Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms
Abstract
1. Introduction
2. Methodology
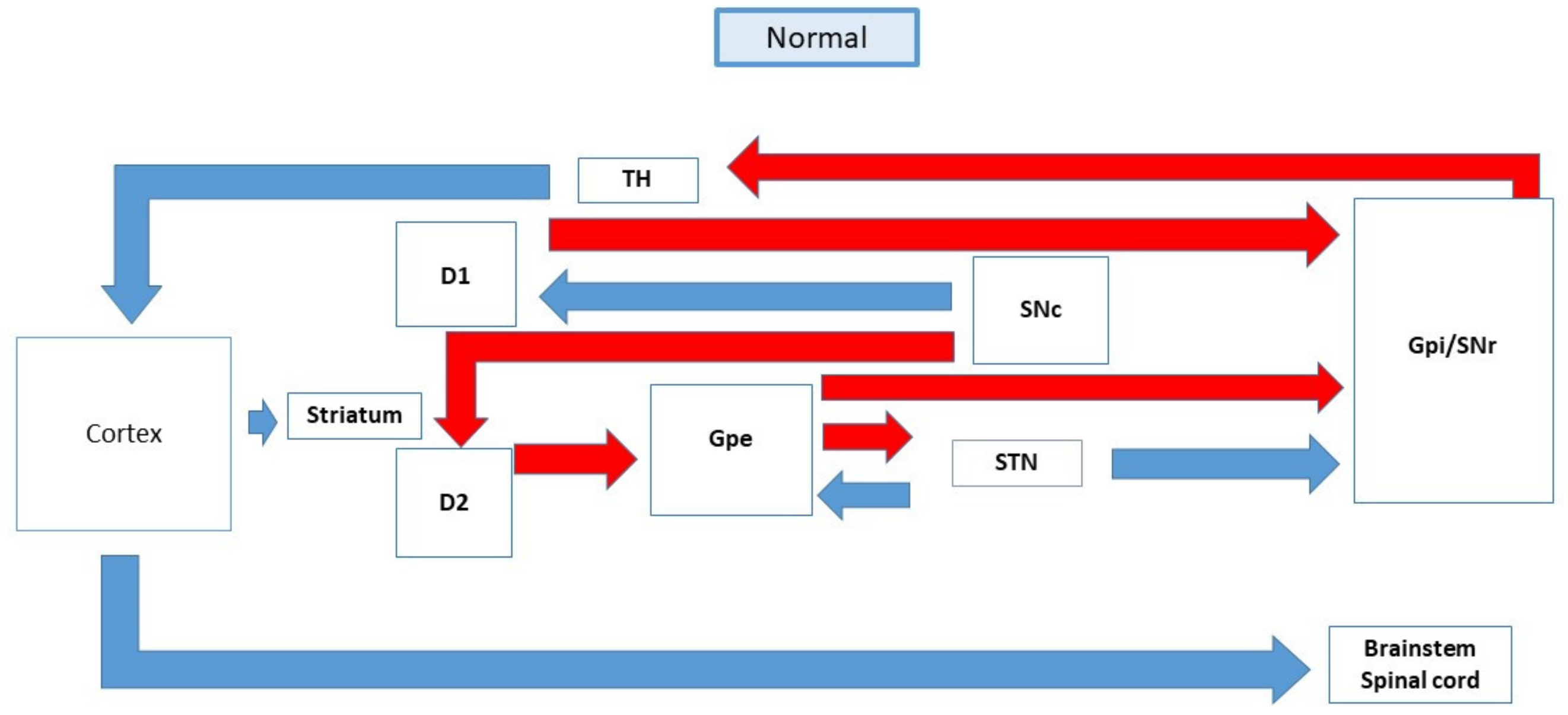
3. Management of Parkinson’s Disease and Its Associated Psychosis
3.1. Mechanism of Action
3.1.1. Levodopa
3.1.2. Dopamine Agonists
3.1.3. Anticholinergics
3.1.4. COMT Inhibitors
3.1.5. MAO-B Inhibitors
3.1.6. Amantadine
3.1.7. Antipsychotics Used for Treating PDP
Clozapine
Olanzapine
Quetiapine
Risperidone
Ziprasidone
3.1.8. Safinamide
3.1.9. Istradefylline
3.1.10. Pimavanserin
3.2. Efficacy
3.2.1. Levodopa
3.2.2. Dopamine Agonists
3.2.3. Anticholinergics
3.2.4. COMT Inhibitors
3.2.5. MAO-B Inhibitors
3.2.6. Amantadine
3.2.7. Antipsychotics Used for Treating PDP
Clozapine
Olanzapine
Quetiapine
Risperidone
Ziprasidone
3.2.8. Safinamide
3.2.9. Istradefylline
3.2.10. Pimavanserin
3.3. Safety
3.3.1. Levodopa
3.3.2. Dopamine Agonists
3.3.3. Anticholinergics
3.3.4. COMT Inhibitors
3.3.5. MOA-B Inhibitors
3.3.6. Amantadine
3.3.7. Antipsychotics Used for Treating PDP
Clozapine
Olanzapine
Quetiapine
Risperidone
Ziprasidone
3.3.8. Safinamide
3.3.9. Istradefylline
3.3.10. Pimavanserin
4. Overall Comparison of Efficacy and Safety of Conventional Drugs Used in Treating PD
5. Overall Comparison of New Drugs Approved from 2016–2019 for the Treatment of PD in Terms of Efficacy and Safety
6. Non-Pharmacological Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno, G.M.; Gandhi, R.; Lessig, S.L.; Wright, B.; Litvan, I.; Nahab, F.B. Mortality in patients with Parkinson disease psychosis receiving pimavanserin and quetiapine. Neurology 2018, 91, 797–799. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Rana, A.Q.; Malik, S.H.; Rizvi, S.F.H.; Akhter, S.; Vannabouathong, C.; Sarfraz, Z.; Rana, R. Comprehensive Examination of Therapies for Pain in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2018, 51, 190–206. [Google Scholar] [CrossRef]
- Kon, T.; Ueno, T.; Haga, R.; Tomiyama, M. The factors associated with impulse control behaviors in Parkinson’s disease: A 2-year longitudinal retrospective cohort study. Brain Behav. 2018, 8, e01036. [Google Scholar] [CrossRef]
- Binde, C.D.; Tvete, I.F.; Gåsemyr, J.; Natvig, B.; Klemp, M. A multiple treatment comparison meta-analysis of monoamine oxidase type B inhibitors for Parkinson’s disease. Br. J. Clin. Pharmacol. 2018, 84, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Riederer, P.; Grünblatt, E. Determination of Monoamine Oxidase A and B Activity in Long-Term Treated Patients With Parkinson Disease. Clin. Neuropharmacol. 2017, 40, 208–211. [Google Scholar] [CrossRef]
