Public Health 2022, 19, 391. https://doi.org/10.3390/ijerph19010391
Ekstedt M, Kirsebom M, Lindqvist G, Kneck Å, Frykholm O, Flink M, Wannheden C. Design and Development of an eHealth Service for Collaborative Self-Management among Older Adults with Chronic Diseases: A Theory-Driven User-Centered Approach. International Journal of Environmental Research and Public Health. 2022; 19(1):391. https://doi.org/10.3390/ijerph19010391
Chicago/Turabian StyleEkstedt, Mirjam, Marie Kirsebom, Gunilla Lindqvist, Åsa Kneck, Oscar Frykholm, Maria Flink, and Carolina Wannheden. 2022. "Design and Development of an eHealth Service for Collaborative Self-Management among Older Adults with Chronic Diseases: A Theory-Driven User-Centered Approach" International Journal of Environmental Research and Public Health 19, no. 1: 391. https://doi.org/10.3390/ijerph19010391
APA StyleEkstedt, M., Kirsebom, M., Lindqvist, G., Kneck, Å., Frykholm, O., Flink, M., & Wannheden, C. (2022). Design and Development of an eHealth Service for Collaborative Self-Management among Older Adults with Chronic Diseases: A Theory-Driven User-Centered Approach. International Journal of Environmental Research and Public Health, 19(1), 391. https://doi.org/10.3390/ijerph19010391






