TMT-Based Quantitative Proteomics Reveals Cochlear Protein Profile Alterations in Mice with Noise-Induced Hearing Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Noise-Induced Hearing Loss (NIHL) Model Construction
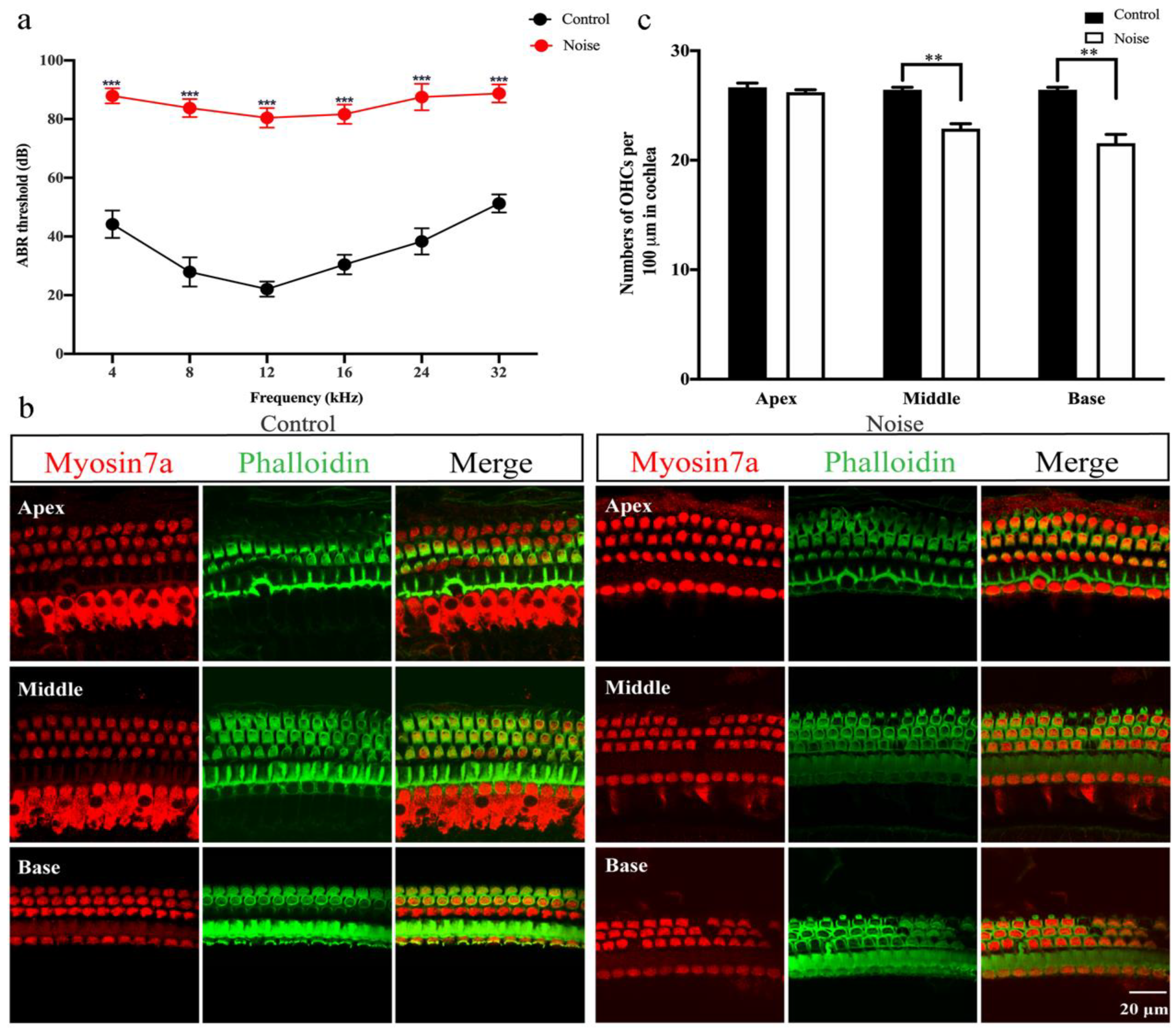
2.2. Auditory Brainstem Response (ABR) Measurement
2.3. Cochlea Surface Preparations and Hair Cells (HCs) Counting
2.4. Protein Extraction and Digestion
2.5. Tandem Mass Tag (TMT) Labeling and HPLC Fractionation
2.6. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.7. Database Search and Bioinformatics Analysis
2.8. Oxidative Stress Markers Levels Assay, and Proinflammatory Cytokines Measurement
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. ABR Thresholds and HCs Immunofluorescence Staining and Counting
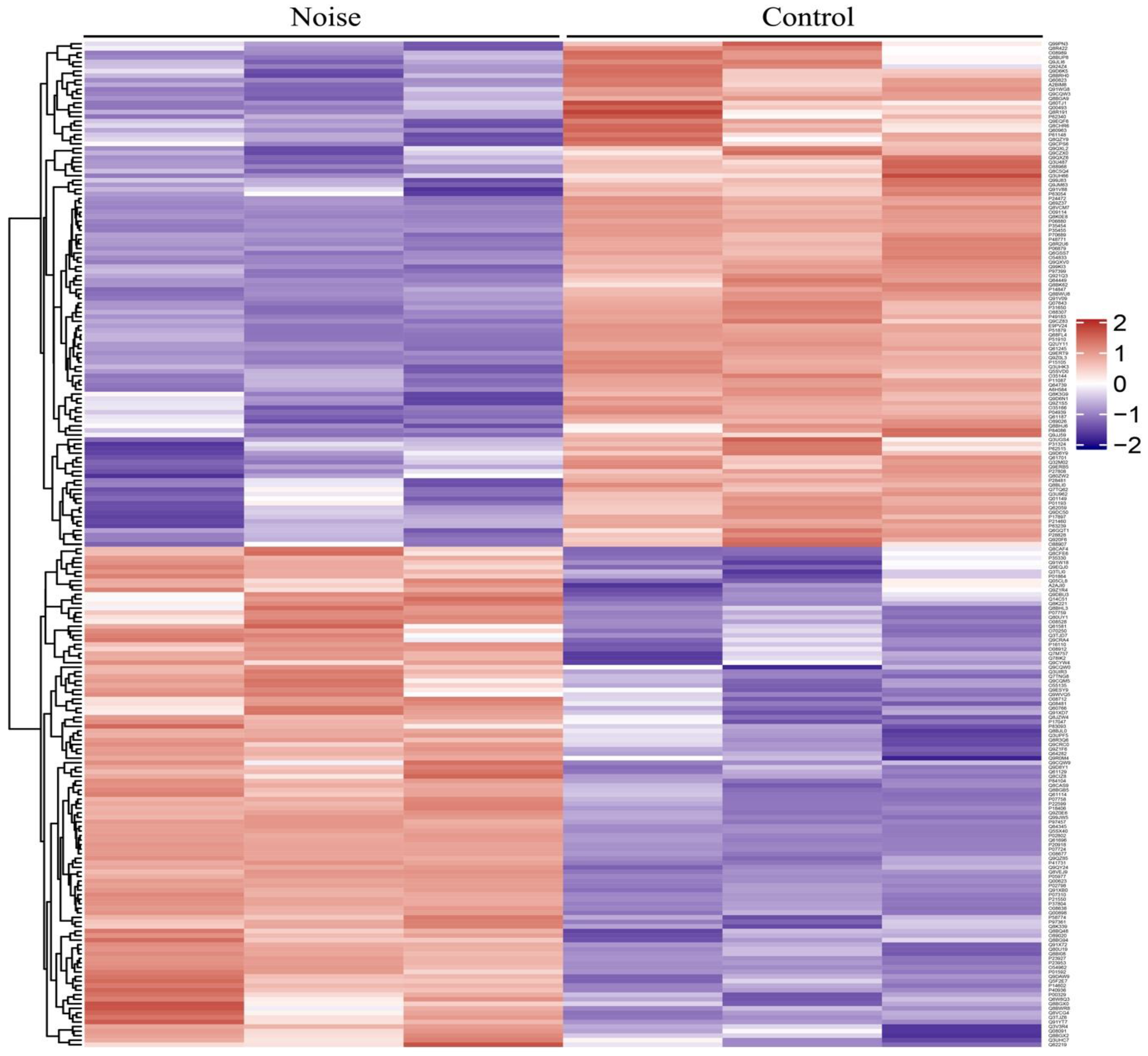
3.2. Identification of Differentially Expressed Proteins (DEPs)
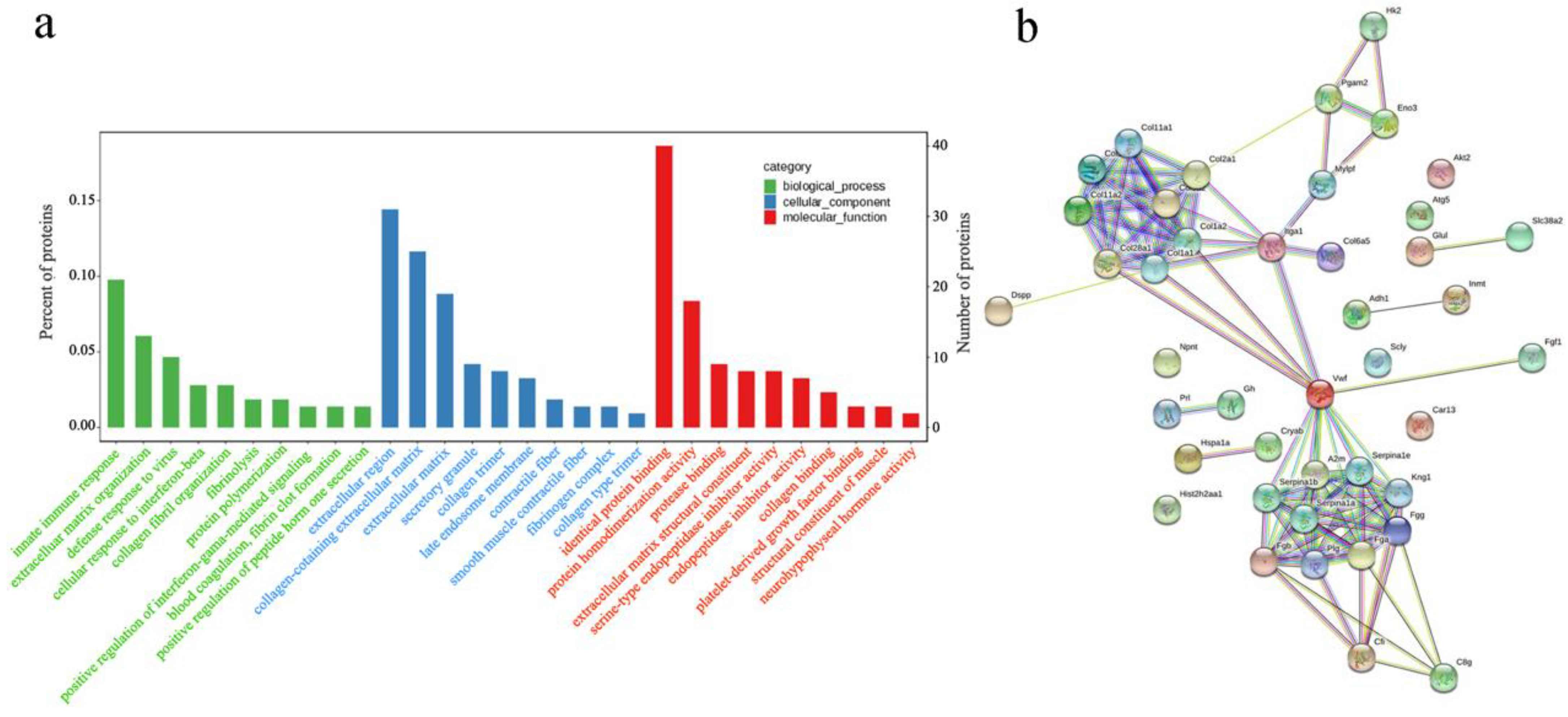
3.3. Functional Enrichment Analysis of DEPs
3.4. Pathway Enrichment and Protein–Protein Interaction (PPI) Network Analysis
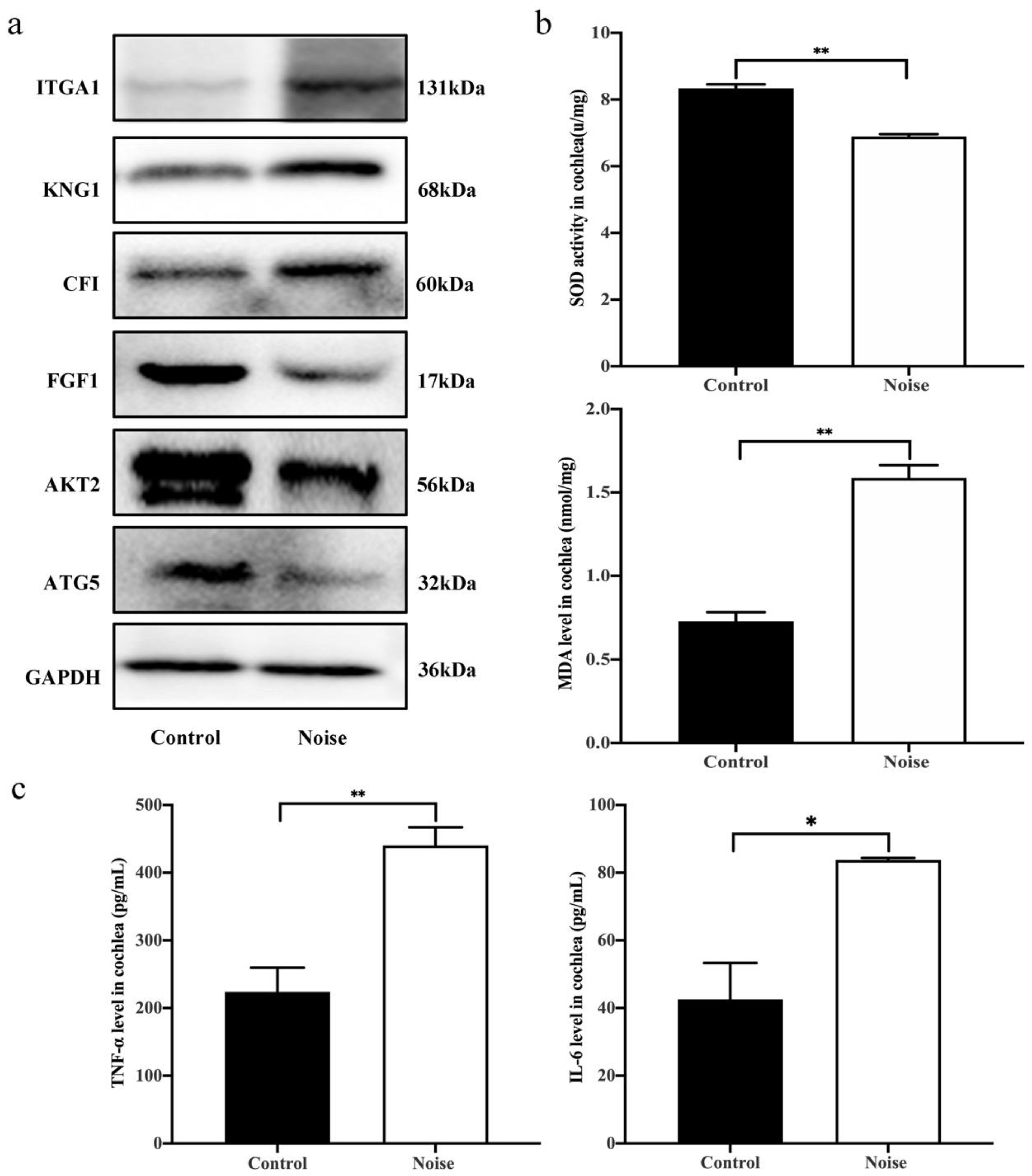
3.5. Validation of DEPs by Western Blot Analysis
3.6. Measurement of Superoxide Dismutase (SOD) Activity, Malondialdehyde (MDA) Level and Proinflammatory Cytokines Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, L.; Ji, J.; Wan, L.; Zhang, J.; Yin, L.; Pu, Y. An overview of research trends and genetic polymorphisms for noise-induced hearing loss from 2009 to 2018. Environ. Sci. Pollut. Res. Int. 2019, 26, 34754–34774. [Google Scholar] [PubMed]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.F. Strengthen the research for the prevention and control of occupational noise-induced hearing loss in our country. