Remote Monitoring of CIEDs—For Both Safety, Economy and Convenience?
Abstract
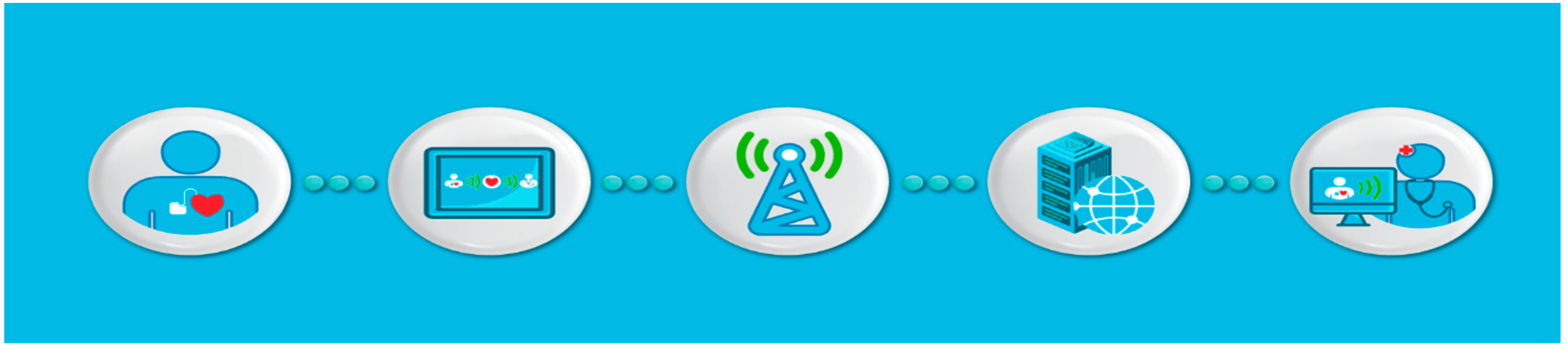
:1. Introduction
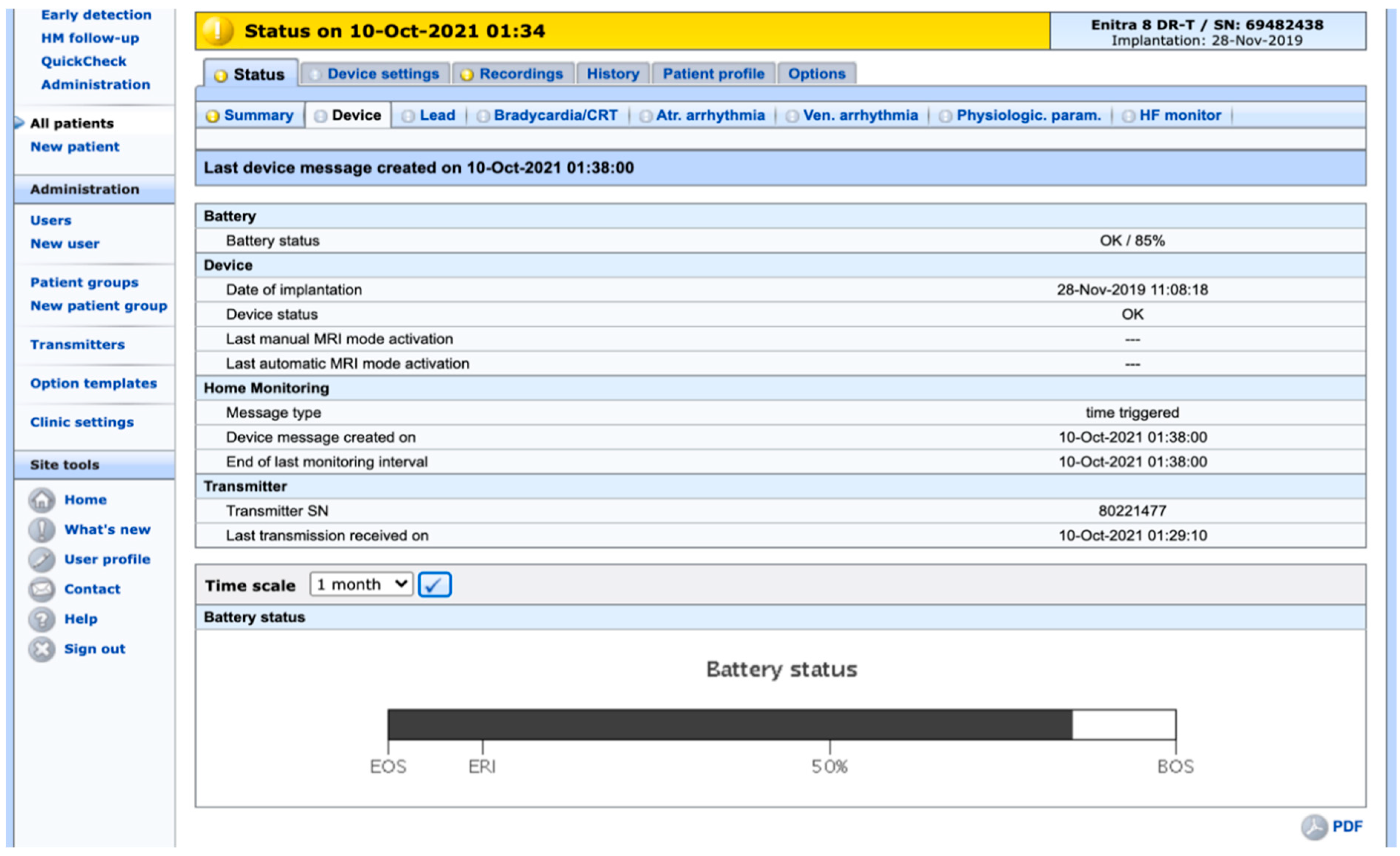
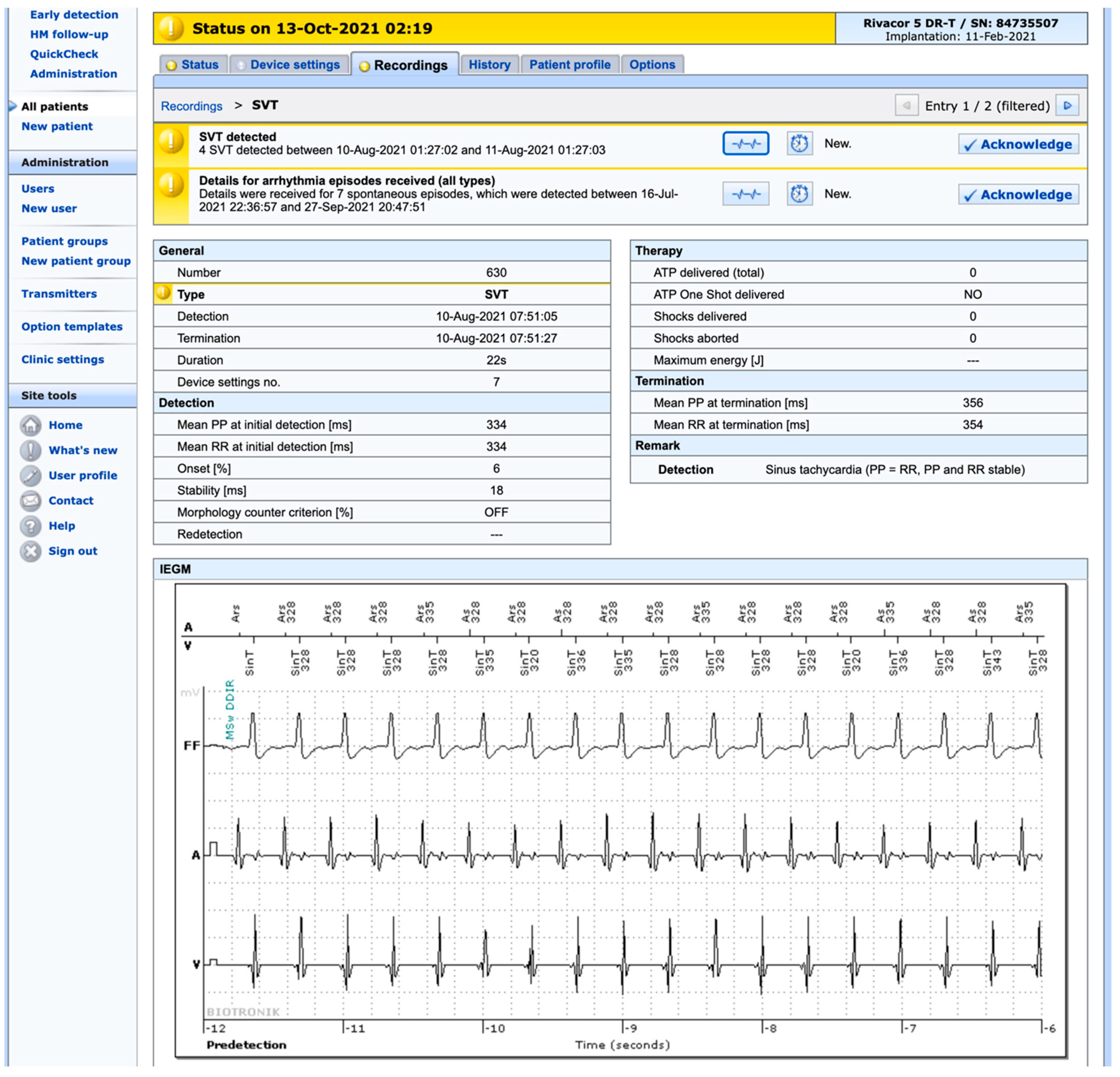
2. Safety
3. Economy
4. Convenience and Patient Satisfaction
5. Pitfalls and Limitations
6. Future Possibilities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsson, B.; Elmqvist, H.; Rydén, L.; Schüller, H. Lessons from the first patient with an implanted pacemaker: 1958–2001. Pacing Clin. Electrophysiol. 2003, 26, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Van Hemel, N.M.; Van der Wall, E.E. 8 October 1958, D Day for the implantable pacemaker. Neth. Heart J. 2008, 16, S3–S4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Camm, J.; Merkely, B.; Raatikainen, P.; Arnar, D.O.; The EHRA white Book 2017. The Current Status of Cardiac Electrophysiology in ESC Member Countries. 2017. Available online: https://www.escardio.org/static-file/Escardio/Subspecialty/EHRA/Publications/Documents/2017/ehra-white-book-2017.pdf (accessed on 14 October 2021).
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Tolosana, J.M. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.J.; Silka, M.J.; Silva, J.A.; Balaji, S.; Beach, C.M.; Benjamin, M.N.; Wackel, P.L. 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients. Heart Rhythm. 2021, 18, 1925–1950. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Potpara, T.S.; Boveda, S.; Deharo, J.-C.; Chen, J.; Dobreanu, D.; Fumagalli, S.; Lenarczyk, R.; Madrid, A.H.; Larsen, T.B.; et al. Patients’ knowledge and attitudes regarding living with implantable electronic devices: Results of a multicentre, multinational patient survey conducted by the European Heart Rhythm Association. Europace 2018, 20, 386–391. [Google Scholar] [CrossRef]
- Maines, M.; Tomasi, G.; Moggio, P.; Peruzza, F.; Catanzariti, D.; Angheben, C.; Simoncelli, M.; Degiampietro, M.; Piffer, L.; Valsecchi, S.; et al. Implementation of remote follow-up of cardiac implantable electronic devices in clinical practice: Organizational implications and resource consumption. J. Cardiovasc. Med. 2020, 21, 648–653. [Google Scholar] [CrossRef]
- Nishii, N.; Miyoshi, A.; Kubo, M.; Miyamoto, M.; Morimoto, Y.; Kawada, S.; Nakagawa, K.; Watanabe, A.; Nakamura, K.; Morita, H.; et al. Analysis of arrhythmic events is useful to detect lead failure earlier in patients followed by remote monitoring. J. Cardiovasc. Electrophysiol. 2018, 29, 463–470. [Google Scholar] [CrossRef]
