Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review
Abstract
1. Introduction
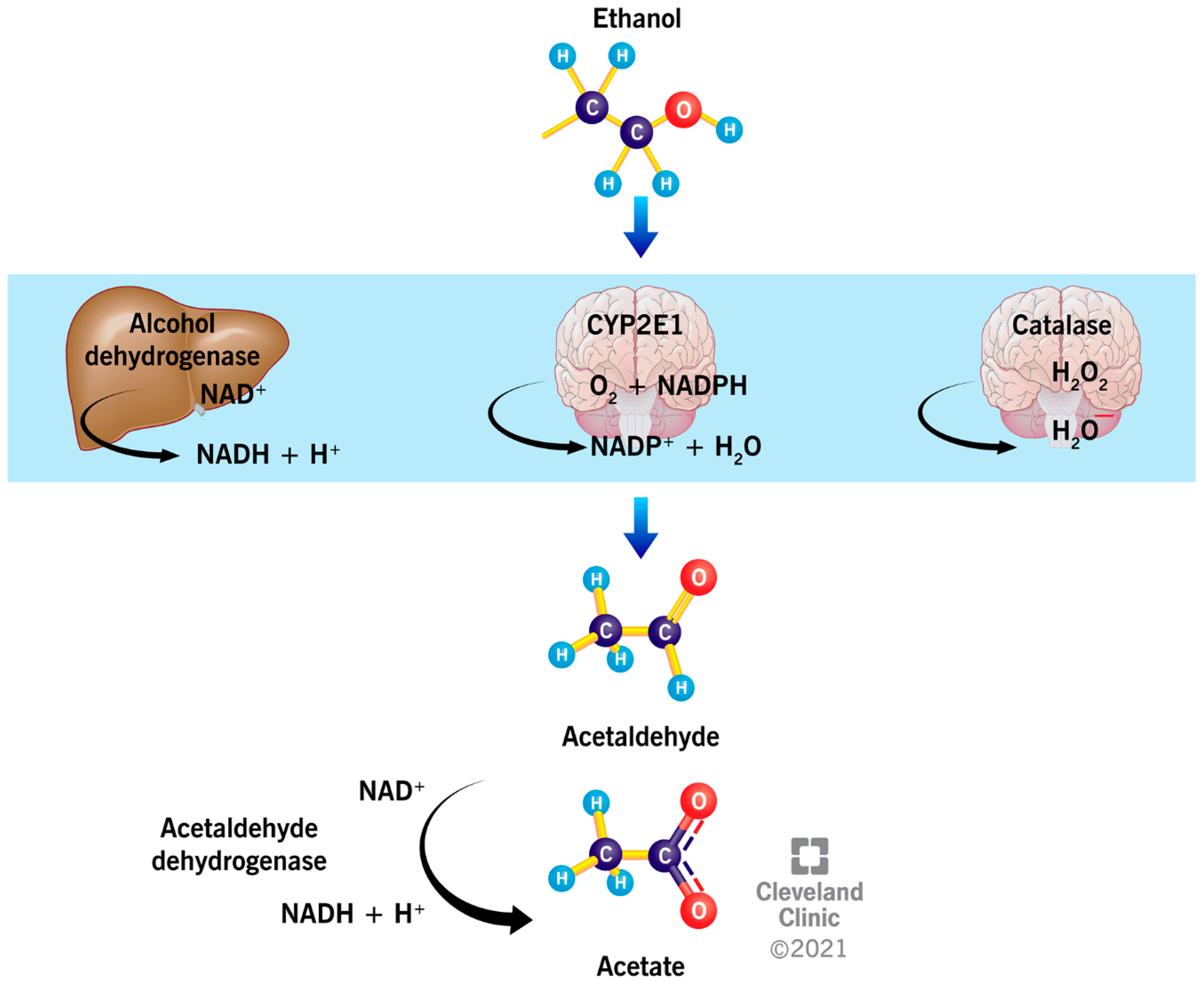
2. Metabolism of Ethanol
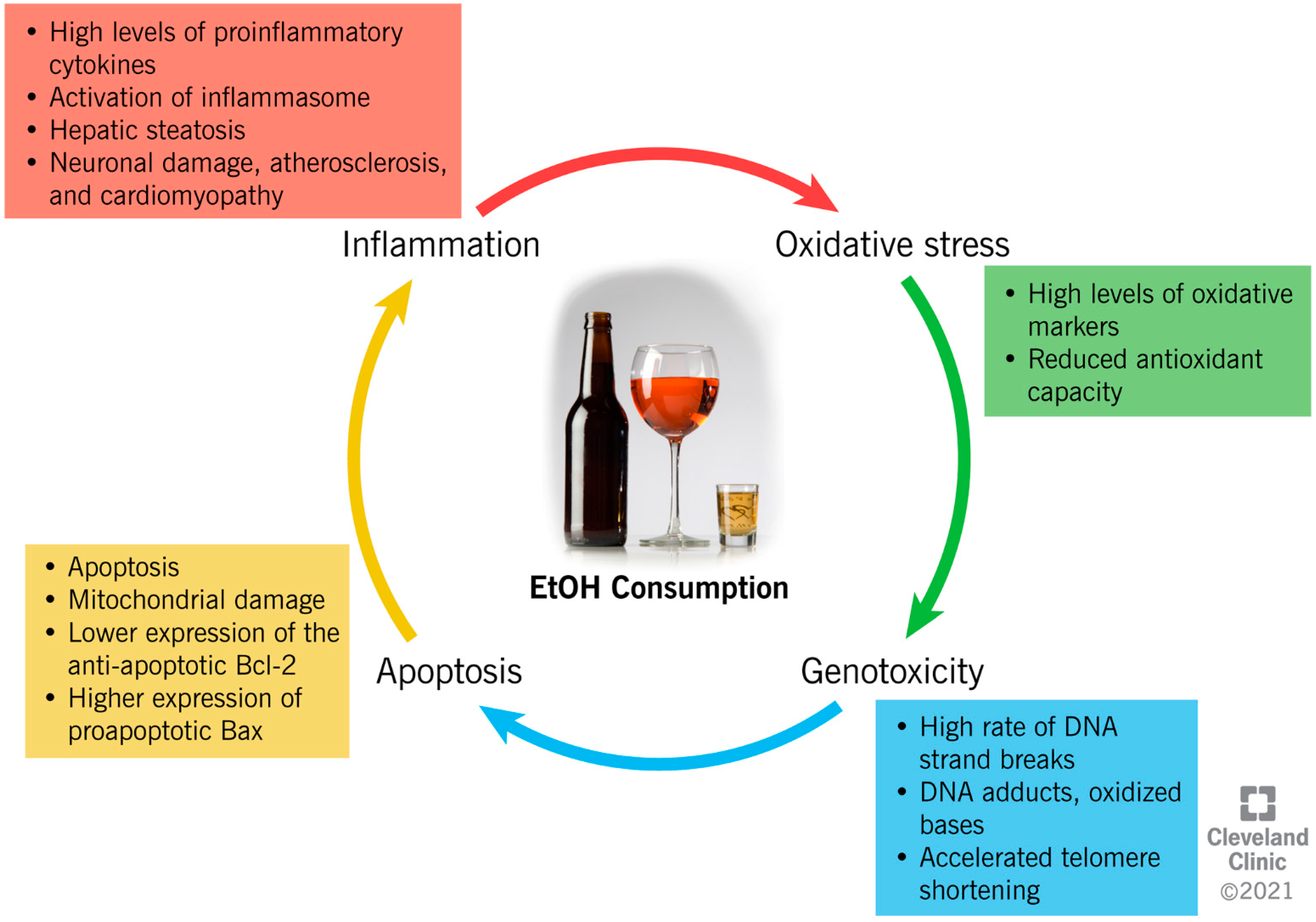
3. Ethanol-Induced Mechanisms of Cellular Damage
4. Alcohol Consumption and Male Infertility: Evidence from Animal and Human Studies
4.1. Impact of Alcohol on Reproductive Hormonal Regulation
4.2. Impact of Alcohol Consumption on Semen Quality
4.3. Impact of Alcohol Consumption on Gene Transcription, Genetics, and Epigenetics Regulation
4.4. Consequences of Paternal Alcohol Consumption on the Offspring
5. Limitations of the Published Human Studies and Future Areas of Investigation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2018.
- EU Citizens’ Attitudes towards Alcohol. Available online: https://ec.europa.eu/health/sites/default/files/alcohol/docs/ebs_331_en.pdf (accessed on 9 February 2021).
- National Institute on Alcohol Abuse and Alcoholism Drinking Levels Defined. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 2 September 2021).
- National Institute on Alcohol Abuse and Alcoholism Alcohol Facts and Statistics. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics (accessed on 2 September 2021).
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Touvier, M.; Druesne-Pecollo, N.; Kesse-Guyot, E.; Andreeva, V.A.; Galan, P.; Hercberg, S.; Latino-Martel, P. Demographic, socioeconomic, disease history, dietary and lifestyle cancer risk factors associated with alcohol consumption. Int. J. Cancer 2014, 134, 445–459. [Google Scholar] [CrossRef]
