Quality of Life in Cohabitants of Patients Suffering Inflammatory Bowel Disease: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Study Variables
2.3. Data Analysis
3. Results
3.1. Patients
3.2. Cohabitants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [Green Version]
- Park, K.T.; Ehrlich, O.G.; Allen, J.I.; Meadows, P.; Szigethy, E.M.; Henrichsen, K.; Kim, S.C.; Lawton, R.C.; Murphy, S.M.; Regueiro, M.; et al. The Cost of Inflammatory Bowel Disease: An Initiative From the Crohn’s & Colitis Foundation. Inflamm. Bowel Dis. 2020, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Knowles, S.R.; Graff, L.A.; Wilding, H.; Hewitt, C.; Keefer, L.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part I. Inflamm. Bowel Dis. 2018, 24, 742–751. [Google Scholar] [CrossRef]
- Parekh, N.K.; Shah, S.; McMaster, K.; Speziale, A.; Yun, L.; Nguyen, D.L.; Melmed, G.; Kane, S. Effects of caregiver burden on quality of life and coping strategies utilized by caregivers of adult patients with inflammatory bowel disease. Ann. Gastroenterol. 2017, 30, 89–95. [Google Scholar] [CrossRef]
- Magro, F.; Portela, F.; Lago, P.; Deus, J.; Cotter, J.; Cremers, I.; Vieira, A.; Peixe, P.; Caldeira, P.; Lopes, H.; et al. Inflammatory bowel disease: A patient’s and caregiver’s perspective. Dig. Dis. Sci. 2009, 54, 2671–2679. [Google Scholar] [CrossRef]
- Shukla, R.; Thakur, E.; Bradford, A.; Hou, J.K. Caregiver Burden in Adults With Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zand, A.; Kim, B.J.; van Deen, W.K.; Stokes, Z.; Platt, A.; O’Hara, S.; Khong, H.; Hommes, D.W. The effects of inflammatory bowel disease on caregivers: Significant burden and loss of productivity. BMC Health Serv. Res. 2020, 20, 556. [Google Scholar] [CrossRef]