- Chiu, C.S.L.; Timmermans, I.; Versteeg, H.; Zitron, E.; Mabo, P.; Pedersen, S.S.; Meine, M. Effect of remote monitoring on clinical outcomes in European heart failure patients with an implantable cardioverter-defibrillator: Secondary results of the REMOTE-CIED randomized trial. Europace 2021. online ahead of print. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Albanese, M.; Placidi, F.; Forle, G.B.; Di Biase, L.; Ribatti, V.; Romigi, A. Feasibility of automated detection of sleep apnea using implantable pacemakers and defibrillators: A comparison with simultaneous polysomnography recording. J. Interv. Card. Electrophysiol. 2019, 56, 327–333. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, F.J.; Osca Asensi, J.; Romero, R.; Lozano, I.F.; Larrazabal, J.M.; Ferrer, J.M.; Ortiz, R.; Pombo, M.; Tornés, F.J.; Kolbolandi, M.M. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: A long-term randomized trial (RM-ALONE). Eur. Heart J. 2019, 40, 1837–1846. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Chen, S. Early event detection using a home monitoring system for patients with cardiac pacemakers. Aging Clin. Exp. Res. 2014, 26, 131–135. [Google Scholar] [CrossRef]
- Vogtmann, T.; Stiller, S.; Marek, A.; Kespohl, S.; Gomer, M.; Kühlkamp, V.; Zach, G.; Löscher, S.; Baumann, G. Workload and usefulness of daily, centralized home monitoring for patients treated with CIEDs: Results of the MoniC (Model Project Monitor Centre) prospective multicentre study. EP Eur. 2013, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- De Simone, V.; Guardalben, S.; Guarise, P.; Padovani, N.; Giacopelli, D.; Zanotto, G. Home Monitoring trends during COVID-19 infection. J. Arrhythm. 2021, 37, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.Z.; Crosbie, C.; Kahn, M.; Motwani, M. Protecting the most vulnerable during COVID-19 and beyond: A case report on the remote management of heart failure patients with cardiac implantable electronic devices. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Piro, A.; Magnocavallo, M.; Della Rocca, D.G.; Neccia, M.; Manzi, G.; Mariani, M.V.; Straito, M.; Bernardini, A.; Severino, P.; Iannucci, G.; et al. Management of cardiac implantable electronic device follow-up in COVID-19 pandemic: Lessons learned during Italian lockdown. J. Cardiovasc. Electrophysiol. 2020, 31, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Magnocavallo, M.; Bernardini, A.; Mariani, M.V.; Piro, A.; Marini, M.; Nicosia, A.; Adduci, C.; Rapacciuolo, A.; Saporito, D.; Grossi, S.; et al. Home delivery of the communicator for remote monitoring of cardiac implantable devices: A multicenter experience during the COVID-19 lockdown. Pacing Clin. Electrophysiol. 2021, 44, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Parahuleva, M.S.; Soydan, N.; Divchev, D.; Lüsebrink, U.; Schieffer, B.; Erdogan, A. Home monitoring after ambulatory implanted primary cardiac implantable electronic devices: The home ambulance pilot study. Clin. Cardiol. 