- Graff-Iversen, S.; Jansen, M.D.; Hoff, D.A.; Høiseth, G.; Knudsen, G.P.; Magnus, P.; Mørland, J.; Normann, P.T.; Næss, O.E.; Tambs, K. Divergent associations of drinking frequency and binge consumption of alcohol with mortality within the same cohort. J. Epidemiol. Community Health 2013, 67, 350–357. [Google Scholar] [CrossRef]
- Dugum, M.; McCullough, A. Diagnosis and management of alcoholic liver disease. J. Clin. Transl. Hepatol. 2015, 3, 109–116. [Google Scholar]
- Sawada Feldman, H.; Lyons Jones, K.; Lindsay, S.; Slymen, D.; Klonoff-Cohen, H.; Kao, K.; Rao, S.; Chambers, C. Prenatal alcohol exposure patterns and alcohol-related birth defects and growth deficiencies: A prospective study. Alcohol. Clin. Exp. Res. 2012, 36, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Rivas, I.; Sanvisens, A.; Bolao, F.; Fuster, D.; Tor, J.; Pujol, R.; Torrens, M.; Rey-Joly, C.; Muga, R. Impact of medical comorbidity and risk of death in 680 patients with alcohol use disorders. Alcohol. Clin. Exp. Res. 2013, 37, E221–E227. [Google Scholar] [CrossRef]
- Trudell, J.R.; Messing, R.O.; Mayfield, J.; Harris, R.A. Alcohol dependence: Molecular and behavioral evidence. Trends Pharmacol. Sci. 2014, 35, 317–323. [Google Scholar] [CrossRef]
- Vengeliene, V.; Bilbao, A.; Molander, A.; Spanagel, R. Neuropharmacology of alcohol addiction. Br. J. Pharmacol. 2008, 154, 299–315. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism Alcohol Use Disorder: A Comparison between DSM–IV and DSM–5. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-use-disorder-comparison-between-dsm?fbclid=IwAR0gGIMEub8BuoxUTAMbygBtNBLfJvMh0yOSN1vdLelzZMMHG4v57GjZm9g (accessed on 7 October 2021).