- Masachs, M.; Casellas, F.; Malagelada, J.R. Spanish translation, adaptation, and validation of the 32-item questionnaire on quality of life for inflammatory bowel disease (IBDQ-32). Rev. Esp. Enferm. Dig. 2007, 99, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Vergara, M.; Casellas, F.; Badia, X.; Malagelada, J.R. Assessing the quality of life of household members of patients with inflammatory bowel disease: Development and validation of a specific questionnaire. Am. J. Gastroenterol. 2002, 97, 1429–1437. [Google Scholar] [CrossRef]
- Marasca, C.; Napolitano, M.; Monfrecola, G.; Masara, A.; Annunziata, M.C.; Donnarumma, M.; Fabbrocini, G. Quality of life in people living with patients suffering from hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e342–e343. [Google Scholar] [CrossRef]
- Ramos-Alejos-Pita, C.; Arias-Santiago, S.; Molina-Leyva, A. Quality of Life in Cohabitants of Patients with Hidradenitis Suppurativa: A Cross-sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 6000. [Google Scholar] [CrossRef]
- Sprangers, M.A.; de Regt, E.B.; Andries, F.; van Agt, H.M.; Bijl, R.V.; de Boer, J.B.; Foets, M.; Hoeymans, N.; Jacobs, A.E.; Kempen, G.I.; et al. Which chronic conditions are associated with better or poorer quality of life? J. Clin. Epidemiol. 2000, 53, 895–907. [Google Scholar] [CrossRef] [Green Version]
- Knowles, S.R.; Keefer, L.; Wilding, H.; Hewitt, C.; Graff, L.A.; Mikocka-Walus, A. Quality of Life in Inflammatory Bowel Disease: A Systematic Review and Meta-analyses-Part II. Inflamm. Bowel Dis. 2018, 24, 966–976. [Google Scholar] [CrossRef]
