Diagnostic Accuracy of 10/66 Dementia Protocol in Fijian-Indian Elders Living in New Zealand
Abstract
1. Introduction
2. Materials and Methods
2.1. Index Test: 10/66 Dementia Protocol
Translation and Adaptation of the 10/66 Dementia Protocol
2.2. Validity Study
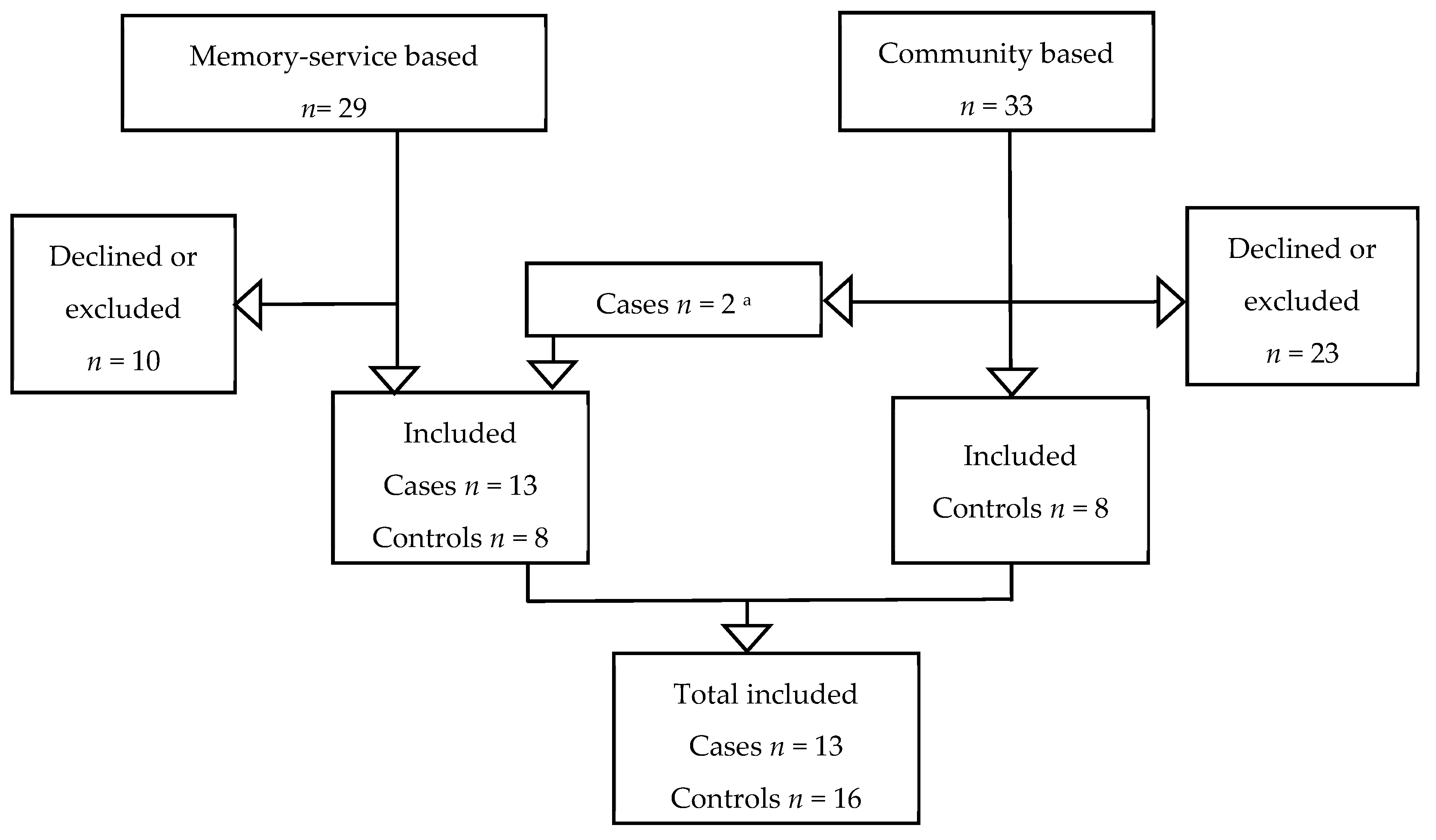
2.2.1. Settings and Participants
2.2.2. Memory Service-Based Participants
Eligibility Criteria for Memory Service-Based Dementia Cases
Eligibility Criteria for Memory Service-Based Controls
2.2.3. Community-Based Participants
Eligibility Criteria for Community-Based Controls
2.2.4. Participants Unable to Give Informed Consent
2.2.5. Informants
2.2.6. Blinding
2.2.7. Interviewers
2.2.8. Interviewing Process
2.3. Data Analysis
2.3.1. Dementia 10/66 Diagnosis
2.3.2. Statistical Analysis
3. Results
3.1. Translation and Adaptation
3.2. Demographic Characteristics
3.3. Diagnostic Test Accuracy
3.3.1. 10/66 Dementia Diagnosis
3.3.2. 10/66 Dementia Protocol Sub-Components Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia: A Public Health Priority; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and meta-analysis. Alzheimers Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Chiew, H.J.; Lim, L.; Rosa-Neto, P.; Kandiah, N.; Gauthier, S. The influence of language and culture on cognitive assessment tools in the diagnosis of early cognitive impairment and dementia. Expert Rev. Neurother. 2018, 18, 859–869. [Google Scholar] [CrossRef]