- Mukherjee, S. Alcoholism and its effects on the central nervous system. Curr. Neurovasc. Res. 2013, 10, 256–262. [Google Scholar] [CrossRef]
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef]
- Koob, G.F.; Colrain, I.M. Alcohol use disorder and sleep disturbances: A feed-forward allostatic framework. Neuropsychopharmacology 2020, 45, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A. Clinical review 33: Clinically important effects of alcohol on endocrine function. J. Clin. Endocrinol. Metab. 1992, 74, 957–960. [Google Scholar] [PubMed]
- Close, C.E.; Roberts, P.L.; Berger, R.E. Cigarettes, alcohol and marijuana are related to pyospermia in infertile men. J. Urol. 1990, 144, 900–903. [Google Scholar] [CrossRef]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef]
- Grover, S.; Mattoo, S.K.; Pendharkar, S.; Kandappan, V. Sexual dysfunction in patients with alcohol and opioid dependence. Indian J. Psychol. Med. 2014, 36, 355–365. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Wilkinson, P.K.; Sedman, A.J.; Sakmar, E.; Kay, D.R.; Wagner, J.G. Pharmacokinetics of ethanol after oral administration in the fasting state. J. Pharmacokinet. Biopharm. 1977, 5, 207–224. [Google Scholar] [CrossRef]
- Eriksson, C.J.P.; Fukunaga, T.; Sarkola, T.; Chen, W.J.; Chen, C.C.; Ju, J.M.; Cheng, A.T.; Yamamoto, H.; Kohlenberg-Muller, K.; Kimura, M.; et al. Functional relevance of human ADH polymorphism. Alcohol. Clin. Exp. Res. 2001, 25, 157S–163S. [Google Scholar] [CrossRef]
- Lieber, C.S. Perspectives: Do alcohol calories count? Am. J. Clin. Nutr. 1991, 54, 976–982. [Google Scholar] [CrossRef]
- Zimatkin, S.M.; Pronko, S.P.; Vasiliou, V.; Gonzalez, F.J.; Deitrich, R.A. Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol. Clin. Exp. Res. 2006, 30, 1500–1505. [Google Scholar] [CrossRef]
- Oneta, C.M.; Lieber, C.S.; Li, J.J.; Rüttimann, S.; Schmid, B.; Lattmann, J.; Rosman, A.S.; Seitz, H.K. Dynamics of cytochrome P4502E1 activity in man: Induction by ethanol and disappearance during withdrawal phase. J. Hepatol. 2002, 36, 47–52. [Google Scholar] [CrossRef]
- Deng, X.S.; Deitrich, R.A. Putative role of brain acetaldehyde in ethanol addiction. Curr. Drug Abuse Rev. 2008, 1, 3–8. [Google Scholar] [CrossRef][Green Version]
- U.S. Department of Health and Human Services. Disulfiram. In Incorporating Alcohol Pharmacotherapies into Medical Practice: Treatment Improvement Protocol Series (TIP 49); Lulu.com: Rockville, MD, USA, 2009. [Google Scholar]
- Jiang, L.; Gulanski, B.I.; De Feyter, H.M.; Weinzimer, S.A.; Pittman, B.; Guidone, E.; Koretski, J.; Harman, S.; Petrakis, I.L.; Krystal, J.H.; et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J. Clin. Investig. 2013, 123, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, S.F.W.; O’Boyle, G.; Mann, J.; Zeybel, M.; Palmer, J.; Jones, D.E.J.; Day, C.P. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology 2010, 51, 1988–1997. [Google Scholar] [CrossRef]
- Best, C.; Laposata, M. Fatty acid ethyl esters: Toxic non-oxidative metabolites of ethanol and markers of ethanol intake. Front. Biosci. 2003, 8, e202–e217. [Google Scholar]
- Heier, C.; Xie, H.; Zimmermann, R. Nonoxidative ethanol metabolism in humans—from biomarkers to bioactive lipids. IUBMB Life 2016, 68, 916–923. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W.; et al. Kupffer cells: Inflammation pathways and cell-cell interactions in alcohol-associated liver disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Rocco, A.; Compare, D.; Angrisani, D.; Sanduzzi Zamparelli, M.; Nardone, G. Alcoholic disease: Liver and beyond. World J. Gastroenterol. 2014, 20, 14652–14659. [Google Scholar] [CrossRef]
- Seth, D.; Haber, P.; Syn, W.; Diehl, A.; Day, C. Pathogenesis of alcohol-induced liver disease: Classical concepts and recent advances. J. Gastroenterol. Hepatol. 2011, 26, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Saha, B. Alcohol’s effect on host defense. Alcohol Res. 2015, 37, 159–170. [Google Scholar]
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef]
- Xu, G.; Li, C.; Parsiola, A.L.; Li, J.; McCarter, K.D.; Shi, R.; Mayhan, W.G.; Sun, H. Dose-dependent influences of ethanol on ischemic stroke: Role of inflammation. Front. Cell. Neurosci. 2019, 13, 6. [Google Scholar] [CrossRef]
- Ren, T.; Mackowiak, B.; Lin, Y.; Gao, Y.; Niu, J.; Gao, B. Hepatic injury and inflammation alter ethanol metabolism and drinking behavior. Food Chem. Toxicol. 2020, 136, 111070. [Google Scholar] [CrossRef]