- Pokrzywinski, R.M.; Harding, G.; Brooks, A.; Goodwin, B.; Williams, J. Documenting the Journey of Patients with Eosinophilic Esophagitis and the Impact of the Disease on Patients and their Caregivers: A Cross-sectional, Qualitative Research Study. Adv. Ther. 2020, 37, 4458–4478. [Google Scholar] [CrossRef]
- Mukkada, V.; Falk, G.W.; Eichinger, C.S.; King, D.; Todorova, L.; Shaheen, N.J. Health-Related Quality of Life and Costs Associated With Eosinophilic Esophagitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2018, 16, 495–503.e8. [Google Scholar] [CrossRef] [Green Version]
- Dunn, J. Impact of mobility impairment on the burden of caregiving in individuals with multiple sclerosis. Expert Rev. Pharmacoecon. Outcomes Res. 2010, 10, 433–440. [Google Scholar] [CrossRef]
- Uzuner, S.; Durcan, G.; Sahin, S.; Bahali, K.; Barut, K.; Kilicoglu, A.G.; Adrovic, A.; Bilgic, A.; Kasapcopur, O. Caregiver burden and related factors in caregivers of patients with childhood-onset systemic lupus erythematosus. Clin. Rheumatol. 2021, 40, 5025–5032. [Google Scholar] [CrossRef]
- Stanton, A.L.; Revenson, T.A.; Tennen, H. Health psychology: Psychological adjustment to chronic disease. Annu. Rev. Psychol. 2007, 58, 565–592. [Google Scholar] [CrossRef] [Green Version]
- Lix, L.M.; Sajobi, T.T.; Sawatzky, R.; Liu, J.; Mayo, N.E.; Huang, Y.; Graff, L.A.; Walker, J.R.; Ediger, J.; Clara, I.; et al. Relative importance measures for reprioritization response shift. Qual. Life Res. 2013, 22, 695–703. [Google Scholar] [CrossRef]
- Carr, A.J.; Gibson, B.; Robinson, P.G. Measuring quality of life: Is quality of life determined by expectations or experience? BMJ 2001, 322, 1240–1243. [Google Scholar] [CrossRef]