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2016, 34, 881–883. [Google Scholar] [PubMed]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.M.; Sha, S.H. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, B.; Ji, J.; Wan, L.; Yin, L.; Zhu, B.; Zhang, J.; Pu, Y. CARD8 polymorphism rs2043211 protects against noise-induced hearing loss by causing the dysfunction of CARD8 protein. Environ. Sci. Pollut. Res. Int. 2021, 28, 8626–8636. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ao, X.; Lin, S.; Guan, S.; Zheng, L.; Han, X.; Ye, H. Quantitative Comparative Proteomics Reveal Biomarkers for Dengue Disease Severity. Front. Microbiol. 2019, 10, 2836. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, J.J.; Schoem, S.R.; Warnock, G.R. Squamous cell carcinoma arising in a benign teratoma of the maxilla. Otolaryngol. Head Neck Surg. 1996, 114, 447–452. [Google Scholar] [CrossRef]
- Muenchhoff, J.; Poljak, A.; Thalamuthu, A.; Gupta, V.B.; Chatterjee, P.; Raftery, M.; Masters, C.L.; Morris, J.C.; Bateman, R.J.; Fagan, A.M.; et al. Changes in the plasma proteome at asymptomatic and symptomatic stages of autosomal dominant Alzheimer’s disease. Sci. Rep. 2016, 6, 29078. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Zhang, W.; Zhang, F.; Roepstorff, P.; Yang, F.; Lu, Z.; Ding, W. TMT-Based Quantitative Proteomics Analysis Reveals Airborne PM2.5-Induced Pulmonary Fibrosis. Int. J. Environ. Res. Public Health 2018, 16, 98. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.; Schafer, J.; Kuhn, K.; Kienle, S.; Schwarz, J.; Schmidt, G.; Neumann, T.; Johnstone, R.; Mohammed, A.K.; Hamon, C. Tandem mass tags: A novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 2003, 75, 1895–1904. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Han, J.J.; Lee, S.Y.; Jung, G.; Min, H.J.; Song, J.J.; Koo, J.W. Outcomes of Peptide Vaccine GV1001 Treatment in a Murine Model of Acute Noise-Induced Hearing Loss. Antioxidants 2020, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scimemi, P.; Santarelli, R.; Selmo, A.; Mammano, F. Auditory brainstem responses to clicks and tone bursts in C57 BL/6J mice. Acta Otorhinolaryngol. Ital. 2014, 34, 264–271. [Google Scholar] [PubMed]
- Miao, L.; Wang, B.; Zhang, J.; Yin, L.; Pu, Y. Plasma metabolomic profiling in workers with noise-induced hearing loss: A pilot study. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef]
- Yamashita, D.; Jiang, H.Y.; Schacht, J.; Miller, J.M. Delayed production of free radicals following noise exposure. Brain Res. 2004, 1019, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Prell, C.G.; Yamashita, D.; Minami, S.B.; Yamasoba, T.; Miller, J.M. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 2007, 226, 22–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Choung, Y.H.; Taura, A.; Pak, K.; Choi, S.J.; Masuda, M.; Ryan, A.F. Generation of highly-reactive oxygen species is closely related to hair cell damage in rat organ of Corti treated with gentamicin. Neuroscience 2009, 161, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Czerska, M.; Mikolajewska, K.; Zielinski, M.; Gromadzinska, J.; Wasowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bahaloo, M.; Rezvani, M.E.; Farashahi Yazd, E.; Zare Mehrjerdi, F.; Davari, M.H.; Roohbakhsh, A.; Mollasadeghi, A.; Nikkhah, H.; Vafaei, M.; Mehrparvar, A.H. Effect of myricetin on the gene expressions of NOX3, TGF-beta1, prestin, and HSP-70 and anti-oxidant activity in the cochlea of noise-exposed rats. Iran. J. Basic Med. Sci. 2020, 23, 594–599. [Google Scholar] [PubMed]