- Roberto, M.; Patel, R.R.; Bajo, M. Ethanol and cytokines in the central nervous system. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2017; Volume 248, pp. 397–431. [Google Scholar]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. Curr. Rev. 2015, 37, 331–351. [Google Scholar]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D. Oxidative stress. Annu. Rev. Bioch 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Mantle, D.; Preedy, V. Free radicals as mediators of alcohol toxicity. Advers. Druf. React. Toxicol. Rev. 1999, 18, 235–252. [Google Scholar]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhao, Y.; Zhang, X. Acetaldehyde induces cytotoxicity of SH-SY5Y cells via inhibition of Akt activation and induction of oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 4512309. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Chang, X.; Gou, W.; Zhou, X.; Liu, Z.; Li, Z.; Wu, Y.; Zuo, D. Acetaldehyde induces neurotoxicity in vitro via oxidative stress- and Ca2+ imbalance-mediated endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2019, 2019, 2593742. [Google Scholar] [CrossRef] [PubMed]
- Clavijo-Cornejo, D.; Gutiérrez-Carrera, M.; Palestino-Domínguez, M.; Dominguez-Perez, M.; Nuño, N.; Souza, V.; Miranda, R.U.; Kershenobich, D.; Gutiérrez-Ruiz, M.C.; Bucio, L.; et al. Acetaldehyde targets superoxide dismutase 2 in liver cancer cells inducing transient enzyme impairment and a rapid transcriptional recovery. Food Chem. Toxicol. 2014, 69, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Yeligar, S.M.; Harris, F.L.; Hart, C.M.; Brown, L.A.S. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J. Immunol. 2012, 188, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Luo, J. Autophagy and ethanol neurotoxicity. Autophagy 2014, 10, 2099–2108. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, X.; Liu, J.; Feng, W.; Cai, L.; Wu, X.; Chen, S.Y. Sulforaphane restores acetyl-histone H3 binding to Bcl-2 promoter and prevents apoptosis in ethanol-exposed neural crest cells and mouse embryos. Exp. Neurol. 2018, 300, 60–66. [Google Scholar] [CrossRef]
- Kapasi, A.A.; Patel, G.; Goenka, A.; Nahar, N.; Modi, N.; Bhaskaran, M.; Reddy, K.; Franki, N.; Patel, J.; Singhal, P.C. Ethanol promotes T cell apoptosis through the mitochondrial pathway. Immunology 2003, 108, 313–320. [Google Scholar] [CrossRef]
- Han, J.Y.; Joo, Y.; Kim, Y.S.; Lee, Y.K.; Kim, H.J.; Cho, G.J.; Choi, W.S.; Kang, S.S. Ethanol induces cell death by activating caspase-3 in the rat cerebral cortex. Mol. Cells 2005, 20, 189–195. [Google Scholar]
- Steiner, J.L.; Lang, C.H. Etiology of alcoholic cardiomyopathy: Mitochondria, oxidative stress and apoptosis. Int. J. Biochem. Cell Biol. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Khirug, S.; Soni, S.; Saez Garcia, M.; Tessier, M.; Zhou, L.; Kulesskaya, N.; Rauvala, H.; Lindholm, D.; Ludwig, A.; Molinari, F.; et al. Protective role of low ethanol administration following ischemic stroke via recovery of KCC2 and p75NTR expression. Mol. Neurobiol. 2021, 58, 1145–1161. [Google Scholar] [CrossRef]
- Kotova, N.; Vare, D.; Schultz, N.; Meesters, D.G.; Stepnik, M.; Grawé, J.; Helleday, T.; Jenssen, D. Genotoxicity of alcohol is linked to DNA replication-associated damage and homologous recombination repair. Carcinogenesis 2013, 34, 325–330. [Google Scholar] [CrossRef]
- Santovito, A.; Cervella, P.; Delpero, M. Evidence of genotoxicity in lymphocytes of non-smoking alcoholics. Mol. Biol. Rep. 2015, 42, 53–59. [Google Scholar] [CrossRef][Green Version]
- Ceni, E.; Mello, T.; Galli, A. Pathogenesis of alcoholic liver disease: Role of oxidative metabolism. World J. Gastroenterol. 2014, 20, 17756–17772. [Google Scholar] [CrossRef] [PubMed]
- Peccerella, T.; Arslic-Schmitt, T.; Mueller, S.; Linhart, K.B.; Seth, D.; Bartsch, H.; Seitz, H.K. Chronic ethanol consumption and generation of Etheno-DNA adducts in cancer-prone tissues. Adv. Exp. Med. Biol. 2018, 1032, 81–92. [Google Scholar] [PubMed]
- Sapkota, M.; Wyatt, T.A. Alcohol, aldehydes, adducts and airways. Biomolecules 2015, 5, 2987–3008. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Zhang, J.; Rane, G.; Dai, X.; Shanmugam, M.K.; Arfuso, F.; Samy, R.P.; Lai, M.K.P.; Kappei, D.; Kumar, A.P.; Sethi, G. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 2016, 25, 55–69. [Google Scholar] [CrossRef]
- Yamaki, N.; Matsushita, S.; Hara, S.; Yokoyama, A.; Hishimoto, A.; Higuchi, S. Telomere shortening in alcohol dependence: Roles of alcohol and acetaldehyde. J. Psychiatr. Res. 2019, 109, 27–32. [Google Scholar] [CrossRef]
- Dixit, S.; Whooley, M.A.; Vittinghoff, E.; Roberts, J.D.; Heckbert, S.R.; Fitzpatrick, A.L.; Lin, J.; Leung, C.; Mukamal, K.J.; Marcus, G.M. Alcohol consumption and leukocyte telomere length. Sci. Rep. 2019, 9, 1404. [Google Scholar] [CrossRef]
- Salonen, I.; Pakarinen, P.; Huhtaniemi, I. Effect of chronic ethanol diet on expression of gonadotropin genes in the male rat. J. Pharmacol. Exp. Ther. 1992, 260, 463–467. [Google Scholar]
- Van Thiel, D.H.; Lester, R.; Sherins, R.J. Hypogonadism in alcoholic liver disease: Evidence for a double defect. Gastroenterology 1974, 67, 1188–1199. [Google Scholar] [CrossRef]
- Gordon, G.; Altman, K.; Southren, L.; Rupin, E.; Lieber, C. Effect of alcohol (ethanol) administration on sex-hormone metabolism in normal men. N. Engl. J. Med. 1976, 295, 793–797. [Google Scholar] [CrossRef]