- Buzgova, R.; Kozakova, R.; Bar, M. Satisfaction of Patients With Severe Multiple Sclerosis and Their Family Members With Palliative Care: Interventional Study. Am. J. Hosp. Palliat. Care 2021, 38, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabi, S.W.; Seleit, D.I.; Badran, A.; Koni, A.; Zyoud, S.H. Impact of socio-demographic and clinical characteristics on functional disability and health-related quality of life in patients with rheumatoid arthritis: A cross-sectional study from Palestine. Health Qual. Life Outcomes 2021, 19, 241. [Google Scholar] [CrossRef]

| Variables | Patients (n = 56) | Cohabitants (n = 82) |
|---|---|---|
| Sex | ||
| Male | 50% (28) | 37 (45%) |
| Female | 50% (28) | 82 (55%) |
| Educational | ||
| None | 3 (5%) | 8 (10%) |
| Primary | 13 (23%) | 20 (24%) |
| Secondary | 18 (32%) | 19 (23%) |
| University or Higher | 22 (39%) | 35 (43%) |
| Occupation | ||
| Employed | 35 (62%) | 56 (69%) |
| Unemployed | 8 (14%) | 11 (13%) |
| Retired | 13 (23%) | 15 (18%) |
| Marital Status | ||
| Married | 42 (75%) | 58 (71%) |
| Single | 14 (25%) | 24 (29%) |
| Variables (n) | IBDQ 32 (Mean ± SD) | p |
|---|---|---|
| Sex Female (28) Male (28) | 127 ± 34 140.6 ± 35 | 0.16 |
| Age | –0.24 ± 0.33 | 0.46 |
| Marital Status Single (14) Married (42) | 141 ± 31 132 ± 36 | 0.41 |
| Surgical treatments No (42) Yes (13) | 137 ± 35 123 ± 36 | 0.21 |
| Number of flares in the last year ≤2 (20) >2 (36) | 145 ± 31 128 ± 36 | 0.008 |
| Number of hospital admissions in the last year None (25) ≥1 (31) | 134 ± 31 134 ± 39 | 0.99 |
| Extraintestinal diseases No (45) Yes (11) | 134 ± 36 135 ± 34 | 0.9 |
| Type of IBD Ulcerative colitis (22) Crohn’s disease (32) Indeterminate colitis (2) | 150.55 ± 32 121.5 ± 33 150.5 ± 36 | 0.007 0.009 0.006 0.8 |
