Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
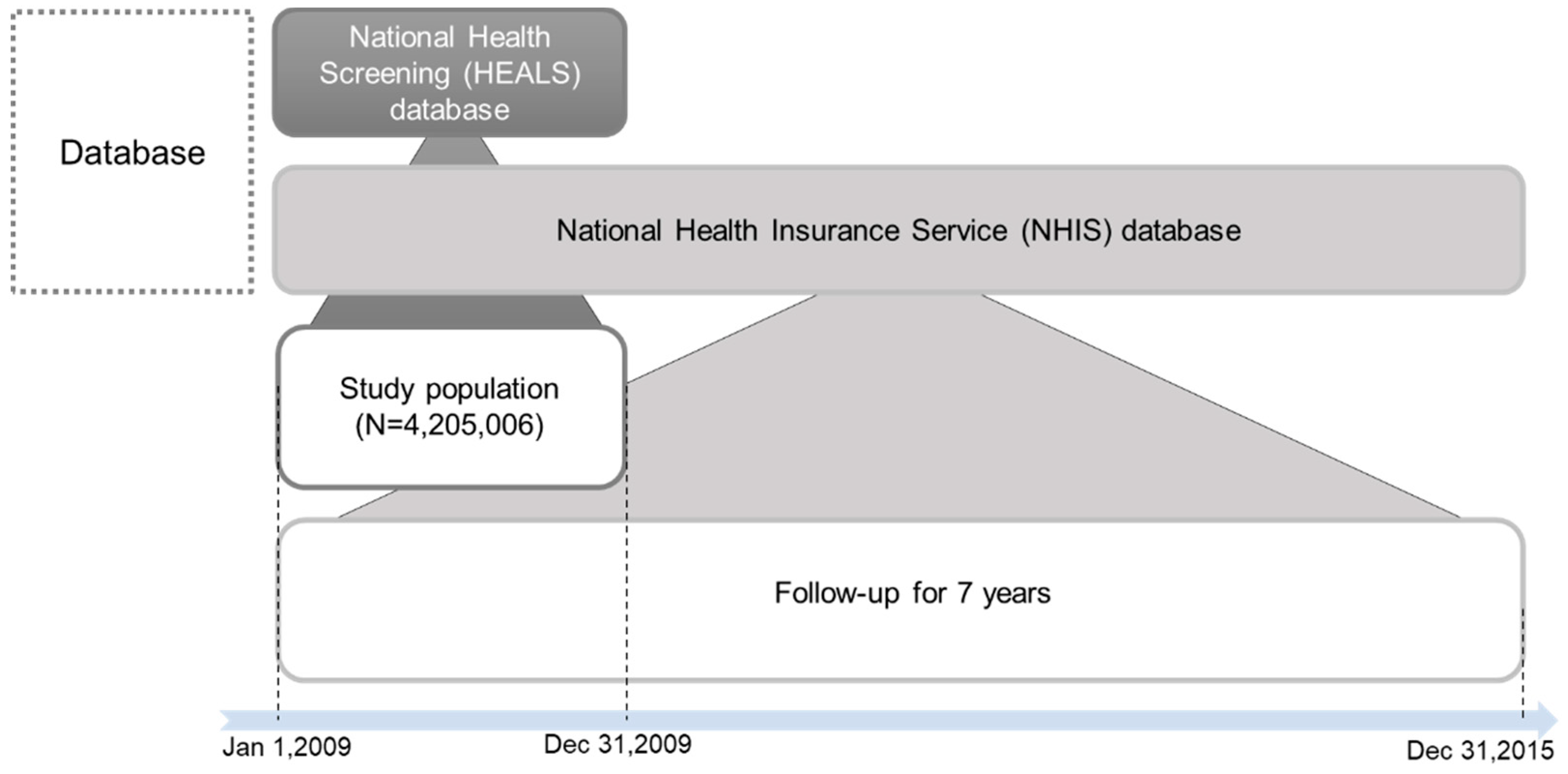
2.1. Study Design and Data Source
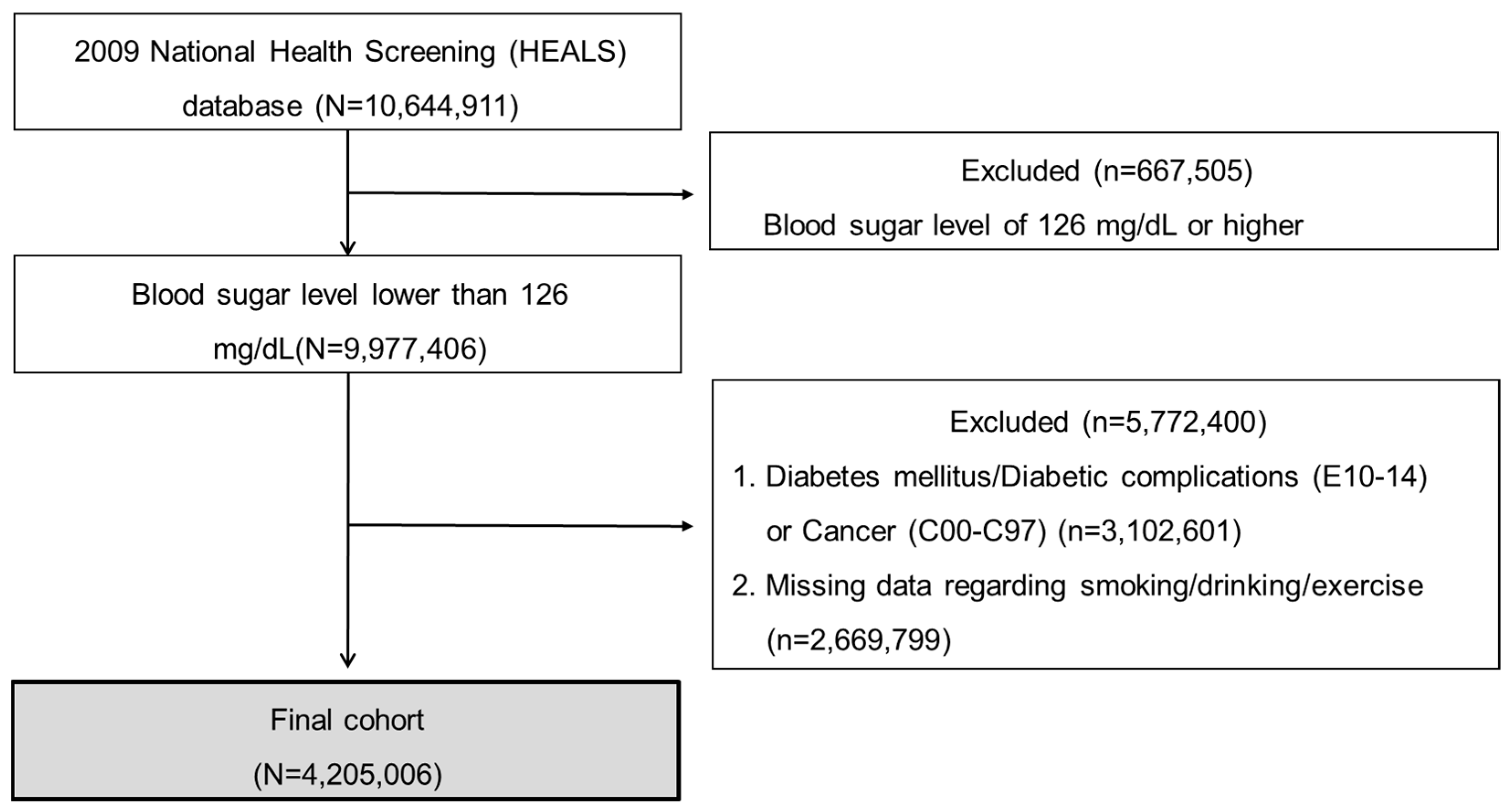
2.2. Study Subjects and Setting
2.3. Variables
2.3.1. Outcome Variables
2.3.2. Household Income
2.3.3. Metabolic Syndrome
2.3.4. Current Smoking, High-Risk Drinking, and Proper Exercise
2.4. Statistical Analyses
3. Results
3.1. Baseline Demographics
3.2. Incidence and Characteristics of Progression from Non-Diabetes to Diabetes
3.3. Risk Factors of Progression from Non-Diabetes to Diabetes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Zand, A.; Ibrahim, K.; Patham, B. Prediabetes: Why Should We Care? Methodist Debakey Cardiovasc. J. 2018, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- ADA, 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar]
- ADA, 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S32–S36. [Google Scholar]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for developing diabetes. Lancet 2012, 379, 2279. [Google Scholar] [CrossRef]
- Azami, M.; Sharifi, A.; Norozi, S.; Mansouri, A.; Sayehmiri, K. Prevalence of diabetes, impaired fasting glucose and impaired glucose tolerance in patients with thalassemia major in Iran: A meta-analysis study. Casp. J. Intern. Med. 2017, 8, 1. [Google Scholar]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatrics 2020, 174, e194498. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Violante, R.; Rodríguez-Morán, M. Distribution of fasting plasma glucose and prevalence of impaired fasting glucose, impaired glucose tolerance and type 2 diabetes in the Mexican paediatric population. Paediatr. Perinat. Epidemiol. 2009, 23, 363–369. [Google Scholar] [CrossRef]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef]