2017, 40, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Callum, K.; Graune, C.; Bowman, E.; Molden, E.; Leslie, S.J. Remote monitoring of implantable defibrillators is associated with fewer inappropriate shocks and reduced time to medical assessment in a remote and rural area. World J. Cardiol. 2021, 13, 46–54. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; D’Onofrio, A.; Calò, L.; Vaccari, D.; Zanotto, G.; Curnis, A.; Buja, G.; Rovai, N.; Gargaro, A. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: The HomeGuide Registry. Europace 2013, 15, 970–977. [Google Scholar] [CrossRef]
- Vergara, P.; Solimene, F.; D’Onofrio, A.; Pisanò, E.C.; Zanotto, G.; Pignalberi, C.; Iacopino, S.; Maglia, G.; Della Bella, P.; Calvi, V.; et al. Are Atrial High-Rate Episodes Associated with Increased Risk of Ventricular Arrhythmias and Mortality. JACC Clin. Electrophysiol. 2019, 5, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Yamazaki, F.; Goto, T.; Asai, T.; Yamamoto, T.; Hirooka, K.; Ando, K. Remote Management of Pacemaker Patients with Biennial In-Clinic Evaluation: Continuous Home Monitoring in the Japanese At-Home Study: A Randomized Clinical Trial. Circ. Arrhythmia Electrophysiol. 2020, 13, e007734. [Google Scholar] [CrossRef]
- Zanotto, G.; D’Onofrio, A.; Della Bella, P.; Solimene, F.; Pisanò, E.C.; Iacopino, S.; Dondina, C.; Giacopelli, D.; Gargaro, A.; Ricci, R.P. Organizational model and reactions to alerts in remote monitoring of cardiac implantable electronic devices: A survey from the Home Monitoring Expert Alliance project. Clin. Cardiol. 2019, 42, 76–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, S.; Piccini, J.P.; Snell, J.; Prillinger, J.B.; Dalal, N.; Varma, N. Improved survival in patients enrolled promptly into remote monitoring following cardiac implantable electronic device implantation. J. Interv. Card. Electrophysiol. 2016, 46, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.Z.; Sammut-Powell, C.; Kwok, C.S.; Tay, T.; Motwani, M.; Martin, G.P.; Taylor, J.K. Remote monitoring data from cardiac implantable electronic devices predicts all-cause mortality. Europace 2021, euab160, online ahead of print. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining Home Monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. Europace 2021, euab170, online ahead of print. [Google Scholar] [CrossRef]
- Forleo, G.B.; Solimene, F.; Pisanò, E.C.; Zanotto, G.; Calvi, V.; Pignalberi, C.; D’Onofrio, A. Long-term outcomes after prophylactic ICD and CRT-D implantation in nonischemic patients: Analysis from a nationwide database of daily remote-monitoring transmissions. J. Cardiovasc. Electrophysiol. 2019, 30, 1626–1635. [Google Scholar] [CrossRef]
- O’Shea, C.J.; Middeldorp, M.E.; Thomas, G.; Harper, C.; Elliott, A.D.; Ray, N.; Campbell, K.; Lau, D.H.; Sanders, P. Atrial fibrillation burden during the coronavirus disease 2019 pandemic. Europace 2021, 23, 1493–1501. [Google Scholar] [CrossRef]