| Time from diagnosis | –0.05 ± 0.03 | 0.05 |
| Ulcerative colitis extension E1 (3) E2 (4) E3 (15) | 161 124 ± 42 93 ± 21 | 0.14 |
| Ulcerative colitis severity S1 (15) S2 (6) S3 (1) | 151 ± 32 151.5 ± 38 143 | 0.97 |
| Mayo index | –3.1 ± 3.2 | 0.35 |
| Crohn’s phenotype B1 (18) B2 Stenosing (8) B3 Penetrating (6) | 117 ± 28 117 ± 43 140 ± 29 | 0.319 |
| Perianal Crohn’s disease No (29) Yes (3) | 120.5 ± 32 131 ± 49 | 0.6 |
| Colonic Crohn’s disease None (19) Colonic Crohn’s (13) | 126 ± 29 115 ± 39 | 0.29 |
| Harvey–Bradshaw index | –5.7 ± 1.5 | 0.001 |
| Extraintestinal diseases No (45) Yes (11) | 134 ± 36 135 ± 34 | 0.89 |
| Articular disease No (50) Yes (6) | 135 ± 36 124 ± 28 | 0.46 |
| Crohn’s Disease (n = 32) | Ulcerative Colitis (n = 22) | p | |
|---|---|---|---|
| Age, Years | 43 ± 13 | 48 ± 15 | 0.21 |
| Sex | |||
| Male | 53% | 46% | 0.58 |
| Female | 47% | 54% | |
| Educational | |||
| None | 6% | 5% | |
| Primary | 22% | 23% | 0.82 |
| Secondary | 38.50% | 27% | |
| University or Higher | 34% | 46% | |
| Occupation | |||
| Employee | 56.30% | 73% | |
| Unemployed | 15.60% | 13.60% | 0.26 |
| Retired | 28.10% | 13.60% | |
| Marital Status | |||
| Partner | 71.90% | 72.30% | 0.65 |
| Single | 28.10% | 22.30% | |
| Previous surgery | 34.40% | 9.10% | 0.033 |
| PCR | 7.15 ± 4.75 | 4.11 ± 3.21 | 0.35 |
| Calprotectin | 1121 ± 608 | 3909 ± 1338 | 0.075 |
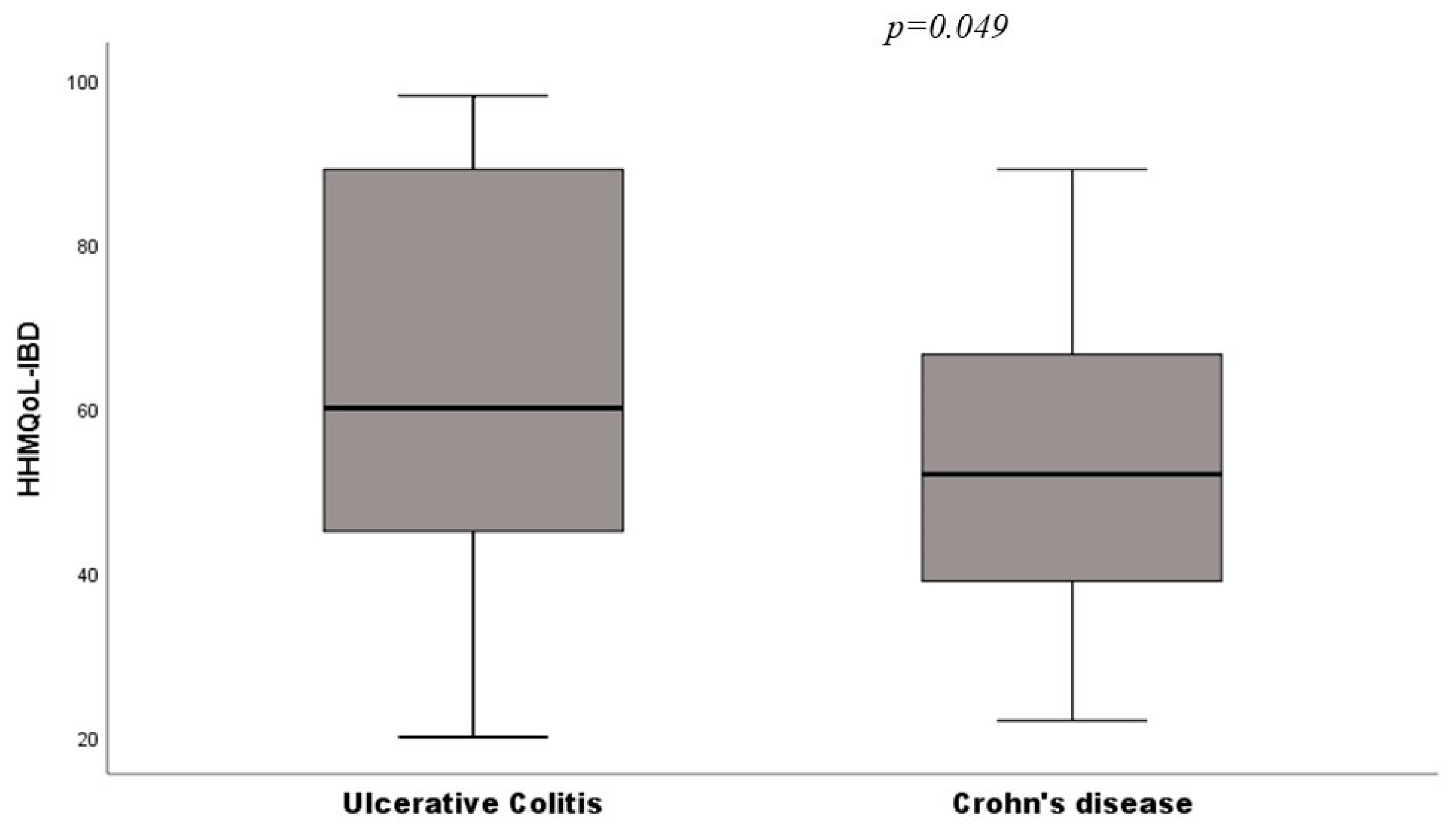
| HHMQoL-IBD | 53 ± 18 | 62 ± 23 | 0.049 |
| IBDQ Score | 121.5 ± 33 | 150.5 ± 32 | 0.002 |
| N° Flares since diagnosis | 5.5 ± 4.4 | 3.8 ± 3.3 | 0.024 |
| N° Hospital Admissions since diagnosis | 2.1 ± 3.1 | 0.689 ± 0.99 | 0.13 |