- Anjana, R.M.; Shanthi Rani, C.S.; Deepa, M.; Pradeepa, R.; Sudha, V.; Divya Nair, H.; Lakshmipriya, N.; Subhashini, S.; Binu, V.S.; Unnikrishnan, R.; et al. Incidence of Diabetes and Prediabetes and Predictors of Progression Among Asian Indians: 10-Year Follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 2015, 38, 1441–1448. [Google Scholar] [CrossRef]
- Tillin, T.; Hughes, A.D.; Wang, Q.; Wurtz, P.; Ala-Korpela, M.; Sattar, N.; Forouhi, N.G.; Godsland, I.F.; Eastwood, S.V.; McKeigue, P.M.; et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015, 58, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Kurotani, K.; Miyamoto, T.; Kochi, T.; Eguchi, M.; Imai, T.; Nishihara, A.; Tomita, K.; Uehara, A.; Yamamoto, M.; Murakami, T.; et al. Metabolic syndrome components and diabetes incidence according to the presence or absence of impaired fasting glucose: The Japan Epidemiology Collaboration on Occupational Health Study. J. Epidemiol. 2017, 27, 408–412. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.-H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service–National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2016, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.C.; Kim, Y.Y.; Park, S.K.; Khang, Y.H.; Kim, H.C.; Park, J.H.; Kang, H.J.; Do, C.H.; Song, J.S.; Lee, E.J.; et al. Cohort profile: The National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017, 7, e016640. [Google Scholar] [CrossRef]