- O’Shea, C.J.; Middeldorp, M.E.; Hendriks, J.M.; Brooks, A.G.; Lau, D.H.; Emami, M.; Sanders, P. Remote monitoring alert burden: An analysis of transmission in >26,000 patients. Clin. Electrophysiol. 2021, 7, 226–234. [Google Scholar] [CrossRef]
- Pron, G.; Ieraci, L.; Kaulback, K.; Medical Advisory Secretariat HQO. Internet-based device-assisted remote monitoring of cardiovascular implantable electronic devices: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2012, 12, 1–86. [Google Scholar]
- Mairesse, G.H.; Braunschweig, F.; Klersy, K.; Cowie, M.R.; Leyva, F. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: A survey from the health economics committee of the European Heart Rhythm Association. Europace 2015, 17, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.E.; Campbell, D.; Duhn, L.J.; Giddens, K.; Gillis, A.M.; AbdelWahab, A.; Nault, I.; Raj, S.R.; Lockwood, E.; Basta, J.; et al. Remote Monitoring of Cardiovascular Implantable Electronic Devices in Canada: Survey of Patients and Device Health Care Professionals. CJC Open 2021, 3, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Zanaboni, P.; Landolina, M.; Marzegalli, M.; Lunati, M.; Perego, G.B.; Guenzati, G.; Curnis, A.; Valsecchi, S.; Borghetti, F.; Borghi, G.; et al. Cost-Utility Analysis of the EVOLVO Study on Remote Monitoring for Heart Failure Patients with Implantable Defibrillators: Randomized Controlled Trial. J. Med. Internet Res. 2013, 15, e106. [Google Scholar] [CrossRef] [PubMed]
- Naraparaju, V.; Almnajam, M.; Joseph, L.; Vernon, G.; Wakefield, D.; Magnano, A.R.; Tolat, A. A survey on patient preferences towards CIED implantation. Indian Pacing Electrophysiol. J. 2021, 21, 227–231. [Google Scholar] [CrossRef]
- Burri, H.; Sticherling, C.; Wright, D.; Makino, K.; Smala, A.; Tilden, D. Cost-consequence analysis of daily continuous remote monitoring of implantable cardiac defibrillator and resynchronization devices in the UK. Europace 2013, 15, 1601–1608. [Google Scholar] [CrossRef]
- Bautista-Mesa, R.J.; Lopez-Villegas, A.; Peiro, S.; Catalan-Matamoros, D.; Robles-Musso, E.; Lopez-Liria, R.; Leal-Costa, C. Long-term cost-utility analysis of remote monitoring of older patients with pacemakers: The PONIENTE study. BMC Geriatr. 2020, 20, 474. [Google Scholar] [CrossRef]
- Dario, C.; Delise, P.; Gubian, L.; Saccavini, C.; Brandolino, G.; Mancin, S. Large Controlled Observational Study on Remote Monitoring of Pacemakers and Implantable Cardiac Defibrillators: A Clinical, Economic, and Organizational Evaluation. Interact. J. Med. Res. 2016, 5, e4. [Google Scholar] [CrossRef]
- Lopez-Villegas, A.; Catalan-Matamoros, D.; Robles-Musso, E.; Bautista-Mesa, R.; Peiro, S. Cost-utility analysis on telemonitoring of users with pacemakers: The PONIENTE study. J. Telemed. Telecare 2019, 25, 204–212. [Google Scholar] [CrossRef]