- Li, Y.; Zhu, W.; Wei, B.; Su, Y.; Gao, Y.; Feng, Y.; Liu, Y. Effect of noise on antioxidant capacity of brain tissue in guinea pigs. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2015, 33, 276–278. [Google Scholar] [PubMed]
- Vethanayagam, R.R.; Yang, W.; Dong, Y.; Hu, B.H. Toll-like receptor 4 modulates the cochlear immune response to acoustic injury. Cell Death Dis. 2016, 7, e2245. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Cai, Q.; Vethanayagam, R.R.; Wang, J.; Yang, W.; Hu, B.H. Immune defense is the primary function associated with the differentially expressed genes in the cochlea following acoustic trauma. Hear. Res. 2016, 333, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef]
- Bas, E.; Martinez-Soriano, F.; Lainez, J.M.; Marco, J. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Otolaryngol. 2009, 129, 385–389. [Google Scholar] [CrossRef]
- Arslan, H.H.; Satar, B.; Serdar, M.; Yilmaz, E. Changes in Proinflammatory Cytokines in the Cochlea in Relation to Hearing Thresholds in Noise-Exposed Rats. J. Int. Adv. Otol. 2017, 13, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.A.; Lyu, A.R.; Jeong, S.H.; Kim, T.H.; Park, M.J.; Park, Y.H. Acoustic Trauma Modulates Cochlear Blood Flow and Vasoactive Factors in a Rodent Model of Noise-Induced Hearing Loss. Int. J. Mol. Sci. 2019, 20, 5316. [Google Scholar] [CrossRef] [Green Version]
- Warnecke, A.; Prenzler, N.K.; Schmitt, H.; Daemen, K.; Keil, J.; Dursin, M.; Lenarz, T.; Falk, C.S. Defining the Inflammatory Microenvironment in the Human Cochlea by Perilymph Analysis: Toward Liquid Biopsy of the Cochlea. Front. Neurol. 2019, 10, 665. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Wang, B.; Zhang, J.; Yin, L.; Pu, Y. A functional SNP in miR-625-5p binding site of AKT2 3′UTR is associated with noise-induced hearing loss susceptibility in the Chinese population. Environ. Sci. Pollut. Res. Int. 2021, 28, 40782–40792. [Google Scholar] [CrossRef]
- Fazleabas, A.T.; Bell, S.C.; Fleming, S.; Sun, J.; Lessey, B.A. Distribution of integrins and the extracellular matrix proteins in the baboon endometrium during the menstrual cycle and early pregnancy. Biol. Reprod. 1997, 56, 348–356. [Google Scholar] [CrossRef]
- Billings, L.K.; Hsu, Y.H.; Ackerman, R.J.; Dupuis, J.; Voight, B.F.; Rasmussen-Torvik, L.J.; Hercberg, S.; Lathrop, M.; Barnes, D.; Langenberg, C.; et al. Impact of common variation in bone-related genes on type 2 diabetes and related traits. Diabetes 2012, 61, 2176–2186. [Google Scholar] [CrossRef] [Green Version]
- Yim, D.H.; Zhang, Y.W.; Eom, S.Y.; Moon, S.I.; Yun, H.Y.; Song, Y.J.; Youn, S.J.; Hyun, T.; Park, J.S.; Kim, B.S.; et al. ITGA1 polymorphisms and haplotypes are associated with gastric cancer risk in a Korean population. World J. Gastroenterol. 2013, 19, 5870–5876. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell. Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Xu, A. Screening of genes associated with inflammatory responses in the endolymphatic sac reveals underlying mechanisms for autoimmune inner ear diseases. Exp. Ther. Med. 2018, 16, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.M.; Rullo, J.; Chen, M.; Ghazarian, M.; Bak, S.; Xiao, H.; Hay, J.B.; Cybulsky, M.I. α1β1 integrin-mediated adhesion inhibits macrophage exit from a peripheral inflammatory lesion. J. Immunol. 