- Ching, M.; Valenca, M.; Negro-Vilar, A. Acute ethanol treatment lowers hypophyseal portal plasma luteinizing hormone-releasing hormone (LH-RH) and systemic plasma LH levels in orchidectomized rats. Brain Res. 1988, 443, 325–328. [Google Scholar] [CrossRef]
- Salonen, I.; Huhtaniemi, I. Effects of chronic ethanol diet on pituitary-testicular function of the rat. Biol. Reprod. 1990, 42, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, M.; Tentler, J.; Halloran, M.; Emanuele, N.; Wallock, L.; Kelley, M. The effect of acute in vivo ethanol exposure on follicle stimulating hormone transcription and translation. Alcohol. Clin. Exp. Res. 1992, 16, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.; Southren, A.L.; Vittek, J.; Lieber, C.S. The effect of alcohol ingestion on hepatic aromatase activity and plasma steroid hormones in the rat. Metabolism 1979, 28, 20–24. [Google Scholar] [CrossRef]
- Badr, F.; Bartke, A.; Dalterio, S.; Bulger, W. Suppression of testosterone production by ethyl alcohol. Possible mode of action. Steroids 1977, 30, 647–655. [Google Scholar] [CrossRef]
- Cobb, C.; Ennis, M.; Van Thiel, D.; Gavaler, J.; Lester, R. Acetaldehyde and ethanol are direct testicular toxins. Surg. Forum. 1978, 29, 641–644. [Google Scholar]
- Castilla-Cortazar, I.; Quiroga, J.; Prieto, J. Insulin-like growth factor-I, liver function, and hypogonadism in rats with experimentally induced cirrhosis. Hepatology 2000, 31, 1379. [Google Scholar] [CrossRef]
- Gordon, G.; Vittek, J.; Southren, A.L.; Munnangi, P.; Lieber, C.S. Effect of chronic alcohol ingestion on the biosynthesis of steroids in rat testicular homogenate in vitro. Endocrinology 1980, 106, 1880–1885. [Google Scholar] [CrossRef] [PubMed]
- Gavaler, J.S.; Perez, H.A.; Estes, L.; Van Thiel, D.H. Morphologic alterations of rat Leydig cells induced by ethanol. Pharmacol. Biochem. Behav. 1983, 18, 341–347. [Google Scholar] [CrossRef]
- Muthusami, K.R.; Chinnaswamy, P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil. Steril. 2005, 84, 919–924. [Google Scholar] [CrossRef]
- Maneesh, M.; Dutta, S.; Chakrabarti, A.; Vasudevan, D. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J. Physiol. Pharmacol. 2006, 50, 291–296. [Google Scholar]
- Jensen, T.K.; Swan, S.; Jørgensen, N.; Toppari, J.; Redmon, B.; Punab, M.; Drobnis, E.Z.; Haugen, T.B.; Zilaitiene, B.; Sparks, A.E.; et al. Alcohol and male reproductive health: A cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014, 29, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Rohrmann, S.; Menke, A.; Selvin, E.; Crespo, C.J.; Rifai, N.; Dobs, A.; Feinleib, M.; Guallar, E.; Platz, E.A. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control 2009, 20, 877–886. [Google Scholar] [CrossRef]
- Hansen, M.L.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J.; Håkonsen, L.B.; Ramlau-Hansen, C.H. Does last week’s alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod. Toxicol. 2012, 34, 457–462. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Balercia, G.; Vicari, E.; Calogero, A.E. Does alcohol have any effect on male reproductive function? A review of literature. Asian J. Androl. 2013, 15, 221–225. [Google Scholar] [CrossRef]
- Rahimipour, M.; Talebi, A.R.; Anvari, M.; Sarcheshmeh, A.A.; Omidi, M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 423–428. [Google Scholar] [CrossRef]
- Franco Punhagui, A.P.; Rodrigues Vieira, H.; Eloisa Munhoz De Lion Siervo, G.; da Rosa, R.; Scantamburlo Alves Fernandes, G. Ethanol exposure during peripubertal period increases the mast cell number and impairs meiotic and spermatic parameters in adult male rats. Microsc. Res. Tech. 2016, 79, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.R.; Sarcheshmeh, A.A.; Khalili, M.A.; Tabibnejad, N. Effects of ethanol consumption on chromatin condensation and DNA integrity of epididymal spermatozoa in rat. Alcohol 2011, 45, 403–409. [Google Scholar] [CrossRef]
- Bai, S.; Wan, Y.; Zong, L.; Li, W.; Xu, X.; Zhao, Y.; Hu, X.; Zuo, Y.; Xu, B.; Tong, X.; et al. Association of alcohol intake and semen parameters in men with primary and secondary infertility: A cross-sectional study. Front. Physiol. 2020, 11, 566625. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Dehò, F.; Montanari, E.; Montorsi, F.; Salonia, A. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019, 21, 478–485. [Google Scholar] [PubMed]
- Borges, E.; de Braga, D.P.A.F.; Provenza, R.R.; de Cassia Savio Figueira, R.; Iaconelli, A.; Setti, A.S. Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia 2018, 50, e13090. [Google Scholar] [CrossRef] [PubMed]
- Sermondade, N.; Elloumi, H.; Berthaut, I.; Mathieu, E.; Delarouzire, V.; Ravel, C.; Mandelbaum, J. Progressive alcohol-induced sperm alterations leading to spermatogenic arrest, which was reversed after alcohol withdrawal. Reprod. Biomed. Online 2010, 20, 324–327. [Google Scholar] [CrossRef]