- Hur, K.Y.; Moon, M.K.; Park, J.S.; Kim, S.K.; Lee, S.H.; Yun, J.S.; Baek, J.H.; Noh, J.; Lee, B.W.; Oh, T.J.; et al. 2021 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2021, 45, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Son, K.Y. Effects of different definitions of low muscle mass on its association with metabolic syndrome in older adults: A Korean nationwide study. Geriatr. Gerontol. Int. 2021, 21, 1003–1009. [Google Scholar] [CrossRef]
- Statistics Korea. Korean Statistical Information Service. Available online: https://kosis.kr/eng (accessed on 27 October 2021).
- Lee, S.; Kim, J.-S.; Jung, J.-G.; Oh, M.-K.; Chung, T.-H.; Kim, J. Korean alcohol guidelines for moderate drinking based on facial flushing. Korean J. Fam. Med. 2019, 40, 204–211. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.W. Exercise and type 2 diabetes: ACSM and ADA joint position statement. J. Korean Diabetes 2012, 13, 61–68. [Google Scholar] [CrossRef][Green Version]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Bae, N.-K.; Kim, K.-H.; Kwon, I.-S.; Cho, Y.-C. Changes in Prevalence of Obesity for 10 Years (1997~2007) and Its Related Factors in Health Checkup Examinees. J. Korea Acad. Ind. Coop. Soc. 2009, 10, 1091–1099. [Google Scholar]
- Han, S.J.; Kim, H.J.; Kim, D.J.; Lee, K.W.; Cho, N.H. Incidence and predictors of type 2 diabetes among Koreans: A 12-year follow up of the Korean Genome and Epidemiology Study. Diabetes Res. Clin. Pract. 2017, 123, 173–180. [Google Scholar] [CrossRef] [PubMed]
- HIRA Statistics about Diabetes. Available online: http://opendata.hira.or.kr/op/opc/olapMfrnIntrsIlnsInfo.do (accessed on 27 October 2021).