- Perl, S.; Stiegler, P.; Rotman, B.; Prenner, G.; Lercher, P.; Anelli-Monti, M.; Sereinigg, M.; Riegelnik, V.; Kvas, E.; Kos, C.; et al. Socio-economic effects and cost saving potential of remote patient monitoring (SAVE-HM trial). Int. J. Cardiol. 2013, 169, 402–407. [Google Scholar] [CrossRef]
- Ricci, R.P.; Morichelli, L.; D’Onofrio, A.; Calò, L.; Vaccari, D.; Zanotto, G.; Curnis, A.; Buja, G.; Rovai, N.; Gargaro, A. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: Data from the HomeGuide Registry. J. Cardiovasc. Electrophysiol. 2014, 25, 1216–1223. [Google Scholar] [CrossRef]
- Sapp, J.A.; Gillis, A.M.; AbdelWahab, A.; Nault, I.; Nery, P.B.; Healey, J.S.; Raj, S.R.; Lockwood, E.; Sterns, L.D.; Sears, S.F.; et al. Remote-only monitoring for patients with cardiac implantable electronic devices: A before-and-after pilot study. CMAJ Open 2021, 9, E53–E61. [Google Scholar] [CrossRef] [PubMed]
- Heidbuchel, H.; Hindricks, G.; Broadhurst, P.; Van Erven, L.; Fernandez-Lozano, I.; Rivero-Ayerza, M.; Malinowski, K.; Marek, A.; Garrido, R.F.R.; Löscher, S.; et al. EuroEco (European Health Economic Trial on Home Monitoring in ICD Patients): A provider perspective in five European countries on costs and net financial impact of follow-up with or without remote monitoring. Eur. Heart J. 2015, 36, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Villegas, A.; Catalan-Matamoros, D.; Peiro, S.; Lappegard, K.T.; Lopez-Liria, R. Cost-utility analysis of telemonitoring versus conventional hospital-based follow-up of patients with pacemakers. The NORDLAND randomized clinical trial. PLoS ONE 2020, 15, e0226188. [Google Scholar]
- Timmermans, I.; Meine, M.; Szendey, I.; Aring, J.; Roldán, J.R.; van Erven, L.; Kahlert, P.; Zitron, E.; Mabo, P.; Denollet, J.; et al. Remote monitoring of implantable cardioverter defibrillators: Patient experiences and preferences for follow-up. Pacing Clin. Electrophysiol. 2019, 42, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, R.P.; Morichelli, L.; Quarta, L.; Porfili, A.; Magris, B.; Giovene, L.; Torcinaro, S.; Gargaro, A. Effect of daily remote monitoring on pacemaker longevity: A retrospective analysis. Heart Rhythm. 2015, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Braunschweig, F.; Deharo, J.C.; Leyva, F.; Lubinski, A.; Lazzaro, C. Impact of extending device longevity on the long-term costs of implantable cardioverter-defibrillator therapy: A modelling study with a 15-year time horizon. Europace 2013, 15, 1453–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, N.; Love, C.J.; Schweikert, R.; Moll, P.; Michalski, J.; Epstein, A.E. Automatic remote monitoring utilizing daily transmissions: Transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. EP Eur. 2018, 20, 622–628. [Google Scholar] [CrossRef]
