A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza uralensis Extract for Preventing Dental Caries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Extraction of Plant Material
2.3. Study Design and Treatments
2.4. Clinical Examination
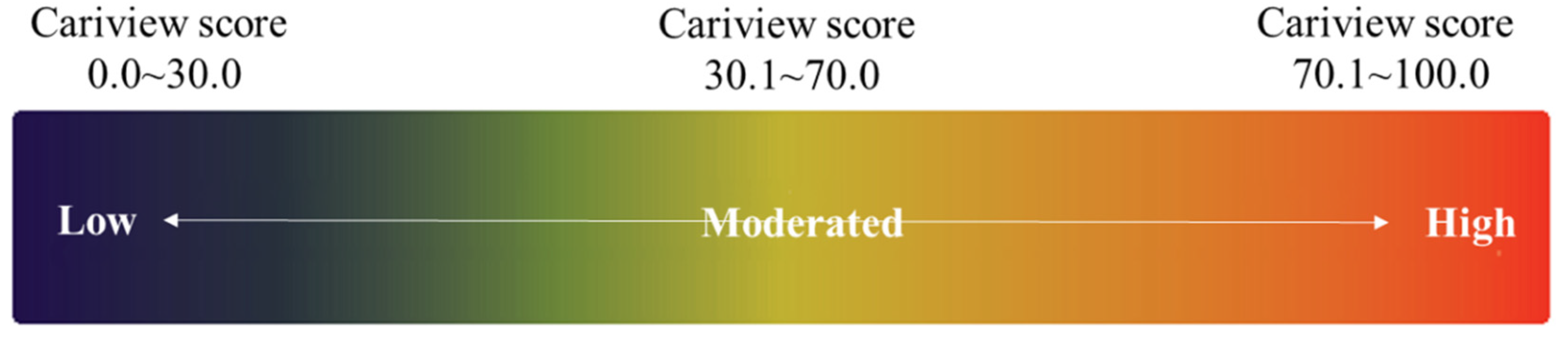
2.5. Cariview Test
2.6. Microbiological Analysist
2.7. Statistical Analysis
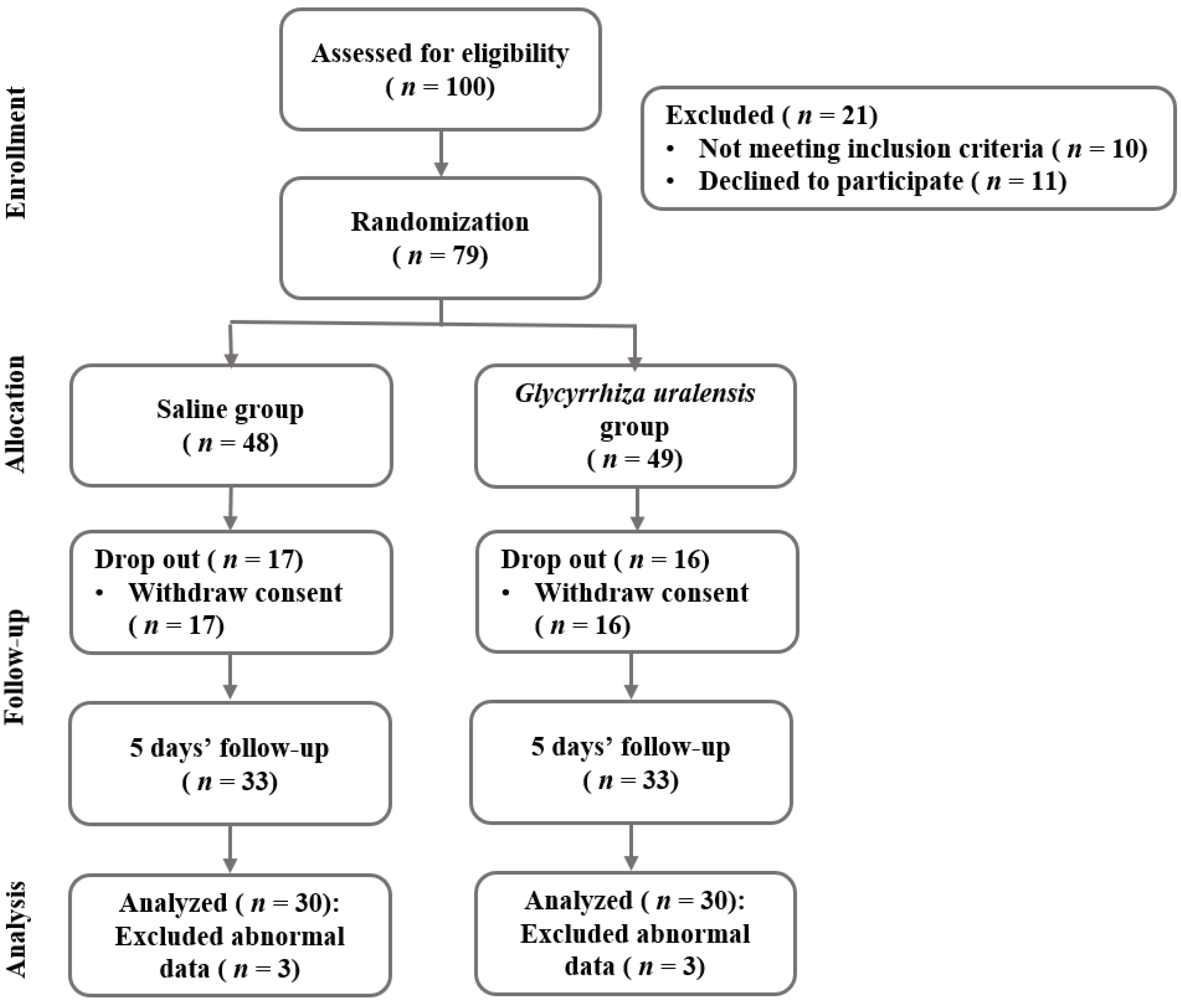
3. Results
3.1. Study Population
3.2. Evaluation of Cariview Activity
3.3. Dental Caries-Causing Bacteria in Subgingival Plaque
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.S.; Park, H.J.; You, J.S.; Park, H.H.; Kwon, I.B.; Lee, H.Y. Isolation of an anticariogenic compound from Magnoliae bark. Korean J. Food Sci. Technol. 1998, 44, 331–384. [Google Scholar]
- Hamada, S.; Slade, H.D. Biology, Immunology and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 60, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2001, 38, 204–211. [Google Scholar] [CrossRef]
- Park, K.M.; You, J.S.; Lee, H.Y.; Beak, N.I.; Hwang, J.K. Kuwanon G: An antibacterial agents from the root bark of Morus alba against oral pathogens. J. Ethnopharmacol. 2003, 84, 181–185. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.G.; Bail, B.J.; Yang, Y.M.; Lee, K.Y.; Lee, Y.H.; Kim, M.A. Antimicrobial effect of essential oils on oral bacteria. J. Korean Acad. Pediatr. Dent. 2009, 36, 1–11. [Google Scholar]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Iniue, M.; Koga, T.; Sato, S.; Hamada, S. Synthesis of adherent insoluble glucan by the concerted action of the two glucosyltransferase components of Streptococcus mutans. FEBS Lett. 1982, 143, 101–104. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, S.C.; Lee, J.R. Antimicrobial effects of sophorae radix extract against oral microorganism. Korea J. Herbol. 2010, 25, 81–88. [Google Scholar] [CrossRef]
- Wenham, D.G.; Davies, R.M.; Cole, J.A. Insoluble glucan synthesis by mutansucrase as determinant of the cariogenicity of Streptococcus mutans. J. Gen. Microbiol. 1981, 127, 407–415. [Google Scholar] [CrossRef]