- Deloitte Report for Alzheimer’s New Zealand. Updated Dementia Economic Impact Report 2016. New Zealand. 2017. Available online: https://www.alzheimers.org.nz/getmedia/79f7fd09-93fe-43b0-a837-771027bb23c0/Economic-Impacts-of-Dementia-2017.pdf/ (accessed on 3 May 2021).
- Sharma, A. Tracing the Relational Ethic in the Postcolonial Life Writing of the Indo-Fijian Diaspora. Life Writ. 2018, 15, 273–283. [Google Scholar] [CrossRef]
- Mishra, S. The Leonidas Fijians: A minor history. J. Pac. His. 2014, 49, 283–300. [Google Scholar] [CrossRef]
- Ethnologue. Ethnologue: Languages of the World, 24th ed.; Eberhard, D.M., Simons, G.F., Fennig, C.D., Eds.; SIL International: Dallas, TX, USA, 2021. [Google Scholar]
- Siegel, J. Indian languages in Fiji: Past, present and future. South Asia J. South Asian Stud. 1998, 21, 181–214. [Google Scholar] [CrossRef]
- Weber, E. Looking north or looking anywhere? Indo-Fijian international relations after the coups of May 2000 and December 2006. Bandung J. Glob. South 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Madraiwiwi, J. The Fijian Elections of 2014: Returning to Democracy… ? J. Pac. His. 2015, 50, 54–60. [Google Scholar] [CrossRef]
- Hundt, M. Home Is Where You’re Born: Negotiating Identity in the Diaspora. Stud. Neophilol. 2014, 86, 125–137. [Google Scholar] [CrossRef][Green Version]
- Statistics New Zealand-Tatauranga Aotearoa. Statistics about Ethnicity Give Information by the Ethnic Groups that People Identify with or Feel They Belong to. New Zealand: Statistics New Zealand. 2018. Available online: https://www.stats.govt.nz/topics/ethnicity (accessed on 17 March 2021).