- Seiler, A.; Biundo, E.; Di Bacco, M.; Rosemas, S.; Nicolle, E.; Lanctin, D.; Hennion, J.; de Melis, M.; Van Heel, L. Clinic Time Required for Remote and In-person Management of Cardiac Device Patients: Time and Motion Workflow Evaluation. JMIR Cardio 2021. online ahead of print. [Google Scholar] [CrossRef]
- Safarikova, I.; Bulava, A.; Hajek, P. Remote monitoring of implantable cardioverters defibrillators: A comparison of acceptance between octogenarians and younger patients. J. Geriatr. Cardiol. 2020, 17, 417–426. [Google Scholar]
- Artico, J.; Zecchin, M.; Zorzin Fantasia, A.; Skerl, G.; Ortis, B.; Franco, S.; Sinagra, G. Long-term patient satisfaction with implanted device remote monitoring: A comparison among different systems. J. Cardiovasc. Med. 2019, 20, 542–550. [Google Scholar] [CrossRef]
- Catalan-Matamoros, D.; Lopez-Villegas, A.; Lappegård, K.T.; Lopez-Liria, R. Assessing Communication during Remote Follow-Up of Users with Pacemakers in Norway: The NORDLAND Study, a Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 7678. [Google Scholar] [CrossRef]
- Versteeg, H.; Timmermans, I.; Widdershoven, J.; Kimman, G.-J.; Prevot, S.; Rauwolf, T.; Scholten, M.F.; Zitron, E.; Mabo, P.; Denollet, J.; et al. Effect of remote monitoring on patient-reported outcomes in European heart failure patients with an implantable cardioverter-defibrillator: Primary results of the REMOTE-CIED randomized trial. Europace 2019, 21, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Kitt, S.; Gill, J.; McComb, J.M.; Ng, G.A.; Raftery, J.; Roderick, P.; Seed, A.; Williams, S.G.; Witte, K.K.; et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 2017, 38, 2352–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Liria, R.; López-Villegas, A.; Leal-Costa, C.; Peiró, S.; Robles-Musso, E.; Bautista-Mesa, R.; Rocamora-Pérez, P.; Lappegård, K.T.; Catalán-Matamoros, D. Effectiveness and Safety in Remote Monitoring of Patients with Pacemakers Five Years after an Implant: The Poniente Study. Int. J. Environ. Res. Public Health 2020, 17, 1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Villegas, A.; Catalan-Matamoros, D.; Lopez-Liria, R.; Enebakk, T.; Thunhaug, H.; Lappegård, K.T. Health-related quality of life on tele-monitoring for users with pacemakers 6 months after implant: The NORDLAND study, a randomized trial. BMC Geriatr. 2018, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Mabo, P.; Victor, F.; Bazin, P.; Ahres, S.; Babuty, D.; Da Costa, A.; Binet, D.; Daubert, J.-C. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur. Heart J. 2012, 33, 1105–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalan-Matamoros, D.; Lopez-Villegas, A.; Tore-Lappegard, K.; Lopez-Liria, R. Patients’ experiences of remote communication after pacemaker implant: The NORDLAND study. PLoS ONE 2019, 14, e0218521. [Google Scholar]