- Jensen, T.K.; Gottschau, M.; Madsen, J.O.B.; Andersson, A.M.; Lassen, T.H.; Skakkebæk, N.E.; Swan, S.H.; Priskorn, L.; Juul, A.; Jørgensen, N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open 2014, 4, e005462. [Google Scholar] [CrossRef]
- Ricci, E.; Al Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef]
- Anifandis, G.; Bounartzi, T.; Messini, C.I.; Dafopoulos, K.; Sotiriou, S.; Messinis, I.E. The impact of cigarette smoking and alcohol consumption on sperm parameters and sperm DNA fragmentation (SDF) measured by Halosperm. Arch. Gynecol. Obstet. 2014, 290, 777–782. [Google Scholar] [CrossRef]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2018, 50, e12926. [Google Scholar] [CrossRef]
- Ricci, E.; Noli, S.; Ferrari, S.; La Vecchia, I.; Cipriani, S.; De Cosmi, V.; Somigliana, E.; Parazzini, F. Alcohol intake and semen variables: Cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology 2018, 6, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, M.; Shirpoor, A.; Naderi, R.; Kheradmand, F.; Gharalari, F.H.; Samadi, M.; Khalaji, N.; Gharaaghaji, R. Long-term ethanol consumption promotes changes in β-defensin isoform gene expression and induces structural changes and oxidative DNA damage to the epididymis of rats. Mol. Reprod. Dev. 2019, 86, 624–631. [Google Scholar] [CrossRef]
- Shayakhmetova, G.M.; Bondarenko, L.B.; Matvienko, A.V.; Kovalenko, V.M. Chronic alcoholism-mediated metabolic disorders in albino rat testes. Interdiscip. Toxicol. 2014, 7, 165–172. [Google Scholar] [CrossRef]
- Koh, P.O.; Won, C.K.; Cho, J.H. Ethanol decreases the expression of pituitary adenylate cyclase activating polypeptide in rat testes. J. Vet. Med. Sci. 2006, 68, 635–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.S.; Shukla, S.D. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 2006, 41, 126–132. [Google Scholar] [CrossRef]
- Rompala, G.R.; Homanics, G.E. Intergenerational effects of alcohol: A review of paternal preconception ethanol exposure studies and epigenetic mechanisms in the male germline. Alcohol. Clin. Exp. Res. 2019, 43, 1032–1045. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Ouko, L.A.; Shantikumar, K.; Knezovich, J.; Haycock, P.; Schnugh, D.J.; Ramsay, M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes—Implications for fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2009, 33, 1615–1627. [Google Scholar] [CrossRef]
- Chang, R.C.; Wang, H.; Bedi, Y.; Golding, M.C. Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Levi Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, M.; Coccurello, R.; Carito, V.; Ciafrè, S.; Ferraguti, G.; Giacovazzo, G.; Mancinelli, R.; Tirassa, P.; Chaldakov, G.N.; Pascale, E.; et al. Paternal alcohol exposure in mice alters brain NGF and BDNF and increases ethanol-elicited preference in male offspring. Addict. Biol. 2016, 21, 776–787. [Google Scholar] [CrossRef]
- Xia, R.; Jin, L.; Li, D.; Liang, H.; Yang, F.; Chen, J.; Yuan, W.; Miao, M. Association between paternal alcohol consumption before conception and anogenital distance of offspring. Alcohol. Clin. Exp. Res. 2018, 42, 735–742. [Google Scholar] [CrossRef]
- Marmorstein, N.; Iacono, W.; McGue, M. Alcohol and illicit drug dependence among parents: Associations with offspring externalizing disorders. Psychol. Med. 2009, 39, 149–155. [Google Scholar] [CrossRef]
- Cservenka, A.; Fair, D.A.; Nagel, B.J. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol. Clin. Exp. Res. 2014, 38, 1912–1923. [Google Scholar] [CrossRef]
- Monaco, A.P. An epigenetic, transgenerational model of increased mental health disorders in children, adolescents and young adults. Eur. J. Hum. Genet. 2021, 29, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Qu, Y.; Han, F.; Bell, E.M.; Zhuang, J.; Chen, J.; François, M.; Lipton, E.; Matale, R.; Cui, W.; et al. Evaluation of interactive effects between paternal alcohol consumption and paternal socioeconomic status and environmental exposures on congenital heart defects. Birth Defects Res. 2020, 112, 1273–1286. [Google Scholar] [CrossRef]
- Infante-Rivard, C.; El-Zein, M. Parental alcohol consumption and childhood cancers: A review. J. Toxicol. Environ. Health—Part B Crit. Rev. 2006, 10, 101–129. [Google Scholar] [CrossRef]
- Akison, L.K.; Moritz, K.M.; Reid, N. Adverse reproductive outcomes associated with fetal alcohol exposure: A systematic review. Reproduction 2019, 153, 329–343. [Google Scholar] [CrossRef]
- Stamatiades, G.A.; Kaiser, U.B. Gonadotropin regulation by pulsatile GnRH: Signaling and gene expression. Mol. Cell. Endocrinol. 2018, 463, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells—Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Pathophysiology of the effects of alcohol abuse on the endocrine system. Alcohol Res. 2017, 38, 255–276. [Google Scholar]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Our experience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Cicero, T.J.; Newman, K.S.; Gerrity, M.; Schmoeker, P.F.; Bell, R.D. Ethanol inhibits the naloxone-induced release of luteinizing hormone-releasing hormone from the hypothalamus of the male rat. Life Sci. 1982, 31, 1587–1596. [Google Scholar] [CrossRef]