- Sadek, B.; Saad, A.; Schwed, J.S.; Weizel, L.; Walter, M.; Stark, H. Anticonvulsant effects of isomeric nonimidazole histamine H3 receptor antagonists. Drug Des. Dev. Ther. 2016, 10, 3633–3651. [Google Scholar] [CrossRef]
- Cattaneo, C.; Barone, P.; Bonizzoni, E.; Sardina, M. Effects of Safinamide on Pain in Fluctuating Parkinson’s Disease Patients: A Post-Hoc Analysis. J. Park. Dis. 2017, 7, 95–101. [Google Scholar] [CrossRef]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Eeden, S.K.V.D.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. Jpn. Park. Dis. 2018, 4, 21. [Google Scholar] [CrossRef]
- Mohanty, D.; Sarai, S.; Naik, S.; Lippmann, S. Pimavanserin for Parkinson Disease Psychosis. Prim. Care Companion CNS Disord. 2019, 21, 18l02355. [Google Scholar] [CrossRef]
- Hammarlund, C.S.; Westergren, A.; Åström, I.; Edberg, A.-K.; Hagell, P. The Impact of Living with Parkinson’s Disease: Balancing within a Web of Needs and Demands. Park. Dis. 2018, 2018, 4598651. [Google Scholar] [CrossRef]
- Fackrell, R.; Carroll, C.B.; Grosset, D.G.; Mohamed, B.; Reddy, P.; Parry, M.; Chaudhuri, K.R.; Foltynie, T. Noninvasive options for ‘wearing-off’ in Parkinson’s disease: A clinical consensus from a panel of UK Parkinson’s disease specialists. Neurodegener. Dis. Manag. 2018, 8, 349–360. [Google Scholar] [CrossRef]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef]
- Fredericks, D.; Norton, J.C.; Atchison, C.; Schoenhaus, R.; Pill, M.W. Parkinson’s disease and Parkinson’s disease psychosis: A perspective on the challenges, treatments, and economic burden. Am. J. Manag. Care 2017, 23 (Suppl. 5), S83–S92. [Google Scholar]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abbasi, N.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Department of Statistics Malaysia. 2018. Available online: https://www.dosm.gov.my/v1/ (accessed on 10 November 2021).
- Papapetropoulos, S.; Adi, N.; Ellul, J.; Argyriou, A.A.; Chroni, E. A Prospective Study of Familial versus Sporadic Parkinson’s Disease. Neurodegener. Dis. 2007, 4, 424–427. [Google Scholar] [CrossRef]
- Causes of Parkinson’s Disease. The Michael J. Fox Foundation for Parkinson’s Research|Parkinson’s Disease. Available online: https://www.michaeljfox.org/causes (accessed on 2 February 2021).
- Johnson, M.E.; Stecher, B.; Labrie, V.; Brundin, L.; Brundin, P. Triggers, Facilitators, and Aggravators: Redefining Parkinson’s Disease Pathogenesis. Trends Neurosci. 2019, 42, 4–13. [Google Scholar] [CrossRef]
- Shin, H.-W.; Chung, S.J. Drug-Induced Parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef]
- Association EPD. Vascular (Arteriosclerotic) Parkinsonism. Available online: https://www.epda.eu.com/about-parkinsons/types/vascular-arteriosclerotic-parkinsonism/ (accessed on 2 February 2020).