- Quan, J.; Li, T.K.; Pang, H.; Choi, C.H.; Siu, S.C.; Tang, S.Y.; Wat, N.M.S.; Woo, J.; Johnston, J.M.; Leung, G.M. Diabetes incidence and prevalence in Hong Kong, China during 2006–2014. Diabet. Med. A J. Br. Diabet. Assoc. 2017, 34, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Goto, M.; Noda, M.; Tsugane, S. Incidence of type 2 diabetes in Japan: A systematic review and meta-analysis. PLoS ONE 2013, 8, e74699. [Google Scholar] [CrossRef]
- Thomas, A.; Ashcraft, A. Type 2 Diabetes Risk among Asian Indians in the US: A Pilot Study. Nurs. Res. Pract. 2013, 2013, 492893. [Google Scholar] [CrossRef]
- Selph, S.; Dana, T.; Bougatsos, C.; Blazina, I.; Patel, H.; Chou, R. Screening for Type 2 Diabetes Mellitus: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2015, 162, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, A.A. Epidemiology of conventional cardiovascular risk factors among hypertensive subjects with normal and impaired fasting glucose. S. Afr. Med. J. 2010, 100, 594–597. [Google Scholar] [CrossRef] [PubMed]
- NICE. Preventing Type 2 Diabetes Overview; National Institute for Health and Care Excellence: London, UK, 2021. [Google Scholar]
- Ramachandran, A.; Snehalatha, C.; Satyavani, K.; Sivasankari, S.; Vijay, V. Metabolic syndrome does not increase the risk of conversion of impaired glucose tolerance to diabetes in Asian Indians—Result of Indian diabetes prevention programme. Diabetes Res. Clin. Pract. 2007, 76, 215–218. [Google Scholar] [CrossRef]
- Maddatu, J.; Anderson-Baucum, E.; Evans-Molina, C. Smoking and the risk of type 2 diabetes. Transl. Res. 2017, 184, 101–107. [Google Scholar] [CrossRef]
- Śliwińska-Mossoń, M.; Milnerowicz, H. The impact of smoking on the development of diabetes and its complications. Diab. Vasc. Dis. Res. 2017, 14, 265–276. [Google Scholar] [CrossRef]
- Akter, S.; Goto, A.; Mizoue, T. Smoking and the risk of type 2 diabetes in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 553–561. [Google Scholar] [CrossRef]
- Kim, J.H.; Noh, J.; Choi, J.W.; Park, E.C. Association of Education and Smoking Status on Risk of Diabetes Mellitus: A Population-Based Nationwide Cross-Sectional Study. Int. J. Env. Res. Public. Health 2017, 14, 655. [Google Scholar] [CrossRef]
- Cai, X.; Chen, Y.; Yang, W.; Gao, X.; Han, X.; Ji, L. The association of smoking and risk of diabetic retinopathy in patients with type 1 and type 2 diabetes: A meta-analysis. Endocrine 2018, 62, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015, 132, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Yu, F.F.; Zhou, Y.H.; He, J. Association between alcohol consumption and the risk of incident type 2 diabetes: A systematic review and dose-response meta-analysis. Am. J. Clin. Nutr. 2016, 103, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Jenkins, D.W.; Jenks, A. Exercise and Diabetes: A Narrative Review. J. Foot. Ankle Surg 2017, 56, 968–974. [Google Scholar] [CrossRef]