- López-Liria, R.; López-Villegas, A.; Enebakk, T.; Thunhaug, H.; Lappegård, K.T.; Catalán-Matamoros, D. Telemonitoring and Quality of Life in Patients after 12 Months Following a Pacemaker Implant: The Nordland Study, a Randomised Trial. Int. J. Environ. Res. Public Health 2019, 16, 2001. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Villegas, A.; Catalán-Matamoros, D.; Robles-Musso, E.; Peiró, S. Workload, time and costs of the informal cares in patients with tele-monitoring of pacemakers: The PONIENTE study. Clin. Res. Cardiol. 2016, 105, 307–313. [Google Scholar] [CrossRef]
- Fraiche, A.M.; Matlock, D.D.; Gabriel, W.; Rapley, F.A.; Kramer, D.B. Patient and Provider Perspectives on Remote Monitoring of Pacemakers and Implantable Cardioverter-Defibrillators. Am. J. Cardiol. 2021, 149, 42–46. [Google Scholar] [CrossRef]
- Dilaveris, P.; Casado-Arroyo, R.; Lumens, J. A roadmap to nationwide monitoring of Cardiovascular Implantable Electronic Devices in Greece: Staying safe in the era of COVID-19 pandemic. Hell. J. Cardiol. 2020, 61, 396. [Google Scholar] [CrossRef]
- Iacopino, S.; Placentino, F.; Colella, J.; Pesce, F.; Pardeo, A.; Filannino, P.; Petretta, A. Remote monitoring of cardiac implantable devices during COVID-19 outbreak: “keep people safe” and “focus only on health care needs”. Acta Cardiol. 2021, 76, 158–161. [Google Scholar] [CrossRef]
- Mugnai, G.; Volpiana, A.; Cavedon, S.; Paolini, C.; Perrone, C.; Bilato, C. Boosting telemedicine through remote monitoring of cardiac electronic devices during the Italian COVID-19 outbreak. Cardiol. J. 2021, 28, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Providencia, R.; Barra, S.; Kircanski, B.; Guerra, J.M.; Conte, G.; Duncker, D.; Marijon, E.; Anic, A.; Boveda, S. The use of remote monitoring of cardiac implantable devices during the COVID-19 pandemic: An EHRA physician survey. EP Eur. 2021, euab215, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mantini, N.; Borne, R.T.; Varosy, P.D.; Rosenberg, M.A.; Marzec, L.N.; Sauer, W.H.; Nguyen, D.T. Use of cell phone adapters is associated with reduction in disparities in remote monitoring of cardiac implantable electronic devices. J. Interv. Card. Electrophysiol. 2021, 60, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Martignani, C. Cybersecurity in cardiac implantable electronic devices. Expert Rev. Med. Devices 2019, 16, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.C.; Kautzner, J.; Casado-Arroyo, R.; Burri, H.; Callens, S.; Cowie, M.R.; Fraser, A.G. Remote monitoring of cardiac implanted electronic devices: Legal requirements and ethical principles—ESC Regulatory Affairs Committee/EHRA joint task force report. Europace 2020, 22, 1742–1758. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.G.; Gerke, S.; Kramer, D.B. Ethical and Legal Implications of Remote Monitoring of Medical Devices. Milbank Q. 2020, 98, 1257–1289. [Google Scholar] [CrossRef]