- Galvan, A.; Wichmann, T. Pathophysiology of Parkinsonism. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 1459–1474. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- Bamford, N.S.; Robinson, S.; Palmiter, R.D.; Joyce, J.; Moore, C.; Meshul, C.K. Dopamine Modulates Release from Corticostriatal Terminals. J. Neurosci. 2004, 24, 9541–9552. [Google Scholar] [CrossRef]
- Etiology and Pathogenesis of Parkinson Disease—UpToDate. Available online: https://www.uptodate.com/contents/etiology-and-pathogenesis-of-parkinson-disease (accessed on 17 January 2020).
- Kenneth, L. Neuropsychopharmacology: The Fifth Generation of Progress, 5th ed.; Zigmoid, M.J., Burke, R.E., Eds.; Pathophysiology of Parkinson’s disease; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 1781–1793. [Google Scholar]
- Obeso, J.A.; Oroz, M.C.R.; Rodriguez, M.; Lanciego, J.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef]
- Bezard, E.; Gross, C.E.; Brotchie, J. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003, 26, 215–221. [Google Scholar] [CrossRef]
- Adams, J.R.; van Netten, H.; Schulzer, M.; Mak, E.; Mckenzie, J.; Strongosky, A.; Sossi, V.; Ruth, T.J.; Lee, C.S.; Farrer, M.; et al. PET in LRRK2 mutations: Comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain J. Neurol. 2005, 128 Pt 12, 2777–2785. [Google Scholar] [CrossRef]
- Hashimoto, M.; Rockenstein, E.; Crews, L.; Masliah, E. Role of Protein Aggregation in Mitochondrial Dysfunction and Neurodegeneration in Alzheimer’s and Parkinson’s Diseases. NeuroMolecular Med. 2003, 4, 21–36. [Google Scholar] [CrossRef]
- Antonini, A. Levodopa in the treatment of Parkinson’s disease: An old drug still going strong. Clin. Interv. Aging 2010, 5, 229–238. [Google Scholar] [CrossRef]
- Hauser, R.A. Levodopa: Past, Present, and Future. Eur. Neurol. 2009, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sgroi, S.; Kaelin-Lang, A.; Capper-Loup, C. Spontaneous locomotor activity and L-DOPA-induced dyskinesia are not linked in 6-OHDA parkinsonian rats. Front. Behav. Neurosci. 2014, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D. Dopamine agonists: Their role in the treatment of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2000, 68, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B.J.; Duker, A.; Chiu, P. Dopamine Agonists and Pathologic Behaviors. Park. Dis. 2012, 2012, 603631. [Google Scholar] [CrossRef][Green Version]
- Katzenschlager, R.; Sampaio, C.; Costa, J.; Lees, A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst. Rev. 2002, 2002, CD003735. [Google Scholar] [CrossRef]
- Dong, J.; Cui, Y.; Li, S.; Le, W. Current Pharmaceutical Treatments and Alternative Therapies of Parkinson’s Disease. Curr. Neuropharmacol. 2016, 14, 339–355. [Google Scholar] [CrossRef]
- Rivest, J.; Barclay, C.L.; Suchowersky, O. COMT Inhibitors in Parkinson’s Disease. Can. J. Neurol. Sci. 1999, 26, S34–S38. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Ayyannan, S.R. Monoamine oxidase-B inhibitors as potential neurotherapeutic agents: An overview and update. Med. Res. Rev. 2019, 39, 1603–1706. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, L.; Vecsei, L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord.—Drug Targets 2017, 16, 425–439. [Google Scholar] [CrossRef]
- Chang, C.; Ramphul, K. Amantadine. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499953/ (accessed on 14 February 2020).
- Crosby, N.J.; Deane, K.; Clarke, C. Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst. Rev. 2003, 2003, CD003467. [Google Scholar] [CrossRef] [PubMed]
- Divac, N.; Stojanović, R.; Vujović, K.S.; Medić, B.; Damjanović, A.; Prostran, M. The Efficacy and Safety of Antipsychotic Medications in the Treatment of Psychosis in Patients with Parkinson’s Disease. Behav. Neurol. 2016, 2016, 4938154. [Google Scholar] [CrossRef]
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23, 2087. [Google Scholar] [CrossRef] [PubMed]
- Accessdata.fda.gov. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019758s062lbl.pdf (accessed on 6 March 2020).
- Ballanger, B.; Strafella, A.P.; van Eimeren, T.; Zurowski, M.; Rusjan, P.M.; Houle, S.; Fox, S.H. Serotonin 2A Receptors and Visual Hallucinations in Parkinson Disease. Arch. Neurol. 2010, 67, 416–421. [Google Scholar] [CrossRef]
- Thomas, K.; Saadabadi, A. Olanzapine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK532903/ (accessed on 30 January 2020).