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002495. [Google Scholar] [CrossRef]
- Groos, S.; Kretschmann, J.; Macare, C.; Weber, A.; Hagen, B. Qualitätssicherungsbericht 2017 Disease Management Programme in Nordrhein: Tabellenband, Version 1c; Zentralinstitut für die kassenärztliche Versorgung in Deutschland: Berlin, Germany, 2018. [Google Scholar]
| Variables | Total | Male | Female | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Total (n) | 4,205,006 | 100.0 | 3,019,103 | 71.8% | 1,185,903 | 28.2% | ||
| IFG | No | 3,179,247 | 75.6% | 2,192,290 | 69.0% | 986,957 | 31.0% | <0.0001 |
| Yes | 1,025,759 | 24.4% | 826,813 | 80.6% | 198,946 | 19.4% | ||
| Age group | 40.1 ± 12.2 | 40.4 ± 11.9 | 39.3 ± 12.8 | <0.0001 | ||||
| 18~29 | 977,664 | 23.3% | 615,826 | 63.0% | 361,838 | 37.0% | <0.0001 | |
| 30~39 | 1,254,309 | 29.8% | 999,030 | 79.6% | 255,279 | 20.4% | ||
| 40~49 | 1,078,746 | 25.7% | 754,509 | 69.9% | 324,237 | 30.1% | ||
| 50~59 | 585,787 | 13.9% | 421,115 | 71.9% | 164,672 | 28.1% | ||
| 60~69 | 237,616 | 5.7% | 177,846 | 74.8% | 59,770 | 25.2% | ||
| ≥70 | 70,884 | 1.7% | 50,777 | 1.68 | 20,107 | 28.4% | ||
| Household income | 1 | 647,627 | 15.4% | 400,233 | 61.8% | 247,394 | 38.2% | <0.0001 |
| 2 | 747,199 | 17.8% | 455,816 | 61.0% | 291,383 | 39.0% | ||
| 3 | 928,682 | 22.1% | 669,523 | 72.1% | 259,159 | 27.9% | ||
| 4 | 989,910 | 23.5% | 775,989 | 78.4% | 213,921 | 21.6% | ||
| 5 | 891,588 | 21.2% | 717,542 | 80.5% | 174,046 | 19.5% | ||
| Hypertension | No | 3,901,156 | 92.8% | 2,784,454 | 71.4% | 1,116,702 | 28.6% | <0.0001 |
| Yes | 303,850 | 7.2% | 234,649 | 77.2% | 69,201 | 22.8% | ||
| Systolic blood pressure | <120 | 1,720,188 | 40.9% | 1,012,586 | 58.9% | 707,602 | 41.1% | <0.0001 |
| 120~139 | 2,083,991 | 49.6% | 1,673,166 | 80.3% | 410,825 | 19.7% | ||
| ≥140 | 400,827 | 9.5% | 333,351 | 83.2% | 67,476 | 16.8% | ||
| Diastolic blood pressure | <80 | 2,279,381 | 54.2% | 1,441,408 | 63.2% | 837,973 | 36.8% | <0.0001 |
| 80~89 | 1,522,799 | 36.2% | 1,236,603 | 81.2% | 286,196 | 18.8% | ||
| ≥90 | 402,826 | 9.6% | 341,092 | 84.7% | 61,734 | 15.3% | ||
| Dyslipidemia | No | 4,108,644 | 97.7% | 2,939,918 | 71.6% | 1,168,726 | 28.4% | <0.0001 |
| Yes | 96,362 | 2.3% | 79,185 | 82.2% | 17,177 | 17.8% | ||
| Triglyceride | <150 | 2,926,610 | 69.6% | 1,887,318 | 64.5% | 1,039,292 | 35.5% | <0.0001 |
| ≥150 | 1,278,396 | 30.4% | 1,131,785 | 88.5% | 146,611 | 11.5% | ||
| HDL | M < 40, F < 50 | 594,305 | 14.1% | 362,835 | 61.1% | 231,470 | 38.9% | <0.0001 |
| M ≥ 40, F ≥ 50 | 3,610,701 | 85.9% | 2,656,268 | 73.6% | 954,433 | 26.4% | ||
| BMI | 23.6 ± 3.2 | 24.1 ± 3.0 | 22.4 ± 3.2 | <0.0001 | ||||