| Steroid response | |||
| Steroid refractory | 4% | 0% | |
| Steroid dependency | 22% | 23% | 0.33 |
| Number of cohabitants | |||
| 1 | 59% | 77% | |
| 2 | 31% | 9% | 0.17 |
| ≥3 | 9% | 14% |
| Variables (n) | HHMQoL-IBD (Mean ± SD) | p |
|---|---|---|
| Sex | ||
| Female (45) | 56 ± 18 | 0.09 |
| Male (36) | 56 ± 22 | |
| Age | –0.14 ± 0.13 | 0.68 |
| Marital Status | ||
| Single (24) | 57 ±19 | 0.65 |
| Married (58) | 55 ± 20 | |
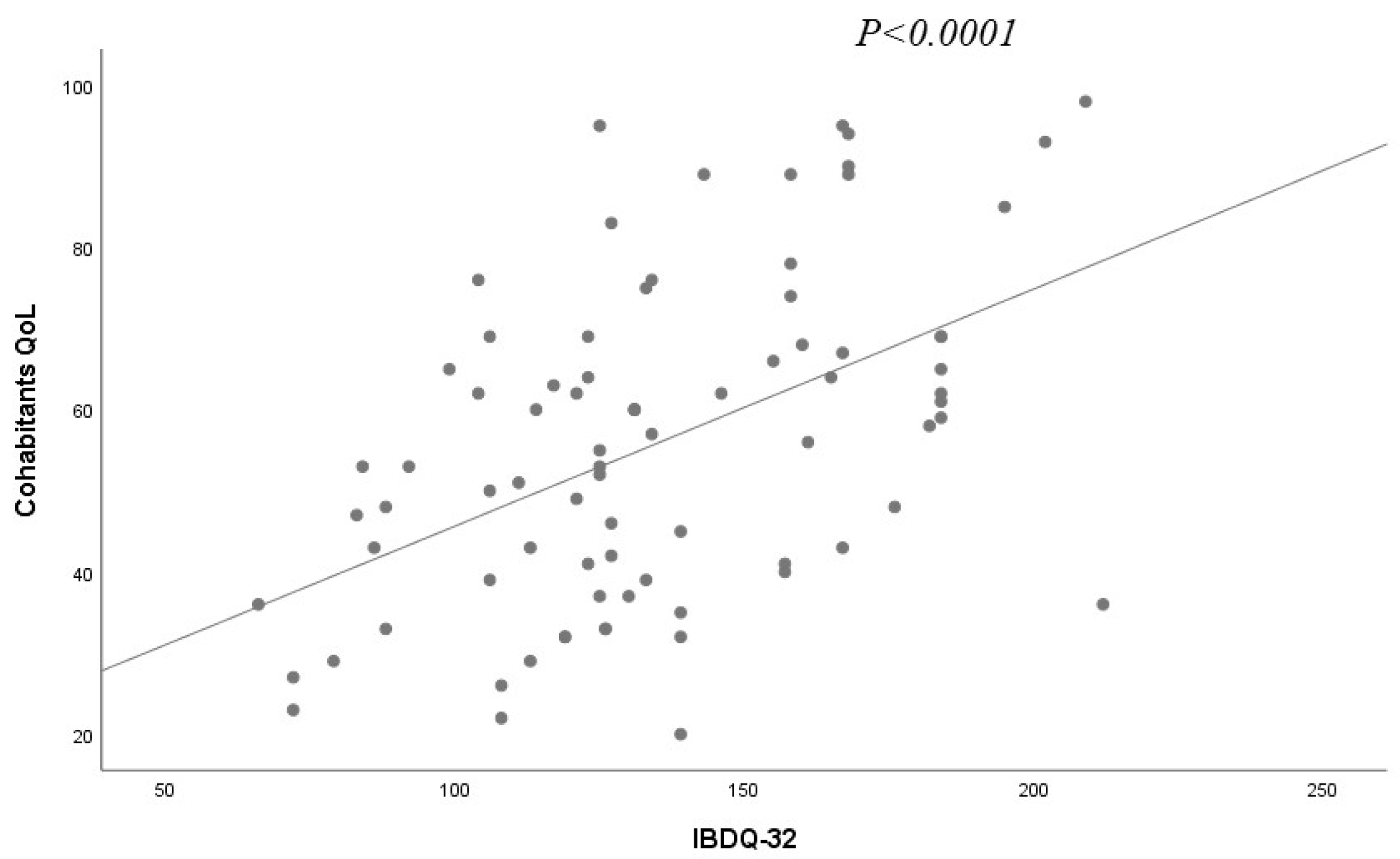
| IBDQ 32 (patients) | 0.28 ± 0.05 | <0.0001 |
| Surgical treatments | ||
| No (59) | 56 ± 21 | 0.7 |
| Yes (21) | 55 ± 17 | |
| Number of flares in the last year | ||
| ≤2 (25) | 58 ± 22 | 0.52 |
| >2 (56) | 55 ± 19 | |
| Number of hospital admissions in the last year | ||
| None (33) | ||
| ≥1 (48) | 53 ± 22 | 0.2 |
| 58 ± 18 | ||
| Extraintestinal diseases | ||
| No (66) | 59 ± 19 | 0.01 |
| Yes (15) | 44 ± 19 | |
| Type of IBD | ||
| Ulcerative colitis (29) | 62 ± 23 | 0.15 |
| Crohn’s disease (59) | 53 ± 8 | |
| Indeterminate colitis (2) | 50.5 ± 3.5 | |
| Ulcerative colitis extension | ||
| E1 (3) | 59 ± 27 | |
| E2 (7) | 74 ± 32 | 0.27 |
| E3 (19) | 58 ± 17 | |
| Ulcerative colitis severity | ||
| S1 (19) | 25 ± 6 | 0.2 |
| S2 (9) | 52 ± 12 | |
| S3 (1) | ||
| Mayo index | –2.68 ± 1-18 | 0.16 |
| Crohn’s phenotype | ||
| B1 (27) | 52 ± 18 | |
| B2 Stenosing (10) | 44 ± 20 | 0.075 |
| B3 Penetrating (13) | 61 ± 13 | |
| Harvey–Bradshaw index | –1.46 ± 0.75 | 0.056 |
| Perianal Crohn’s disease | ||
| No (42) | 51.5 ± 19 | 0.12 |
| Yes (8) | 59.5 ± 12 | |
| Colonic Crohn’s disease | ||
| None (34) | 55 ± 17 | 0.12 |
| Yes (16) | 47 ± 19 | |
| Articular disease | ||
| No (52) | 57.5 ± 20 | 0.04 |