2013, 190, 4305–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, T.T.; Horton, M.A.; Choy, M.Y.; Richman, P.I. Increased expression of laminin/collagen receptor (VLA-1) on epithelium of inflamed human intestine. J. Clin. Pathol. 1990, 43, 313–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhauser, F.; Gob, E.; Kraft, P.; Geis, C.; Schmitt, J.; Brede, M.; Gobel, K.; Helluy, X.; Pham, M.; Bendszus, M.; et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood 2012, 120, 4082–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Huo, M.; Chen, X.; Zhang, Y.; Qiao, Y. Mechanism of tanshinones and phenolic acids from Danshen in the treatment of coronary heart disease based on co-expression network. BMC Complement. Med. Ther. 2020, 20, 28. [Google Scholar] [CrossRef]
- Shi, K.; Chen, X.; Xie, B.; Yang, S.S.; Liu, D.; Dai, G.; Chen, Q. Celastrol Alleviates Chronic Obstructive Pulmonary Disease by Inhibiting Cellular Inflammation Induced by Cigarette Smoke via the Ednrb/Kng1 Signaling Pathway. Front. Pharmacol. 2018, 9, 1276. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef]
- Hakobyan, S.; Harding, K.; Aiyaz, M.; Hye, A.; Dobson, R.; Baird, A.; Liu, B.; Harris, C.L.; Lovestone, S.; Morgan, B.P. Complement Biomarkers as Predictors of Disease Progression in Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 54, 707–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altmann, T.; Torvell, M.; Owens, S.; Mitra, D.; Sheerin, N.S.; Morgan, B.P.; Kavanagh, D.; Forsyth, R. Complement factor I deficiency: A potentially treatable cause of fulminant cerebral inflammation. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e689. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.; Yonemitsu, Y.; Okano, S.; Nakagawa, K.; Nakashima, Y.; Irisa, T.; Iwamoto, Y.; Nagai, Y.; Hasegawa, M.; Sueishi, K. Fibroblast growth factor-2 determines severity of joint disease in adjuvant-induced arthritis in rats. J. Immunol. 2002, 168, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, S.Z.; Wang, N.; Zhou, R.M.; Wang, T.; Wang, D.J.; Li, X.F.; Bui, J.; Li, Y. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum. Reprod. 2010, 25, 1806–1811. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, H.; Talaska, A.E.; Hill, K.; Sha, S.H. Increased Sensitivity to Noise-Induced Hearing Loss by Blockade of Endogenous PI3K/Akt Signaling. J. Assoc. Res. Otolaryngol. 2015, 16, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| KEGG Pathway | Protein | Count | p Value |
|---|---|---|---|
| PI3K/AKT signaling pathway | Col6a5, Prl, Gh1, Col1a1, Col2a1, Fgf1, Col1a2, Col9a2, Akt2, Itga1, Vwf | 11 | 0.033 |
| ECM-receptor interaction | Col6a5, Col1a1, Col2a1, Dspp, Col1a2, Col9a2, Npnt, Itga1, Vwf | 9 | 9.34 × 10−6 |
| Focal adhesion | Col6a5, Col1a1, Col2a1, Col1a2, Col9a2, Akt2, Mylpf, Itga1, Vwf | 9 | 0.005 |
| Protein digestion and absorption | Col6a5, Col1a1, Col2a1, Col1a2, Col9a2, Col28a1, Col5a2, Col11a1, Col11a2, Slc38a2 | 10 | 2.11 × 10−6 |
| Complement and coagulation cascades | Fga, A2m, Fgb, Fgg, Kng1, Serpina1a, Plg, Serpina1b, Serpina1e, Cfi, Vwf, C8g | 12 | 1.84 × 10−8 |
| Platelet activation | Fga, Col1a1, Col1a2, Akt2, Fgb, Fgg, Vwf | 7 | 0.004 |
| Neutrophil extracellular trap formation | Fga, Akt2, Hist2h2aa1, Fgb, Fgg, Vwf | 6 | 0.041 |
| Nitrogen metabolism | Glul, Ca13 | 2 | 0.033 |
| Longevity regulating pathway | Akt2, Atg5, Hspa1a, Cryab | 4 | 0.020 |
| Selenocompound metabolism | Scly, Inmt | 2 | 0.033 |
| Glycolysis/Gluconeogenesis | Hk2, Pgam2, Adh1, Eno3 | 4 | 0.022 |