- Tollens, F.; Gass, N.; Becker, R.; Schwarz, A.; Risterucci, C.; Künnecke, B.; Lebhardt, P.; Reinwald, J.; Sack, M.; Weber-Fahr, W.; et al. The affinity of antipsychotic drugs to dopamine and serotonin 5-HT2 receptors determines their effects on prefrontal-striatal functional connectivity. Eur. Neuropsychopharmacol. 2018, 28, 1035–1046. [Google Scholar] [CrossRef]
- Martinez-Ramirez, D.; Okun, M.S.; Jaffee, M.S. Parkinson’s disease psychosis: Therapy tips and the importance of communication between neurologists and psychiatrists. Neurodegener. Dis. Manag. 2016, 6, 319–330. [Google Scholar] [CrossRef]
- Yuan, M.; Sperry, L.; Malhado-Chang, N.; Duffy, A.; Wheelock, V.; Farias, S.; O’Connor, K.; Olichney, J.; Shahlaie, K.; Zhang, L. Atypical antipsychotic therapy in Parkinson’s disease psychosis: A retrospective study. Brain Behav. 2017, 7, e00639. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.; Vacca, L.; Stocchi, F. Istradefylline for the treatment of Parkinson’s disease: Is it a promising strategy? Expert Opin. Pharmacother. 2018, 19, 1821–1828. [Google Scholar] [CrossRef]
- Ellis, T.; Cudkowicz, M.E.; Sexton, P.M.; Growdon, J.H. Clozapine and Risperidone Treatment of Psychosis in Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 364–369. [Google Scholar] [CrossRef]
- Pintor, L.; Valldeoriola, F.; Bailles, E.; Martí, M.J.; Muñiz, A.; Tolosa, E. Ziprasidone Versus Clozapine in the Treatment of Psychotic Symptoms in Parkinson Disease. Clin. Neuropharmacol. 2012, 35, 61–66. [Google Scholar] [CrossRef]
- Stocchi, F.; Torti, M. Adjuvant therapies for Parkinson’s disease: Critical evaluation of safinamide. Drug Des. Dev. Ther. 2016, 10, 609–618. [Google Scholar] [CrossRef]
- Cattaneo, C.; Kulisevsky, J.; Tubazio, V.; Castellani, P. Long-term Efficacy of Safinamide on Parkinson’s Disease Chronic Pain. Adv. Ther. 2018, 35, 515–522. [Google Scholar] [CrossRef]
- Agnati, L.F.; Ferre, S.; Lluis, C.; Franco, R.; Fuxe, K. Molecular Mechanisms and Therapeutical Implications of Intramembrane Receptor/Receptor Interactions among Heptahelical Receptors with Examples from the Striatopallidal GABA Neurons. Pharmacol. Rev. 2003, 55, 509–550. [Google Scholar] [CrossRef]
- Jenner, P. Istradefylline, a novel adenosine A2Areceptor antagonist, for the treatment of Parkinson’s disease. Expert Opin. Investig. Drugs 2005, 14, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Kondo, T.; The Japanese Istradefylline Study Group. Adenosine A2Areceptor antagonist istradefylline reduces daily OFF time in Parkinson’s disease. Mov. Disord. 2013, 28, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Ballard, C.; Tariot, P.; Owen, R.; Foff, E.; Youakim, J.; Norton, J.; Stankovic, S. Pimavanserin: Potential Treatment for Dementia-Related Psychosis. J. Prev. Alzheimer’s Dis. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Vanover, K.E.; Weiner, D.M.; Makhay, M.; Veinbergs, I.; Gardell, L.R.; Lameh, J.; Del Tredici, A.L.; Piu, F.; Schiffer, H.H.; Ott, T.R.; et al. Pharmacological and Behavioral Profile of N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) Carbamide (2R,3R)-Dihydroxybutanedioate (2:1) (ACP-103), a Novel 5-Hydroxytryptamine2A Receptor Inverse Agonist. J. Pharmacol. Exp. Ther. 2006, 317, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Jalal, B. The neuropharmacology of sleep paralysis hallucinations: Serotonin 2A activation and a novel therapeutic drug. Psychopharmacology 2018, 235, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef] [PubMed]
- PD MED Collaborative Group incl. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): A large, open-label, pragmatic randomised trial. Lancet 2014, 384, 1196–1205. [Google Scholar] [CrossRef]
- Fahn, S.; Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Lang, A.; Olanow, C.W.; Tanner, C.; Marek, K.; Parkinson Study Group. Levodopa and the Progression of Parkinson’s Disease. N. Engl. J. Med. 2004, 351, 2498–2508. [Google Scholar] [CrossRef]
- Fox, S.H.; Katzenschlager, R.; Lim, S.-Y.; Ravina, B.; Seppi, K.; Coelho, M.; Poewe, W.; Rascol, O.; Goetz, C.G.; Sampaio, C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the motor symptoms of Parkinson’s disease. Mov. Disord. 2011, 26 (Suppl. 3), S2–S41. [Google Scholar] [CrossRef]
- Parkinson Study Group CALM Cohort Investigators. Long-term Effect of Initiating Pramipexole vs. Levodopa in Early Parkinson Disease. Arch. Neurol. 2009, 66, 563–570. [Google Scholar] [CrossRef]
- Rascol, O.; Brooks, D.; Korczyn, A.; De Deyn, P.P.; Clarke, C.E.; Lang, A. A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. N. Engl. J. Med. 2000, 342, 1484–1491. [Google Scholar] [CrossRef]
- Oertel, W.H.; Wolters, E.; Sampaio, C.; Gimenez-Roldan, S.; Bergamasco, B.; Dujardin, M.; Grosset, D.; Arnold, G.; Leenders, K.L.; Hundemer, H.-P.; et al. Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET study. Mov. Disord. 2005, 21, 343–353. [Google Scholar] [CrossRef]
- Lang, A.E.; Lees, A. Management of Parkinson’s disease: An evidence-based review. Mov. Disord. 2002, 17, S1–S166. [Google Scholar] [CrossRef]
- Katsaiti, I.; Nixon, J. Are There Benefits in Adding Catechol-O Methyltransferase Inhibitors in the Pharmacotherapy of Parkinson’s Disease Patients? A Systematic Review. J. Park. Dis. 2018, 8, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Tolcapone. Ncbi.nlm.nih.gov. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548573/ (accessed on 6 March 2020).