- Park, J.S.; Park, S.H.; Park, J.W.; Lee, C.Y. The remineralization aspect of enamel according to change of the degree of saturation of the organic acid buffering solution in pH 5.5. J. Korean Acad. Conserv. Dent. 2010, 35, 96–105. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoo, Y.S.; Joo, K.B.; Lee, K.J. The evaluation of efficacy on oral cavity cleaner (dental cleaner). Korea J. Waters 2011, 2, 1–8. [Google Scholar]
- Hassan, S.; Danishuddin, M.; Adil, M.; Singh, K.; Verma, P.K.; Khan, A.U. Efficacy of E. officinalis on the cariogenic properties of Streptococcus mutans a novel and alternative approach to suppress quorum sensing mechanism. PLoS ONE 2012, 7, e40319. [Google Scholar] [CrossRef]
- Park, S.N.; Lim, Y.K.; Freire, M.O.; Cho, E.; Jin, D.; Kook, J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe 2012, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Paula, V.; Modesto, A.; Santos, K.R.; Gleiser, R. Antimicrobial effects of the combination of chlorhexidine and xylitol. Br. Dent. J. 2010, 209, 19–23. [Google Scholar] [CrossRef][Green Version]
- Zhiyan, H.; Qian, W.; Yuejian, H.; Jingping, L.; Yuntao, J.; Rui, M. Use of the quorum sensing inhibitor furanone C-30 to interfere with biofilm formation by Streptococcus mutans and its luxS mutant strain. Int. J. Antimicrob. Agents 2012, 40, 30–35. [Google Scholar] [CrossRef]
- Yu, Y.E.; Park, E.Y.; Jung, D.H.; Byun, S.H.; Kim, S.C.; Park, S.M. Antibacterial effect of oriental medicinal herbs on dental pathogens. Korean J. Microbiol. 2010, 46, 200–206. [Google Scholar]
- Jung, E.J.; Hong, S.J.; Choi, J.L.; Jeong, S.S.; Lee, H.J.; Choi, C.H. In vitro growth inhibition of Streptococcus mutans by extract of prickly pear. J. Korean Acad. Oral Health 2010, 34, 28–35. [Google Scholar]
- Kim, S.J.; Park, Y.M.; Jung, S.T. Anticariogenic effects and inhibition of glucosyltransferase activity of Chrysanthemun indicum L. extracts. J. Korean Soc. Food Cult. 2005, 20, 341–345. [Google Scholar]
- Poureslami, H. The effects of plant extracts on dental plaque and caries. In Contemporary Approach to Dental Caries; Li, M.Y., Ed.; InTech Europe: Rijeka, Croatia, 2012; pp. 395–402. [Google Scholar] [CrossRef][Green Version]
- Kiuchi, F.; Chen, X.; Tsuda, Y. Four new phenolic constituents from licorice (root of Glycyrrhiza sp.). Heterocycles 1990, 31, 629–636. [Google Scholar] [CrossRef]