- Ricci, R.P.; Vaccari, D.; Morichelli, L.; Zanotto, G.; Calò, L.; D’Onofrio, A.; Curnis, A.; Pisanò, E.C.; Nangah, R.; Brieda, M.; et al. Stroke incidence in patients with cardiac implantable electronic devices remotely controlled with automatic alerts of atrial fibrillation. A sub-analysis of the HomeGuide study. Int. J. Cardiol. 2016, 219, 251–256. [Google Scholar] [CrossRef]
- Rovaris, G.; Solimene, F.; D’Onofrio, A.; Zanotto, G.; Ricci, R.P.; Mazzella, T.; Iacopino, S.; Della Bella, P.; Maglia, G.; Senatore, G.; et al. Does the CHA2DS2-VASc score reliably predict atrial arrhythmias? Analysis of a nationwide database of remote monitoring data transmitted daily from cardiac implantable electronic devices. Heart Rhythm. 2018, 15, 971–979. [Google Scholar] [CrossRef]
- Mendez-Zurita, F.; Alonso-Martin, C.; Ramirez de Diego, I.; Rodriguez-Font, E.; Campos-Garcia, B.; Guerra-Ramos, J.M.; Moreno-Weidmann, Z.; Viñolas, X. Remote monitoring in a patient with multiple leadless pacemakers. J. Arrhythm. 2021, 37, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Afzal, M.R.; Hummel, J.D.; Daoud, E.G.; Houmsse, M.; Kalbfleisch, S.J.; Augostini, R.S. First clinical use of real-time remote programming in cardiac implantable electronic devices. J. Cardiovasc. Electrophysiol. 2020, 31, 2759–2761. [Google Scholar] [CrossRef] [PubMed]
| Abbott (Sylmar, CA, USA) | Merlin.Net™ |
| Biotronik (Berlin, Germany) | Home Monitoring™ |
| Boston Scientific (Marlborough, MA, USA) | Latitude™ |
| LivaNova/MicroPort (Paris, France) | Smartview™ |
| Medico (Padova, Italy) | Ermes™ |
| Medtronic (Minneapolis, MN, USA) | CareLink™ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappegård, K.T.; Moe, F. Remote Monitoring of CIEDs—For Both Safety, Economy and Convenience? Int. J. Environ. Res. Public Health 2022, 19, 312. https://doi.org/10.3390/ijerph19010312
Lappegård KT, Moe F. Remote Monitoring of CIEDs—For Both Safety, Economy and Convenience? International Journal of Environmental Research and Public Health. 2022; 19(1):312. https://doi.org/10.3390/ijerph19010312
Chicago/Turabian StyleLappegård, Knut Tore, and Frode Moe. 2022. "Remote Monitoring of CIEDs—For Both Safety, Economy and Convenience?" International Journal of Environmental Research and Public Health 19, no. 1: 312. https://doi.org/10.3390/ijerph19010312
APA StyleLappegård, K. T., & Moe, F. (2022). Remote Monitoring of CIEDs—For Both Safety, Economy and Convenience? International Journal of Environmental Research and Public Health, 19(1), 312. https://doi.org/10.3390/ijerph19010312