- Rocha, J.F.; Almeida, L.; Falcão, A.; Palma, P.N.; Loureiro, A.I.; Pinto, R.; Bonifácio, M.J.; Wright, L.C.; Nunes, T.; Soares-Da-Silva, P. Opicapone: A short lived and very long acting novel catechol-O-methyltransferase inhibitor following multiple dose administration in healthy subjects. Br. J. Clin. Pharmacol. 2013, 76, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Lees, A.; Rocha, J.-F.; Poewe, W.; Rascol, O.; Soares-Da-Silva, P. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: A randomised, double-blind, controlled trial. Lancet Neurol. 2016, 15, 154–165. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Rascol, O. Efficacy and safety of amantadine for the treatment of l-DOPA-induced dyskinesia. J. Neural Transm. 2018, 125, 1237–1250. [Google Scholar] [CrossRef]
- Pajo, A.T.; I Espiritu, A.; Jamora, R.D.G. Efficacy and safety of extended-release amantadine in levodopa-induced dyskinesias: A meta-analysis. Neurodegener. Dis. Manag. 2019, 9, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Eggert, K.; Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Trenkwalder, C.; Ehret, R.; Azulay, J.P.; Isaacson, S.; Felt, L.; et al. Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov. Disord. 2017, 32, 1701–1709. [Google Scholar] [CrossRef]
- Parkinson Study Group. Low-Dose Clozapine for the Treatment of Drug-Induced Psychosis in Parkinson’s Disease. N. Engl. J. Med. 1999, 340, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Pollak, P.; Tison, F.; Rascol, O.; Destée, A.; Péré, J.J.; Senard, J.-M.; Durif, F.; Bourdeix, I. Clozapine in drug induced psychosis in Parkinson’s disease: A randomised, placebo controlled study with open follow up. J. Neurol. Neurosurg. Psychiatry 2004, 75, 689–695. [Google Scholar] [CrossRef]
- Hermanowicz, N.; Alva, G.; Pagan, F.; Patel, A.; Madrid, K.C.; Kremens, D.; Kenney, J.; Arquette, S.; Tereso, G.; Farnum, C.; et al. The Emerging Role of Pimavanserin in the Management of Parkinson’s Disease Psychosis. J. Manag. Care Spec. Pharm. 2017, 23 (Suppl. 6-b), S2–S8. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Weintraub, D.; Ballard, C.; Aarsland, D. The psychosis spectrum in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 81–95. [Google Scholar] [CrossRef]
- Samudra, N.; Patel, N.; Womack, K.; Khemani, P.; Chitnis, S. Psychosis in Parkinson Disease: A Review of Etiology, Phenomenology, and Management. Drugs Aging 2016, 33, 855–863. [Google Scholar] [CrossRef] [PubMed]
- UpToDate. Uptodate.com. 2020. Available online: https://www.uptodate.com/contents/medical-management-of-motor-fluctuations-and-dyskinesia-in-parkinson-disease# (accessed on 6 March 2020).
- Frieling, H.; Hillemacher, T.; Ziegenbein, M.; Neundörfer, B.; Bleich, S. Treating dopamimetic psychosis in Parkinson’s disease: Structured review and meta-analysis. Eur. Neuropsychopharmacol. 2007, 17, 165–171. [Google Scholar] [CrossRef]
- Recommendations|Parkinson’s Disease in Adults Guidance|NICE. 2020. Available online: https://www.nice.org.uk/guidance/ng71/chapter/Recommendations#pharmacological-management-of-motor-symptoms (accessed on 6 March 2020).