- Shipley, G.P.; Taylor, D.A.; Tyagi, A.; Tiwari, G.; Redd, A.J. Genetic structure among Fijian island populations. J. Hum. Genet. 2015, 60, 69–75. [Google Scholar] [CrossRef]
- Morrell, S.; Lin, S.; Tukana, I.; Linhart, C.; Taylor, R.; Vatucawaqa, P.; Magliano, D.J.; Zimmet, P. Diabetes incidence and projections from prevalence surveys in Fiji. Popul. Health Metric. 2016, 14, 45. [Google Scholar] [CrossRef]
- Lin, S.; Tukana, I.; Linhart, C.; Morrell, S.; Taylor, R.; Vatucawaqa, P.; Magliano, D.J.; Zimmet, P. Diabetes and obesity trends in Fiji over 30 years. J. Diabet. 2016, 8, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Collins, V.R.; Dowse, G.K.; Ram, P.; Cabealawa, S.; Zimmet, P.Z. Non-insulin-dependent diabetes and 11-year mortality in Asian Indian and Melanesian Fijians. Diabet. Med. 1996, 13, 125–132. [Google Scholar] [CrossRef]
- Thornley, S.; Chan, W.C.; Crengle, S.; Riddell, T.; Ameratunga, S.; Mehta, S.; Gentles, D.; Wells, S.; Marshall, R.; Jackson, R. Sociodemographic differences in prevalence of diagnosed coronary heart disease in New Zealand estimated from linked national health records. N. Z. Med. J. 2011, 124, 21–34. [Google Scholar] [PubMed]
- Prince, M.; Ferri, C.P.; Acosta, D.; Albanese, E.; Arizaga, R.; Dewey, M.; Gavrilova, S.I.; Guerra, M.; Huang, Y.; Jacob, K.S.; et al. The protocols for the 10/66 dementia research group population-based research programme. BMC Public Health 2007, 7, 165. [Google Scholar] [CrossRef]
- Prince, M.J. The 10/66 dementia research group—10 years on. Indian J. Psychiatry 2009, 51 (Suppl. 1), S8–S15. [Google Scholar] [PubMed]
- Prince, M.; Acosta, D.; Chiu, H.; Scazufca, M.; Varghese, M. Dementia diagnosis in developing countries: A cross-cultural validation study. Lancet 2003, 361, 909–917. [Google Scholar] [CrossRef]
- Phung, K.T.; Chaaya, M.; Waldemar, G.; Atweh, S.; Asmar, K.; Ghusn, H.; Karam, G.; Sawaya, R.; Khoury, R.M.; Zeinaty, I.; et al. Validation of the 10/66 Dementia Research Group diagnostic assessment for dementia in Arabic: A study in Lebanon. J. Geriatr. Psychiatry Neurol. 2014, 27, 282–290. [Google Scholar] [CrossRef]
- Khan, Q.U.A.; Prince, M.J.; Khalid, W.; Zulfiqar, M. Translation and Validation of 10/66 Dementia Diagnostic Battery in Urdu in Karachi, Pakistan. Alzheimer Dis. Assoc. Disord. 2020, 34, 163–169. [Google Scholar] [CrossRef]
- Copeland, J.R.; Dewey, M.E.; Griffiths-Jones, H.M. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol. Med. 1986, 16, 89–99. [Google Scholar] [CrossRef]
- Hall, K.S.; Gao, S.; Emsley, C.L.; Ogunniyi, A.O.; Morgan, O.; Hendrie, H.C. Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. Int. J. Geriatr. Psychiatry 2000, 15, 521–531. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar]
- Prince, M.J.; de Rodriguez, J.L.; Noriega, L.; Lopez, A.; Acosta, D.; Albanese, E.; Arizaga, R.; Copeland, J.R.M.; Dewey, M.; Ferri, C.P.; et al. The 10/66 Dementia Research Group’s fully operationalised DSM-IV dementia computerized diagnostic algorithm, compared with the 10/66 dementia algorithm and a clinician diagnosis: A population validation study. BMC Public Health 2008, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Luria, A.R. The Working Brain: An Introduction to Neuropsychology; Allen Lane: London, UK, 1978. [Google Scholar]