- Sung, K.C. A study on the Pharmaceutical Characteristics & Analysis of Glycyrrhizin Extract. J. Korean Appl. Sci. Technol. 2006, 23, 215–222. [Google Scholar] [CrossRef]
- Hatano, T.; Aga, Y.; Shintani, Y.; Ito, H.; Okuda, T.; Yoshida, T. Minor flavonoids from licorice. Phytochemisty 2000, 55, 959–963. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Im, J.S.; Lee, M.H. Physicochemical Compositions of Raw and Dried Wolha Persimmons. Korean J. Food Preserv. 2007, 14, 611–616. [Google Scholar]
- Wang, X.; Zhang, H.; Chen, L.; Shan, L.; Fan, G.; Gao, X. Liquorice, a unique “guide drug” of traditional Chinese medicine: A review of its role in drug interactions. J. Ethnopharmacol. 2013, 150, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in Licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Peters, M.C.; Tallman, J.A.; Braun, T.M.; Jacobson, J.J. Clinical reduction of S. mutans in pre-school children using a novel liquorice root extract lollipop: A pilot study. Eur. Arch. Paediatr. Dent. 2010, 11, 274–278. [Google Scholar] [CrossRef]
- Yang, S.Y.; Choi, Y.R.; Lee, M.J.; Kang, M.K. Antimicrobial effects against oral pathogens and cytotoxicity of Glycyrrhiza uralensis extract. Plants 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Villinski, J.R.; ChantalBergeron, J.; Cannistra, C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Azelmat, J.; Grenier, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Mummolo, S.; Mattia, J.D.; Casalena, F.; Martino, S.D.; Mattei, A.; Marzo, G. Efficacy of essential oil mouthwash with and without alcohol: A 3-Day plaque accumulation model. Trials 2011, 12, 262. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.B. YD Global Life Science Company, Patentee. Composition and Detection Method for Simultaneous Detection of Multiple Oral Disease-Causing Bacteria Using Multiplex Real-Time PCR. KR Patent 10-1706070, 7 February 2017. [Google Scholar]
- Lomax, J.D. (Ed.) Geriatric Ambulatory and Institutional Care; Illustrated edition; Ishiyaku Euroamerica: St. Louis, MO, USA, 1987; Available online: https://www.amazon.com/Geriatric-Ambulatory-Institutional-James-Lomax/dp/0912791330 (accessed on 21 November 2021).