- Ondo, W.G.; Levy, J.K.; Vuong, K.D.; Hunter, C.; Jankovic, J. Olanzapine treatment for dopaminergic-induced hallucinations. Mov. Disord. Off. J. Mov. Disord. Soc. 2002, 17, 1031–1035. [Google Scholar] [CrossRef]
- Goetz, C.; Blasucci, L.; Leurgans, S.; Pappert, E. Olanzapine and clozapine: Comparative effects on motor function in hallucinating PD patients. Neurology 2000, 55, 789–794. [Google Scholar] [CrossRef]
- Sellers, J.; Darby, R.R.; Farooque, A.; Claassen, D.O. Pimavanserin for Psychosis in Parkinson’s Disease-Related Disorders: A Retrospective Chart Review. Drugs Aging 2019, 36, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Esteban, J.C.; Zarranz, J.J.; Velasco, F.; Lezcano, E.; Lachen, M.C.; Rouco, I.; Barcena, J.; Boyero, S.; Ciordia, R.; Allue, I. Use of Ziprasidone in Parkinsonian Patients With Psychosis. Clin. Neuropharmacol. 2005, 28, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.; Fox, S.; Hauser, R.; Jankovic, J.; Jost, W.; Kulisevsky, J.; Pahwa, R.; Poewe, W.; Anand, R. Safinamide add on to L-dopa: At randomized, placebo controlled 24 weeks global trial in patients with Parkinson’s disease and motor fluctuations. In Proceedings of the 65th Annual Meeting of the American Academy of Neurology (AAN), San Diego, CA, USA, 16–23 March 2013. [Google Scholar]
- Pagonabarraga, J.; Kulisevsky, J. Safinamide from daily clinical practice: First clinical steps. Rev. Neurol. 2017, 65, 29130466. [Google Scholar]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov. Disord. 2014, 29, 229–237. [Google Scholar] [CrossRef]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.H.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov. Disord. 2014, 29, 1273–1280. [Google Scholar] [CrossRef]
- Cattaneo, C.; Sardina, M.; Bonizzoni, E. Safinamide as Add-On Therapy to Levodopa in Mid- to Late-Stage Parkinson’s Disease Fluctuating Patients: Post hoc Analysesof Studies 016 and SETTLE. J. Park. Dis. 2016, 6, 165–173. [Google Scholar] [CrossRef]
- Stocchi, F.; Arnold, G.; Onofrj, M.; Kwiecinski, H.; Szczudlik, A.; Thomas, A.; Bonuccelli, U.; Van Dijk, A.; Cattaneo, C.; Sala, P.; et al. Improvement of motor function in early Parkinson disease by safinamide. Neurology 2004, 63, 746–748. [Google Scholar] [CrossRef]
- Stocchi, F.; Vacca, L.; Grassini, P.; De Pandis, M.F.; Battaglia, G.; Cattaneo, C.; Fariello, R.G. Symptom relief in Parkinson disease by safinamide: Biochemical and clinical evidence of efficacy beyond MAO-B inhibition. Neurology 2006, 67, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Dm, R.B.; Onofrj, M.; Schapira, A.H.; Bhatt, M.; Lucini, V.; Giuliani, R.; Anand, R.; for the Study 015 Investigators. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson’s disease patients. Mov. Disord. 2011, 27, 106–112. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Stocchi, F.; Borgohain, R.; Onofrj, M.; Bhatt, M.; Lorenzana, P.; Lucini, V.; Giuliani, R.; Anand, R.; The Study 017 Investigators. Long-term efficacy and safety of safinamide as add-on therapy in early Parkinson’s disease. Eur. J. Neurol. 2013, 20, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.; Fernandez, H.; Ferreira, J.; Mueller, T.; Saint-Hilaire, M.; Stacy, M.; Tolosa, E.; Anand, R. Safinamide as an add-on therapy to a stable dose of a single dopamine agonist: Results from a randomized, placebo-controlled, 24-week multicenter trial in early idiopathic Parkinson disease patients (MOTION Study). In Proceedings of the 65th Annual Meeting of the American Academy of Neurology (AAN), San Diego, CA, USA, 16–23 March 2013. [Google Scholar]
- De La Cruz, J.; Canal, C. Can pimavanserin help patients with Parkinson disease psychosis? J. Am. Acad. Phys. Assist. 2019, 32, 44–45. [Google Scholar] [CrossRef]
- Espay, A.J.; Guskey, M.T.; Norton, J.C.; Coate, B.; Vizcarra, J.A.; Ballard, C.; Factor, S.A.; Friedman, J.H.; Lang, A.E.; Larsen, N.J.; et al. Pimavanserin for Parkinson’s Disease psychosis: Effects stratified by baseline cognition and use of cognitive-enhancing medications. Mov. Disord. 2018, 33, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Patient Education: Parkinson Disease Treatment Options—Medications (Beyond the Basics)—UpToDate. Available online: https://www.uptodate.com/contents/parkinson-disease-treatment-options-medications-beyond-the-basics#H16 (accessed on 14 February 2020).