- Rowe, P.; Racey, P.; Shenton, J.; Ellwood, M.; Lehane, J. Proceedings: Effects of acute administration of alcohol and barbiturates on plasma luteinizing hormone and testosterone in man. J. Endocrinol. 1974, 63, 50P–51P. [Google Scholar]
- McCann, S.M.; Mastronardi, C.; Walczewska, A.; Karanth, S.; Rettori, V.; Yu, W.H. The role of nitric oxide in reproduction. Brazilian J. Med. Biol. Res. 1999, 32, 1367–1379. [Google Scholar] [CrossRef]
- Canteros, G.; Rettori, V.; Franchi, A.; Genaro, A.; Cebral, E.; Faletti, A.; Gimeno, M.; Mccann, S.M. Ethanol inhibits luteinizing hormone-releasing hormone (LHRH) secretion by blocking the response of LHRH neuronal terminals to nitric oxide. Proc. Natl. Acad. Sci. USA 1995, 92, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Kuller, L.H.; May, S.J.; Perper, J.A. The relationship between alcohol, liver disease, and testicular pathology. Am. J. Epidemiol. 1978, 108, 192–199. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Lee, M.A.; Iozzo, R.V.; Lopez, I.; Touchstone, J.C.; Storey, B.T. Ethanol accelerates acrosomal loss in human spermatozoa. J. Androl. 1988, 9, 357–366. [Google Scholar] [CrossRef]
- Tangsrisakda, N.; Iamsaard, S. Effect of ethanol on the changes in testicular protein expression in adult male rats. Andrologia 2020, 52, e13784. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.J.; Cash, M.K.M.; Vaughn, W.K. Ethanol inhibits human and hamster sperm penetration of eggs. Gamete Res. 1987, 16, 97–107. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Fontana, V.A.; Galotto, C.; Cambiasso, M.Y.; Sobarzo, C.M.A.; Calvo, L.; Calvo, J.C.; Cebral, E. Murine sperm capacitation, oocyte penetration and decondensation following moderate alcohol intake. Reproduction 2018, 155, 529–541. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Perelli, S.; Condorelli, R.A.; Barbagallo, F.; Crafa, A.; Cannarella, R.; La Vignera, S.; Calogero, A.E. The role of resveratrol in human male fertility. Molecules 2021, 26, 2495. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Santoro, M.; De Amicis, F.; Guido, C.; Bonofiglio, D.; Lanzino, M.; Cesario, M.G.; Perrotta, I.; Sisci, D.; Morelli, C. Red wine consumption may affect sperm biology: The effects of different concentrations of the phytoestrogen Myricetin on human male gamete function. Mol. Reprod. Dev. 2013, 80, 155–165. [Google Scholar] [CrossRef]
- Lodovici, M.; Guglielmi, F.; Casalini, C.; Meoni, M.; Cheynier, V.; Dolara, P. Antioxidant and radical scavenging properties in vitro of polyphenolic extracts from red wine. Eur. J. Nutr. 2001, 40, 74–77. [Google Scholar] [CrossRef]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef]
- Martini, A.C.; Molina, R.I.; Estofán, D.; Senestrari, D.; Fiol De Cuneo, M.; Ruiz, R.D. Effects of alcohol and cigarette consumption on human seminal quality. Fertil. Steril. 2004, 82, 374–377. [Google Scholar] [CrossRef]
- López Teijón, M.; Garcia, F.; Serra, O.; Moragas, M.; Rabanal, A.; Olivares, R.; Alvarez, J.G. Semen quality in a population of volunteers from the province of Barcelona. Reprod. Biomed. Online 2007, 15, 434–444. [Google Scholar] [CrossRef]
- Wogatzky, J.; Wirleitner, B.; Stecher, A.; Vanderzwalmen, P.; Neyer, A.; Spitzer, D.; Schuff, M.; Schechinger, B.; Zech, N.H. The combination matters—Distinct impact of lifestyle factors on sperm quality: A study on semen analysis of 1683 patients according to MSOME criteria. Reprod. Biol. Endocrinol. 2012, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.O.; Kim, M.O. Ethanol exposure decreases cell proliferation and increases apoptosis in rat testes. J. Vet. Med. Sci. 2006, 68, 1013–1017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chastain, L.G.; Sarkar, D.K. Alcohol effects on the epigenome in the germline: Role in the inheritance of alcohol-related pathology. Alcohol 2017, 60, 53–66. [Google Scholar] [CrossRef]
- Cicero, T. Effects of paternal exposure. Alcohol Health Res. World 1994, 18, 37–41. [Google Scholar] [PubMed]
- Chang, R.C.; Skiles, W.M.; Chronister, S.S.; Wang, H.; Sutton, G.I.; Bedi, Y.S.; Snyder, M.; Long, C.R.; Golding, M.C. DNA methylation-independent growth restriction and altered developmental programming in a mouse model of preconception male alcohol exposure. Epigenetics 2017, 12, 841–853. [Google Scholar] [CrossRef]
- Curley, J.P.; Mashoodh, R.; Champagne, F.A. Epigenetics and the origins of paternal effects. Horm. Behav. 2011, 59, 306–314. [Google Scholar] [CrossRef]