- Moon, J.S.; Song, B.S.; Park, S.N. Oral health behavior and dental health status of preschool children. J. Korean Acad. Community Health Nurs. 2004, 15, 618–627. [Google Scholar]
- Lee, H.S.; Lee, E.S.; Kang, S.M.; Lee, J.H.; Choi, H.J.; Kim, B.I. Clinical assessment of a new caries activity test using dental plaque acidogenicity in children under three years of age. J. Clin. Pediatr. Dent. 2016, 40, 388–392. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacterial. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Carranza, F.A.; Newman, M.G. Periodontal microbiology. In Clinical Periodontology, 8th ed.; W.B Saunders Co.: New York, NY, USA, 1996; pp. 84–103. Available online: https://www.textbooks.com/Clinical-Periodontology-8th-Edition/9780721667287/Fermin-A-Carranza-and-Michael-G-Newman.php (accessed on 21 November 2021).
- Hamada, S.; Koga, T.; Ooshima, T. Virulence Factors of Streptococcus mutans and Dental Caries Prevention. J. Dent. Res. 1984, 63, 407–411. [Google Scholar] [CrossRef]
- Eum, J.S. Antimicrobial activity of medicinal plants extracts against Streptococcus sobrinus KCOM 1157. J. Korean Appl. Sci. Technol. 2020, 37, 279–286. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Kononen, E.; Wade, W.G. Actinomyces and related organisms in human infections. Clin. Microbiol. Rev. 2015, 28, 419–442. [Google Scholar] [CrossRef]
| Bacteria | Target Genes | Primers/Probe Sets | Amplicon Size (bp) |
|---|---|---|---|
| Streptococcus mutans | mannitol-specific enzyme II (mtlA) gene | 5′-CAGCGCATTCAACACAAGCA-3′ 103 5′-TGTCCCATCGTTGCTGAACC-3′ 5′-HEX-TGCGGTCGTTTTTGCTCATGG-BHQ1–3′ | 103 |
| Streptococcus sobrinus | 16S ribosomal RNA gene | 5′-GTACAACGAGTCGCAAGCCG-3′ 149 5′-TACAAGGCCCGGGAACGTAT-3′ 5′-FAM-TAATCGCGGATCAGCACGCC-BHQ1–3′ | 149 |
| Actinomyces viscosus | sialidase (nanH) gene | 5′-GCTCCCTCATGCTCAACTCG-3′ 5′-GATGATCTGGGCGTTGTCCA-3′ 5′-Texas Red-GAGCCGGTCCCCGACAAGAA-BHQ2–3′ | 140 |
| Characteristics | N (%) | |||
|---|---|---|---|---|
| Saline | Glycyrrhiza uralensis | p-Value | ||
| * Gender | Male | 7 (23.3) | 9 (30.0) | 0.771 |
| Female | 23 (76.7) | 21 (70.0) | ||
| ¥ Age (mean ± SD) | 44.27 ± 11.5 | 45.53 ± 11.22 | 0.821 | |
| * Systemic disease | No disease | 24 (80.0) | 26 (86.7) | 0.731 |
| Have a disease | 6 (20.0) | 4 (13.3) | ||
| * Marriage | Single | 14 (46.7) | 13 (43.3) | 1.000 |
| Married | 16 (53.3) | 17 (56.7) | ||
| Variables | Group | Mean ± SD | * p-Value | ||
|---|---|---|---|---|---|
| Baseline | Treatment | After 5 Days | |||
| Cariview scores | Saline | 54.94 ± 6.95 a | 54779 ± 4.01 a | 60.22 ± 2.73 a | 0.056 |
| Glycyrrhiza uralensis | 54.89 ± 7.55 a | 44.04 ± 4.13 b | 45.54 ± 4.91 b | 0.001 | |
| ¥p-Value | 0.990 | <0.001 | <0.001 | ||
| Risk | Saline | 2.07 ± 0.13 a | 2.00 ± 0.00 a | 2.17 ± 0.19 a | 0.057 |
| Glycyrrhiza uralensis | 2.10 ± 0.15 a | 1.90 ± 0.15 b | 1.90 ± 0.15 b | 0.017 | |
| ¥p-Value | 0.705 | 0.083 | 0.004 | ||
| Group | Risk | N (%) | * p-Value | |
|---|---|---|---|---|
| Baseline | After 5 Days | |||
| Saline | Low | 0 (00.0) | 0 (00.0) | 0.425 |
| Moderate | 26 (86.6) | 25 (83.3) | ||
| High | 4 (13.4) | 5 (16.7) | ||
| Glycyrrhiza uralensis | Low | 0 (00.0) | 3 (10.0) | 0.051 |
| Moderate | 27 (90.0) | 27 (90.0) | ||
| High | 3 (10.0) | 0 (00.0) | ||