| Waist circumference | M < 90, F < 85 | 3,524,867 | 83.8% | 2,461,292 | 69.8% | 1,063,575 | 30.2% | <0.0001 |
| M ≥ 90, F ≥ 85 | 680,139 | 16.2% | 557,811 | 82.0% | 122,328 | 18.0% | ||
| Metabolic syndrome | No | 1,643,100 | 39.1% | 1,380,427 | 84.0% | 262,673 | 16.0% | <0.0001 |
| Yes | 2,561,906 | 60.9% | 1,638,676 | 64.0% | 923,230 | 36.0% | ||
| Current smoking | No | 2,540,898 | 60.4% | 1,427,760 | 56.2% | 1,113,138 | 43.8% | <0.0001 |
| Yes | 1,664,108 | 39.6% | 1,591,343 | 95.6% | 72,765 | 4.4% | ||
| High-risk drinking | No | 3,645,544 | 86.7% | 2,495,166 | 68.4% | 1,150,378 | 31.6% | <0.0001 |
| Yes | 559,462 | 13.3% | 523,937 | 93.7% | 35,525 | 6.3% | ||
| Proper exercise | No | 3,452,613 | 82.1% | 2,436,072 | 70.6% | 1,016,541 | 29.4% | <0.0001 |
| Yes | 752,393 | 17.9% | 583,031 | 77.5% | 169,362 | 22.5% | ||
| Follow-Up Period | Follow-Up Population | Newly Diagnosed Diabetes | Censored 1 | Cumulative Incidence of Diabetes | Person-Years 2 | Conversion Rate 3 | |
|---|---|---|---|---|---|---|---|
| Year | N | n | n | n | % | ||
| <1 | 4,205,006 | 55,129 | 4,165 | 55,129 | 1.3% | 4,202,924 | 13.1 |
| 1~2 | 4,145,712 | 88,029 | 4,881 | 143,158 | 3.4% | 4,143,272 | 21.2 |
| 2~3 | 4,052,802 | 93,463 | 5,259 | 236,621 | 5.6% | 4,050,173 | 23.1 |
| 3~4 | 3,954,080 | 95,179 | 5,286 | 331,800 | 7.9% | 3,951,437 | 24.1 |
| 4~5 | 3,853,615 | 102,731 | 4,910 | 434,531 | 10.3% | 3,851,160 | 26.7 |
| 5~6 | 3,745,974 | 104,789 | 4,321 | 539,320 | 12.8% | 3,743,814 | 28.0 |
| 6~7 | 3,636,864 | 47,695 | 3,589,169 | 587,015 | 14.0% | 1,842,280 | 25.9 |
| Total | 4,205,006 | 587,015 | 14.0% | 25,785,058 | 22.8 |
| Variables | Diabetes Diagnosis | No Diabetes Diagnosis | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | ||
| Total (N) | 587,015 | (14.0) | 3,617,991 | (86.0) | ||
| IFG | No | 362,128 | (11.4) | 2,817,119 | (88.6) | <0.0001 |
| Yes | 224,887 | (21.9) | 800,872 | (78.1) | ||
| Age group | 48.0 ± 12.7 | 38.8 ± 11.6 | <0.0001 | |||
| 18–29 | 49,669 | (5.1) | 927,995 | (94.9) | ||
| 30–39 | 107,231 | (8.6) | 1,147,078 | (91.5) | ||
| 40–49 | 169,599 | (15.7) | 909,147 | (84.3) | ||
| 50–59 | 150,062 | (25.6) | 435,725 | (74.4) | ||
| 60–69 | 82,528 | (34.7) | 155,088 | (65.3) | ||
| ≥70 | 27,926 | (39.4) | 42,958 | (60.6) | ||
| Gender | Male | 426,674 | (14.1) | 2,592,429 | (85.9) | <0.0001 |
| Female | 160,341 | (13.5) | 1,025,562 | (86.5) | ||
| Household income | 1 | 96,040 | (14.8) | 551,587 | (85.2) | <0.0001 |
| 2 | 94,698 | (12.7) | 652,501 | (87.3) | ||
| 3 | 116,804 | (12.6) | 811,878 | (87.4) | ||
| 4 | 136,771 | (13.8) | 853,139 | (86.2) | ||
| 5 | 142,702 | (16.0) | 748,886 | (84.0) | ||
| Hypertension | No | 484,769 | (12.4) | 3,416,387 | (87.6) | <0.0001 |
| Yes | 102,246 | (33.7) | 201,604 | (66.4) | ||