- Watkins, P. COMT inhibitors and liver toxicity. Neurology 2000, 55 (Suppl. 4), S51–S52. [Google Scholar]
- Kaefer, V.; Semedo, J.G.; Kahl, V.F.S.; Von Borowsky, R.G.; Gianesini, J.; Kist, T.B.L.; Pereira, P.; Picada, J.N. DNA damage in brain cells and behavioral deficits in mice after treatment with high doses of amantadine. J. Appl. Toxicol. 2010, 30, 745–753. [Google Scholar] [CrossRef]
- Mocci, G.; Jiménez-Sánchez, L.; Adell, A.; Cortes, R.; Artigas, F. Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology 2014, 79, 49–58. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Trieschmann, M.E.; Friedman, J.H. Treatment of psychosis in Parkinson’s disease: Safety considerations. Drug Saf. 2003, 26, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Lertxundi, U.; Ruiz, A.I.; Aspiazu, M.Á.S.; Domingo-Echaburu, S.; García, M.; Aguirre, C.; García-Moncó, J.C. Adverse Reactions to Antipsychotics in Parkinson Disease. Clin. Neuropharmacol. 2015, 38, 69–84. [Google Scholar] [CrossRef]
- Rosin, D.L.; Hettinger, B.D.; Lee, A.; Linden, J. Anatomy of adenosine A2A receptors in brain: Morphological substrates for integration of striatal function. Neurology 2003, 61 (Suppl. 6), S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.H.; Pavese, N.; Lawrence, A.D.; Tai, Y.F.; Appel, S.; Doder, M.; Brooks, D.J.; Lees, A.J.; Piccini, P. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann. Neurol. 2006, 59, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Zaniewska, M.; Frankowska, M.; Wydra, K.; Fuxe, K. The Importance of the Adenosine A2A Receptor-Dopamine D2 Receptor Interaction in Drug Addiction. Curr. Med. Chem. 2012, 19, 317–355. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Isaacson, S.; Mills, R.; Williams, H.; Chi-Burris, K.; Corbett, A.; Dhall, R.; Ballard, C. Pimavanserin for patients with Parkinson’s disease psychosis: A randomised, placebo-controlled phase 3 trial. Lancet 2014, 383, 533–540. [Google Scholar] [CrossRef]
- Bloem, B.R.; De Vries, N.M.; Ebersbach, G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov. Disord. 2015, 30, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Julie, G.P.; Olga, K.; Shrey, P.; American Association of Neurological Surgeons. Deep Brain Stimulation. Available online: https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Deep-Brain-Stimulation (accessed on 16 November 2021).

| Drug Name | Author, Year, Reference Number | Study Design | Population Characteristics | Interventions | Primary Outcome Measured | Efficacy | Safety |
|---|---|---|---|---|---|---|---|
| Safinamide | Schapira A., et al., 2013 [88] | Randomized, placebo-controlled, double-blind international Phase III trial. | Patients who had mid-to-late-stage idiopathic PD (>3 years of disease) and were treated with optimized, stable doses of L-dopa and DA, catechol-O-methyltransferase inhibitor, anticholinergic, and/or amantadine. | Safinamide 50 mg, Safinamide 100 mg, placebo. | Change in daily on time with no or non-troublesome dyskinesia. | Improved on time (without worsening the troublesome dyskinesia), off time, UPDRS part III, CGI-S, CGI-C, PDQ-39 and off time following the first morning L-dopa dose. | Major AEs: Back pain, headache, falls, dyskinesias, nausea, and urinary tract infections |
| Borgohain R., et al., 2014 [90] | Randomized, placebo-controlled, double-blind Phase III trial. (Study 016) | Patients aged 30–80 years, had been diagnosed with PD ≥3 years, had the presence of motor fluctuations with 1.5 h off a day. | Safinamide 50 mg, Safinamide 100 mg, placebo. | Change in mean daily on time with no or non-troublesome dyskinesias in the 18-h recording period. | Improved UPDRS part III in both safinamide 50 mg (p = 0.0138) and 100 mg (p = 0.0006) groups. Significant improvement in off time, CGI-C and CGI-S in both safinamide groups following the morning dose of levodopa. | Major AEs: Back pain, headache, dyskinesia, depression, and hypertension | |
| Borgohain R., et al., 2014 [91] | Randomized, double-blind, placebo-controlled, 18-month extension study. (Study 018) | Patients who had completed the 016 study or patients who had completed efficacy evaluation at weeks 12 and 24 of Study 016. | Safinamide 50 mg, Safinamide 100 mg, placebo. | Mean change from baseline at Study 016 to endpoint of the DRS score during on time. | Improved total daily on time without troublesome dyskinesia from baseline for safinamide 50 mg (p = 0.0031) and safinamide 100 mg (p = 0.0002). Improved off time, CGI-S, CGI-C (for SAF 50 mg), UPDRS part II, part III and part IV total scores and PDQ-39. | Major AEs: Back pain, insomnia, headache, and dyskinesia | |
| Stocchi F., et al., 2004 [93] | Randomized, placebo-controlled, double-blind, Phase II, dose finding study. | Early PD patients. | Safinamide 0.5 mg/kg, Safinamide 1.0 mg/kg, placebo as monotherapy or as adjunct therapy to a single DA. | Proportion of patients considered as treatment responders, for example 30% improvement in UPDRS part III compared with baseline. | Improved UPDRS part III as compared to baseline, more statistically significant between safinamide 1.0 mg/kg and placebo (p = 0.016). | Major AEs: Abdominal pain, dizziness, and musculoskeletal and connective tissue disorders | |