- Chisholm, D.; Knapp, M.R.; Knudsen, H.C.; Amaddeo, F.; Gaite, L.; van Wijngaarden, B. Client Socio-Demographic and Service Receipt Inventory—European Version: Development of an instrument for international research. EPSILON Study 5. European Psychiatric Services: Inputs Linked to Outcome Domains and Needs. Br. J. Psychiatry 2000, 177, s28–s33. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J. Assessing the multidimensionality of the 12-item General Health Questionnaire. Psychol. Rep. 1999, 84, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Beusenberg, M.; Orley, J.H.; World Health Organization. Division of Mental H. A User’s Guide to the Self-Reporting Questionnaire (SRQ/Compiled by M. beusenberg and J. orley; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Zarit, S.H.; Todd, P.A.; Zarit, J.M. Subjective burden of husbands and wives as caregivers: A longitudinal study. Gerontologist 1986, 26, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Zarit, S.H.; Reever, K.E.; Bach-Peterson, J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Whitlatch, C.J.; Zarit, S.H.; von Eye, A. Efficacy of interventions with caregivers: A reanalysis. Gerontologist 1991, 31, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dewey, M.E.; Copeland, J.R. Diagnosis of dementia from the history and aetiology schedule. Int. J. Geriatr. Psychiatry 2001, 16, 912–917. [Google Scholar] [CrossRef]
- Kaufer, D.I.; Cummings, J.L.; Ketchel, P.; Smith, V.; MacMillan, A.; Shelley, T.; Lopez, O.L.; DeKosky, S.T. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 233–239. [Google Scholar] [CrossRef]
- Shahwan, S.B.M.; Subramaniam, M.; Vaingankar, J.; Ann, C.S. Translation of the WHO CIDI into Bahasa Melayu. J. Trop. Psychol. 2015, 5, e3. [Google Scholar] [CrossRef]
- World Health Organization. Process of Translation and Adaptation of Instruments 2020. Available online: https://www.who.int/substance_abuse/research_tools/translation/en/ (accessed on 17 March 2021).
- Bujang, M.A.; Adnan, T.H. Requirements for Minimum Sample Size for Sensitivity and Specificity Analysis. J. Clin. Diagn. Res. 2016, 10, YE01–TE06. [Google Scholar] [CrossRef] [PubMed]
- Cullum, S.; Mullin, K.; Zeng, I.; Yates, S.; Payman, V.; Fisher, M.; Cheung, G. Do community-dwelling Māori and Pacific peoples present with dementia at a younger age and at a later stage compared with NZ Europeans? Int. J. Geriatr. Psychiatry 2018, 33, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mohs, R.C.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Roman, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.-M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef]
- The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 1994, 57, 416–418. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Storey, J.E.; Rowland, J.T.; Basic, D.; Conforti, D.A.; Dickson, H.G. The Rowland Universal Dementia Assessment Scale (RUDAS): A multicultural cognitive assessment scale. Int. Psychogeriatr. 2004, 16, 13–31. [Google Scholar] [CrossRef]
- Jorm, A.F.; Korten, A.E. Assessment of cognitive decline in the elderly by informant interview. Br. J. Psychiatry 1988, 152, 209–213. [Google Scholar] [CrossRef]
- Jorm, A.F. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol. Med. 1994, 24, 145–153. [Google Scholar] [CrossRef]
- Jorm, A.F.; Jacomb, P.A. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Socio-demographic correlates, reliability, validity and some norms. Psychol. Med. 1989, 19, 1015–1022. [Google Scholar] [CrossRef]
- Cheung, G.; Clugston, A.; Croucher, M.; Malone, D.; Mau, E.; Sims, A.; Gee, S. Performance of three cognitive screening tools in a sample of older New Zealanders. Int. Psychogeriatr. 2015, 27, 981–989. [Google Scholar] [CrossRef]