| Systolic blood pressure | <120 | 182,114 | (10.6) | 1,538,074 | (89.4) | <0.0001 |
| 120–139 | 304,390 | (14.6) | 1,779,601 | (85.4) | ||
| ≥140 | 100,511 | (25.1) | 300,316 | (74.9) | ||
| Diastolic blood pressure | <80 | 264,121 | (11.6) | 2,015,260 | (88.4) | <0.0001 |
| 80–89 | 230,515 | (15.1) | 1,292,284 | (84.9) | ||
| ≥90 | 92,379 | (22.9) | 310,447 | (77.1) | ||
| Dyslipidemia | No | 562,230 | (13.7) | 3,546,414 | (86.3) | <0.0001 |
| Yes | 24,785 | (25.7) | 71,577 | (74.3) | ||
| Triglyceride | <150 | 354,226 | (12.1) | 2,572,384 | (87.9) | <0.0001 |
| ≥150 | 232,789 | (18.2) | 1,045,607 | (81.8) | ||
| HDL | M < 40, F < 50 | 103,175 | (17.4) | 491,130 | (82.6) | <0.0001 |
| M ≥ 40, F ≥ 50 | 483,840 | (13.4) | 3,126,861 | (86.6) | ||
| BMI | 24.5 ± 3.3 | 23.4 ± 3.1 | <0.0001 | |||
| Waist circumference | M < 90, F < 85 | 433,978 | (12.3) | 3,090,889 | (87.7) | <0.0001 |
| M ≥ 90, F ≥ 85 | 153,037 | (22.5) | 527,102 | (77.5) | ||
| Metabolic syndrome | No | 300,847 | (18.3) | 1,342,253 | (81.7) | <0.0001 |
| Yes | 286,168 | (11.2) | 2,275,738 | (88.8) | ||
| Current smoking | No | 367,954 | (14.5) | 2,172,944 | (85.5) | <0.0001 |
| Yes | 219,061 | (13.2) | 1,445,047 | (86.8) | ||
| High-risk drinking | No | 492,266 | (13.5) | 3,153,278 | (86.5) | <0.0001 |
| Yes | 94,749 | (16.9) | 464,713 | (83.1) | ||
| Proper exercise | No | 468,582 | (13.6) | 2,984,031 | (86.4) | <0.0001 |
| Yes | 118,433 | (15.7) | 633,960 | (84.3) | ||
| Variables | Hazard * Ratio | 95% Lower CI | 95% Upper CI | p-Value | |
|---|---|---|---|---|---|
| IFG | No | 1.000 | |||
| Yes | 1.552 | 1.542 | 1.563 | <0.0001 | |
| Age group | 18–29 | 1.000 | |||
| 30–39 | 1.509 | 1.492 | 1.525 | <0.0001 | |
| 40–49 | 2.724 | 2.696 | 2.752 | <0.0001 | |
| 50–59 | 4.382 | 4.336 | 4.429 | <0.0001 | |
| 60–69 | 6.047 | 5.977 | 6.119 | <0.0001 | |
| ≥70 | 7.504 | 7.389 | 7.620 | <0.0001 | |
| Gender | Male | 1.000 | |||
| Female | 1.198 | 1.190 | 1.206 | <0.0001 | |
| Household income | 1 | 1.000 | |||
| 2 | 1.003 | 0.994 | 1.012 | 0.5275 | |
| 3 | 1.005 | 0.997 | 1.014 | 0.2438 | |
| 4 | 1.008 | 0.999 | 1.016 | 0.0732 | |
| 5 | 0.931 | 0.924 | 0.939 | <0.0001 | |
| Hypertension | No | 1.000 | |||
| Yes | 1.443 | 1.433 | 1.454 | <0.0001 | |
| Systolic blood pressure | <120 | 1.000 | |||
| 120–139 | 1.051 | 1.043 | 1.059 | <0.0001 | |
| ≥140 | 1.176 | 1.162 | 1.189 | <0.0001 | |
| Diastolic blood pressure | <80 | 1.000 | |||
| 80–89 | 1.023 | 1.016 | 1.030 | <0.0001 | |
| ≥90 | 1.082 | 1.070 | 1.094 | <0.0001 | |
| Dyslipidemia | No | 1.000 | |||
| Yes | 1.256 | 1.239 | 1.272 | <0.0001 | |
| Triglyceride | <150 | 1.000 | |||
| ≥150 | 1.203 | 1.195 | 1.212 | <0.0001 | |
| HDL | M < 40, F < 50 | 1.000 | |||
| M ≥ 40, F ≥ 50 | 0.937 | 0.930 | 0.945 | <0.0001 | |
| BMI | 1.048 | 1.046 | 1.049 | <0.0001 | |