| Stocchi F., et al., 2006 [94] | Single-center, open, pilot trial. | 25 PD patients with Hoehn and Yahr (H&Y) stages III–IV. | Safinamide 100 mg, Safinamide 150 mg, Safinamide 200 mg as adjunct therapy to stable single DA or LD. | Changes in UPDRS part II, part III, and part IV and CGI. | Improved motor performance (evaluated by UPDRS part III) for more than an 8-week period (p < 0.001). | Major AEs: - | |
| Stocchi F., et al., 2012 [95] | Randomized, placebo-controlled, double-blind Phase III trial. (Study 015) | Early PD patients aged 30–80 years, who were diagnosed with idiopathic PD with <5 years of history and had Hoehn and Yahr (H&Y) stages I–II. | Safinamide 100 mg, Safinamide 200 mg, placebo as adjunct therapy to stable single DA. | Changes in UPDRS part III total score from baseline to endpoint (week 24). | Improved UPDRS part II, UPDRS part III and CGI-C total score in safinamide 100 mg group (p = 0.0419). | Major AEs: Nausea, vomiting, headache, dizziness, back pain, gastritis, and abdominal pain | |
| Schapira A., et al., 2013. [96] | Randomized, double-blind, placebo-controlled, 12-month extension study. (Study 017) | Patients who had completed Study 015 or patients who had completed efficacy evaluation at weeks 12 and 24 of Study 015. | Safinamide 100 mg, Safinamide 200 mg, placebo as adjunct therapy to stable single DA. | Time to intervention from baseline. | Lower rate of intervention in the safinamide 100 mg group compared with dopamine agonists monotherapy (p < 0.05). Improved UPDRS part II and part III was greater in safinamide 100 mg group. | Major AEs: Dizziness, nausea, back pain, nausea, and upper abdominal pain | |
| Barone P., et al., 2013 [97] | Randomized, placebo-controlled, double-blind international Phase III trial. | Patients with early idiopathic PD (<5 years) who were treated with a single DA. | Safinamide 50 mg, Safinamide 100 mg, placebo. | Change in UPDRS part III from baseline to week 24. | Improved UPDRS part III (p = 0.0396) and PDQ-39 in the safinamide 100 mg group. | Major AEs: Nausea, dizziness, headache, arthralgia, and back pain | |
| Istradefylline | Mizuno Y., et al., 2013 [57] | Multicenter, placebo-controlled, randomized, double-blind, parallel-group study. | PD patients with motor complication. | Istradefylline 20 or 40 mg/day and placebo. | Change in daily off time. | The change in daily off time was significantly reduced in the istradefylline 20 mg/day (−0.99 h, p = 0.003) and istradefylline 40 mg/day (−0.96 h, p = 0.003) groups. | Major AEs: Dyskinesia, gait disturbance, gastric ulcer, and hallucinations |
| Pimavanserin | Espay A., et al., 2018 [99] | 6-week randomized, double-blind, placebo-controlled, phase 3 trial. | Patients with PD psychosis. | Pimavanserin 34 mg and placebo. | Change in the Scale for the Assessment of Positive Symptoms-PD. | Mean (pimavanserin vs. placebo) change from baseline was larger in the cognitively impaired (n = 50; −6.62 vs. −0.91; p = 0.002) versus the cognitively unimpaired (n = 135; −5.50 vs. −3.23; p = 0.046) group. The mean difference in SAPS-PD score change from baseline for pimavanserin versus placebo was −3.06 at day 43 (p = 0.001). | Major AEs: Urinary tract infection, fall, peripheral edema, hallucinations, nausea, and confusional state |
| Cummings J., et al., 2018 [109] | 6-week, randomized, double-blind, placebo-controlled study. | Patients with PD psychosis. | Pimavanserin 40 mg and placebo. | SAPS-PD score change from baseline to week 6. | Pimavanserin was associated with statistically significant 5.79 point improvement at week 6 as compared to placebo with 2.73 point (p = 0.001) | Major AEs: Nausea, headache, fall, urinary tract infection, peripheral edema, confusional state, and hallucinations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Wey Han, S.A.; Semilan, S.L.A.; Hong, P.K. Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. Int. J. Environ. Res. Public Health 2022, 19, 364. https://doi.org/10.3390/ijerph19010364
Sivanandy P, Leey TC, Xiang TC, Ling TC, Wey Han SA, Semilan SLA, Hong PK. Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. International Journal of Environmental Research and Public Health. 2022; 19(1):364. https://doi.org/10.3390/ijerph19010364
Chicago/Turabian StyleSivanandy, Palanisamy, Tan Choo Leey, Tan Chi Xiang, Tan Chi Ling, Sean Ang Wey Han, Samantha Lia Anak Semilan, and Phoon Kok Hong. 2022. "Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms" International Journal of Environmental Research and Public Health 19, no. 1: 364. https://doi.org/10.3390/ijerph19010364
APA StyleSivanandy, P., Leey, T. C., Xiang, T. C., Ling, T. C., Wey Han, S. A., Semilan, S. L. A., & Hong, P. K. (2022). Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. International Journal of Environmental Research and Public Health, 19(1), 364. https://doi.org/10.3390/ijerph19010364