| Cariview | Female (n = 48) | Male (n = 12) | ||
|---|---|---|---|---|
| Saline (n = 24) | Glycyrrhiza uralensis (n = 24) | Saline (n = 6) | Glycyrrhiza uralensis (n = 6) | |
| Baseline | 51.19 ± 11.06 | 50.11 ± 11.25 | 71.46 ± 13.91 | 74.00 ± 13.91 |
| p-value † | 0.746 | 0.770 | ||
| Treatment | 54.15 ± 8.86 | 44.43 ± 7.94 | 57.35 ± 1.37 | 42.50 ± 10.07 |
| p-value † | <0.001 | 0.005 | ||
| After 5 days | 60.97 ± 5.89 | 44.58 ± 10.28 | 57.35 ± 1.37 | 49.40 ± 7.12 |
| p-value † | <0.001 | 0.023 | ||
| Variables | Group | Mean ± SD | ||||
|---|---|---|---|---|---|---|
| Before | Treatment | After 5 Days | * p-Value | |||
| Streptococcus mutans | Maxilla | Saline | 41.81 ± 34.84 a | 45.30 ± 31.70 a | 35.71 ± 37.00 a | 0.868 |
| Glycyrrhiza uralensis | 64.80 ± 51.50 a | 40.58 ± 21.40 a | 0.00 ± 0.00 b | 0.002 | ||
| ¥p-Value | 0.334 | 0.756 | 0.017 | |||
| Mandibular | Saline | 83.00 ± 57.91 a | 14.50 ± 11.35 b | 16.67 ± 14.30 b | 0.000 | |
| Glycyrrhiza uralensis | 83.00 ± 57.91 a | 10.50 ± 9.79 b | 0.00 ± 0.00 b | 0.000 | ||
| ¥p-Value | 1.000 | 0.490 | 0.003 | |||
| Streptococcus sobrinus | Maxilla | Saline | 127,537.76 ± 98,642.17 a | 119,800.90 ± 110,133.95 a | 124,878.57 ± 71,101.00 a | 0.988 |
| Glycyrrhiza uralensis | 153,728.50 ± 133,372.23 a | 42,408.68 ± 26,692.10 a | 110,578.89 ± 55,820.33 a | 0.059 | ||
| ¥p-Value | 0.688 | 0.071 | 0.677 | |||
| Mandibular | Saline | 56,727.96 ± 30,115.95 a | 56,101.40 ± 26,700.44 a | 56,808.07 ± 23,433.67 a | 0.998 | |
| Glycyrrhiza uralensis | 90,897.70 ± 65,432.98 a | 25,941.37 ± 13,166.15 b | 89,438.63 ± 56,597.73 a | 0.032 | ||
| ¥p-Value | 0.205 | 0.009 | 0.196 | |||
| Actinomyces viscosus | Maxilla | Saline | 387528.97 ± 156553.26 a | 378413.10 ± 203472.95 a | 370,530.17 ± 149,524.05 a | 0.982 |
| Glycyrrhiza uralensis | 279,862.30 ± 152,068.99 a | 253,726.33 ± 140,596.78 a | 245,293.78 ± 127,744.71 a | 0.888 | ||
| ¥p-Value | 0.182 | 0.173 | 0.097 | |||
| Mandibular | Saline | 274,498.13 ± 119,897.44 a | 281,772.50 ± 132,463.89 a | 281,772.50 ± 132,463.89 a | 0.992 | |
| Glycyrrhiza uralensis | 210,164.80 ± 134,786.75 a | 55,076.30 ± 20,612.73 b | 32,903.89 ± 10,128.22 b | 0.000 | ||
| ¥p-Value | 0.333 | 0.000 | 0.000 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-R.; Nam, S.-H. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza uralensis Extract for Preventing Dental Caries. Int. J. Environ. Res. Public Health 2022, 19, 242. https://doi.org/10.3390/ijerph19010242
Kim Y-R, Nam S-H. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza uralensis Extract for Preventing Dental Caries. International Journal of Environmental Research and Public Health. 2022; 19(1):242. https://doi.org/10.3390/ijerph19010242
Chicago/Turabian StyleKim, Yu-Rin, and Seoul-Hee Nam. 2022. "A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza uralensis Extract for Preventing Dental Caries" International Journal of Environmental Research and Public Health 19, no. 1: 242. https://doi.org/10.3390/ijerph19010242
APA StyleKim, Y.-R., & Nam, S.-H. (2022). A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza uralensis Extract for Preventing Dental Caries. International Journal of Environmental Research and Public Health, 19(1), 242. https://doi.org/10.3390/ijerph19010242