- Rehm, J. How should prevalence of alcohol use disorders be assessed globally? Int. J. Methods Psychiatr. Res. 2016, 25, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, C.; Anderson, K.G.; Ladd, B.O.; Yong, Y.M.; David, M. Inaccuracies in survey reporting of alcohol consumption. BMC Public Health 2019, 19, 1639. [Google Scholar] [CrossRef]
- Alcohol Research: Current Reviews Editorial Staff. Drinking patterns and their definitions. Alcohol Res. 2018, 39, 17–18. [Google Scholar]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Panner Selvam, M.K.; Finelli, R.; Agarwal, A.; Henkel, R. Proteomics and metabolomics—Current and future perspectives in clinical andrology. Andrologia 2021, 53, e13711. [Google Scholar] [CrossRef]
- Hetherington, L.; Schneider, E.K.; DeKretser, D.; Muller, C.H.; Hondermarck, H.; Velkov, T.; Baker, M.A. Deficiency in outer dense fiber 1 is a marker and potential driver of idiopathic male infertility. Mol. Cell. Proteom. 2016, 15, 3685–3693. [Google Scholar] [CrossRef]
- Holland, A.; Ohlendieck, K. Comparative profiling of the sperm proteome. Proteomics 2015, 15, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 6. [Google Scholar] [CrossRef]
- Miranda, R.C.; Pietrzykowski, A.Z.; Tang, Y.; Sathyan, P.; Mayfield, D.; Keshavarzian, A.; Sampson, W.; Hereld, D. MicroRNAs: Master regulators of ethanol abuse and toxicity? Alcohol. Clin. Exp. Res. 2010, 34, 575–587. [Google Scholar] [CrossRef]
- Bedi, Y.; Chang, R.C.; Gibbs, R.; Clement, T.M.; Golding, M.C. Alterations in sperm-inherited noncoding RNAs associate with late-term fetal growth restriction induced by preconception paternal alcohol use. Reprod. Toxicol. 2019, 87, 11–20. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lässer, C.; Szabó, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzás, E.I.; Lötvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta—Gen. Subj. 2012, 1820, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.; Liu, C.; O’Reilly, P.; Gao, H.; Song, P.; Xu, B.; Ruggeri, B.; Amin, N.; Jia, T.; Preis, S.; et al. KLB is associated with alcohol drinking, and its gene product β-Klotho is necessary for FGF21 regulation of alcohol preference. Proc. Natl. Acad. Sci. USA 2016, 113, 14372–14377. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.K.; Polimanti, R.; Johnson, E.C.; McClintick, J.N.; Adams, M.J.; Adkins, A.E.; Aliev, F.; Bacanu, S.A.; Batzler, A.; Bertelsen, S.; et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 2018, 21, 1656–1669. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Zhou, H.; Kember, R.L.; Vickers Smith, R.; Justice, A.C.; Damrauer, S.; Tsao, P.S.; Klarin, D.; Baras, A.; Reid, J.; et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat. Commun. 2019, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
| Impact of Alcohol | Animal Studies | Human Studies |
|---|---|---|
| Effects on Reproductive Hormonal Regulation | Reduced levels of LH, FSH [67,68,69,70,71,72]. Reduced levels of testosterone [73,74,75,76,77]. Altered Leydig cell number and morphology [78]. | Contradictory evidence in literature on levels of FSH, LH, and testosterone [79,80,81,82,83]. |
| Effects on Semen Quality | Reduced sperm concentration and motility [84,85,86,87]. Increased abnormal sperm morphology [84,85,86,87]. Defects in chromatin condensation [86,87]. | Reduced sperm concentration [88,89,90]. Altered semen volume and increased abnormal sperm morphology [91,92,93]. Increased sperm DNA fragmentation and defects in chromatin condensation [89,90,94,95]. Moderate consumption associated with better semen volume and concentration [96]. |
| Effects on Gene Transcription, Genetic, and Epigenetic Regulation | Altered expression of RNA involved in sperm function [97,98]. Altered expression of proteins involved in apoptosis [99]. Aberrant gene acetylation of sperm DNA [100]. | Altered expression of RNA involved in sperm function [101]. Aberrant gene methylation in sperm DNA [102,103]. |
| Transgenerational Effects | Low fetal and birth weight, and limited growth in offspring [101,104]. Nervous system anomalies in offspring [105,106]. Altered reproductive development of offspring [107]. | Higher incidence of psychopathological disorders [108,109,110], congenital heart defects [111], cancer [112], and altered reproductive development [113] in the offspring. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 328. https://doi.org/10.3390/ijerph19010328
Finelli R, Mottola F, Agarwal A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(1):328. https://doi.org/10.3390/ijerph19010328
Chicago/Turabian StyleFinelli, Renata, Filomena Mottola, and Ashok Agarwal. 2022. "Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 1: 328. https://doi.org/10.3390/ijerph19010328
APA StyleFinelli, R., Mottola, F., & Agarwal, A. (2022). Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. International Journal of Environmental Research and Public Health, 19(1), 328. https://doi.org/10.3390/ijerph19010328