| Waist circumference | M < 90, F < 85 | 1.000 | |||
| M ≥ 90, F ≥ 85 | 1.183 | 1.173 | 1.193 | <0.0001 | |
| Metabolic syndrome | No | 1.000 | |||
| Yes | 1.040 | 1.030 | 1.049 | <0.0001 | |
| Current smoking | No | 1.000 | |||
| Yes | 1.082 | 1.075 | 1.088 | <0.0001 | |
| High-risk drinking | No | 1.000 | |||
| Yes | 1.140 | 1.132 | 1.148 | <0.0001 | |
| Proper exercise | No | 1.000 | |||
| Yes | 0.999 | 0.993 | 1.005 | 0.7664 | |
| Variables | Hazard * Ratio | 95% Lower CI | 95% Upper CI | p-Value | |
|---|---|---|---|---|---|
| IFG | No | 1.000 | |||
| Yes | 1.521 | 1.509 | 1.533 | <0.0001 | |
| Age | 1.040 | 1.039 | 1.040 | <0.0001 | |
| Gender | Male | 1.000 | |||
| Female | 1.145 | 1.137 | 1.154 | <0.0001 | |
| Household income | 1 | 1.000 | |||
| 2 | 1.001 | 0.990 | 1.011 | 0.8834 | |
| 3 | 1.000 | 0.990 | 1.011 | 0.9368 | |
| 4 | 0.998 | 0.989 | 1.008 | 0.718 | |
| 5 | 0.908 | 0.900 | 0.917 | <0.0001 | |
| Hypertension | No | 1.000 | |||
| Yes | 1.394 | 1.383 | 1.405 | <0.0001 | |
| Systolic blood pressure | <120 | 1.000 | |||
| 120–139 | 1.051 | 1.042 | 1.060 | <0.0001 | |
| ≥140 | 1.134 | 1.120 | 1.149 | <0.0001 | |
| Diastolic blood pressure | <80 | 1.000 | |||
| 80–89 | 1.021 | 1.013 | 1.029 | <0.0001 | |
| ≥90 | 1.068 | 1.054 | 1.081 | <0.0001 | |
| Dyslipidemia | No | 1.000 | |||
| Yes | 1.248 | 1.231 | 1.266 | <0.0001 | |
| Triglyceride | <150 | 1.000 | |||
| ≥150 | 1.176 | 1.167 | 1.186 | <0.0001 | |
| HDL | M < 40, F < 50 | 1.000 | |||
| M ≥ 40, F ≥ 50 | 0.943 | 0.935 | 0.952 | <0.0001 | |
| BMI | 1.042 | 1.041 | 1.043 | <0.0001 | |
| Waist circumference | M < 90, F < 85 | 1.000 | |||
| M ≥ 90, F ≥ 85 | 1.145 | 1.135 | 1.156 | <0.0001 | |
| Metabolic syndrome | No | 1.000 | |||
| Yes | 1.023 | 1.013 | 1.034 | <0.0001 | |
| Current smoking | No | 1.000 | |||
| Yes | 1.103 | 1.096 | 1.111 | <0.0001 | |
| High-risk drinking | No | 1.000 | |||
| Yes | 1.131 | 1.122 | 1.141 | <0.0001 | |
| Proper exercise | No | 1.000 | |||
| Yes | 0.996 | 0.988 | 1.003 | 0.2366 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, M.K.; Park, J.H.; Kim, K.H.; Ahn, S.-K.; Ji, S.M. Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 123. https://doi.org/10.3390/ijerph19010123
Hyun MK, Park JH, Kim KH, Ahn S-K, Ji SM. Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(1):123. https://doi.org/10.3390/ijerph19010123
Chicago/Turabian StyleHyun, Min Kyung, Jong Heon Park, Kyoung Hoon Kim, Soon-Ki Ahn, and Seon Mi Ji. 2022. "Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 1: 123. https://doi.org/10.3390/ijerph19010123
APA StyleHyun, M. K., Park, J. H., Kim, K. H., Ahn, S.-K., & Ji, S. M. (2022). Incidence and Risk Factors for Progression to Diabetes Mellitus: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 19(1), 123. https://doi.org/10.3390/ijerph19010123