| Yes (9) | 43 ± 17 |
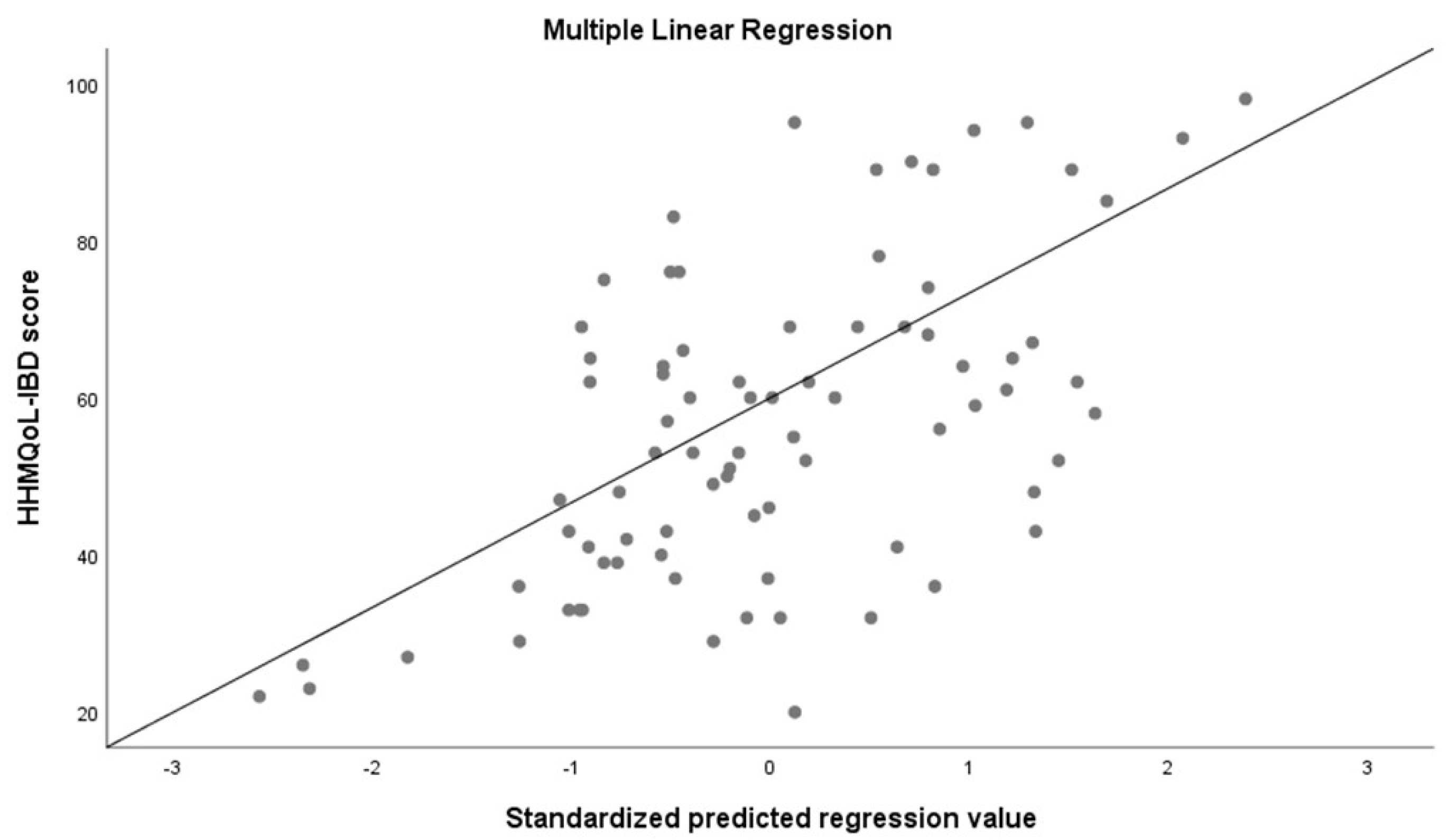
| Variables | Standardized Coefficients (Beta) | p |
|---|---|---|
| Sex | ||
| Male | −0.069 | 0.51 |
| Female | Ref | |
| Age | −0.038 | 0.78 |
| Marital Status | ||
| Married | −0.098 | 0.47 |
| Single | Ref | |
| IBDQ 32 (patients) | 0.064 | <0.0001 |
| Time from diagnosis | −0.018 | 0.867 |
| Education | ||
| University | 0.015 | 0.894 |
| Other (none, primary, secondary) | Ref. | |
| Patient’s occupation | ||
| Active | −0.087 | 0.431 |
| Unemployed | Ref. | |
| Last CRP | −0.084 | 0.433 |
| Number of cohabitants | 0.086 | 0.452 |
| Extraintestinal diseases | −0.21 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Vico, M.; Sánchez-Capilla, A.D.; Redondo-Cerezo, E. Quality of Life in Cohabitants of Patients Suffering Inflammatory Bowel Disease: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 115. https://doi.org/10.3390/ijerph19010115
López-Vico M, Sánchez-Capilla AD, Redondo-Cerezo E. Quality of Life in Cohabitants of Patients Suffering Inflammatory Bowel Disease: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(1):115. https://doi.org/10.3390/ijerph19010115
Chicago/Turabian StyleLópez-Vico, Manuel, Antonio D. Sánchez-Capilla, and Eduardo Redondo-Cerezo. 2022. "Quality of Life in Cohabitants of Patients Suffering Inflammatory Bowel Disease: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 1: 115. https://doi.org/10.3390/ijerph19010115
APA StyleLópez-Vico, M., Sánchez-Capilla, A. D., & Redondo-Cerezo, E. (2022). Quality of Life in Cohabitants of Patients Suffering Inflammatory Bowel Disease: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(1), 115. https://doi.org/10.3390/ijerph19010115