| Protein Accession | Protein Name | Protein Description | Molecular Weight (kDa) | p Value | Fold Change |
|---|---|---|---|---|---|
| Q3V3R4 | ITGA1 | Integrin alpha 1 | 130.81 | 0.013 | 1.22 |
| O08677 | KNG1 | Kininogen 1 | 73.10 | 2.50 × 10−5 | 1.28 |
| Q61129 | CFI | Complement factor I | 67.26 | 0.002 | 1.34 |
| P61148 | FGF1 | Fibroblast growth factor 1 | 17.42 | 0.017 | 0.83 |
| Q60823 | AKT2 | RAC-beta serine/threonine-protein kinase | 55.74 | 0.001 | 0.80 |
| Q99J83 | ATG5 | Autophagy protein 5 | 32.40 | 0.019 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, L.; Zhang, J.; Yin, L.; Pu, Y. TMT-Based Quantitative Proteomics Reveals Cochlear Protein Profile Alterations in Mice with Noise-Induced Hearing Loss. Int. J. Environ. Res. Public Health 2022, 19, 382. https://doi.org/10.3390/ijerph19010382
Miao L, Zhang J, Yin L, Pu Y. TMT-Based Quantitative Proteomics Reveals Cochlear Protein Profile Alterations in Mice with Noise-Induced Hearing Loss. International Journal of Environmental Research and Public Health. 2022; 19(1):382. https://doi.org/10.3390/ijerph19010382
Chicago/Turabian StyleMiao, Long, Juan Zhang, Lihong Yin, and Yuepu Pu. 2022. "TMT-Based Quantitative Proteomics Reveals Cochlear Protein Profile Alterations in Mice with Noise-Induced Hearing Loss" International Journal of Environmental Research and Public Health 19, no. 1: 382. https://doi.org/10.3390/ijerph19010382
APA StyleMiao, L., Zhang, J., Yin, L., & Pu, Y. (2022). TMT-Based Quantitative Proteomics Reveals Cochlear Protein Profile Alterations in Mice with Noise-Induced Hearing Loss. International Journal of Environmental Research and Public Health, 19(1), 382. https://doi.org/10.3390/ijerph19010382