- Jorm, A.F. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): A review. Int. Psychogeriatr. 2004, 16, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, R.M.; Haider, S.; Tomlinson, G.; Alibhai, S. Cognitive assessments in multicultural populations using the Rowland Universal Dementia Assessment Scale: A systematic review and meta-analysis. CMAJ 2015, 187, E169–E175. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.K.; Fearon, P.; Noel-Storr, A.H.; McShane, R.; Stott, D.J.; Quinn, T.J. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Datab. Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.R.; Phung, T.K.; Chaaya, M.; Mackinnon, A.; Waldemar, G. Combining the Rowland Universal Dementia Assessment Scale and the Informant Questionnaire on Cognitive Decline in the Elderly to Improve Detection of Dementia in an Arabic-Speaking Population. Dement. Geriatr. Cogn. Disord. 2016, 41, 46–54. [Google Scholar] [CrossRef]
- Rowland, J.T.; Basic, D.; Storey, J.E.; Conforti, D.A. The Rowland Universal Dementia Assessment Scale (RUDAS) and the Folstein MMSE in a multicultural cohort of elderly persons. Int. Psychogeriatr. 2006, 18, 111–120. [Google Scholar] [CrossRef]
- Ozel-Kizil, E.T.; Turan, E.D.; Yilmaz, E.; Cangoz, B.; Uluc, S. Discriminant validity and reliability of the Turkish version of Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-T). Arch. Clin. Neuropsychol. 2010, 25, 139–145. [Google Scholar] [CrossRef]
- Goncalves, D.C.; Arnold, E.; Appadurai, K.; Byrne, G.J. Case finding in dementia: Comparative utility of three brief instruments in the memory clinic setting. Int. Psychogeriatr. 2011, 23, 788–796. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Lourenco, R.A. Screening for dementia: Brazilian version of the Informant Questionnaire on Cognitive Decline on the Elderly and its psychometric properties. Geriatr. Gerontol. Int. 2013, 13, 687–693. [Google Scholar] [CrossRef]
- Nielsen, T.R.; Andersen, B.B.; Gottrup, H.; Lutzhoft, J.H.; Hogh, P.; Waldemar, G. Validation of the Rowland Universal Dementia Assessment Scale for multicultural screening in Danish memory clinics. Dement. Geriatr. Cogn. Disord. 2013, 36, 354–362. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Sanchez, M.A. Accuracy of the Brazilian version of the informant questionnaire on cognitive decline in the elderly at screening for dementia in community-dwelling elderly participants: Findings from FIBRA-RJ study. J. Geriatr. Psychiatry Neurol. 2014, 27, 212–219. [Google Scholar] [CrossRef]
- Goudsmit, M.; van Campen, J.; Franzen, S.; van den Berg, E.; Schilt, T.; Schmand, B. Dementia detection with a combination of informant-based and performance-based measures in low-educated and illiterate elderly migrants. Clin. Neuropsychol. 2021, 660–678. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Code of Health and Disability Services Consumers’ Rights New Zealand: Health & Disability Commissioner/Te Toihau Hauora, Hauatanga. Available online: https://www.hdc.org.nz/your-rights/about-the-code/code-of-health-and-disability-services-consumers-rights (accessed on 17 March 2021).
- Liu, S.I.; Prince, M.; Chiu, M.J.; Chen, T.F.; Sun, Y.W.; Yip, P.K. Validity and reliability of a Taiwan Chinese version of the community screening instrument for dementia. Am. J. Geriatr. Psychiatry 2005, 13, 581–588. [Google Scholar] [CrossRef]
- Lopez, O.L.; Becker, J.T.; Klunk, W.; Saxton, J.; Hamilton, R.L.; Kaufer, D.I.; Sweet, R.A.; Cidis Meltzer, C.; Wisniewski, S.; Kamboh, M.I.; et al. Research evaluation and diagnosis of probable Alzheimer’s disease over the last two decades: I. Neurology 2000, 55, 1854–1862. [Google Scholar] [CrossRef]
- Beach, T.G.; Monsell, S.E.; Phillips, L.E.; Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012, 71, 266–273. [Google Scholar] [CrossRef]
- Takada, L.T.; Caramelli, P.; Fichman, H.C.; Porto, C.S.; Bahia, V.S.; Anghinah, R.; Carthery-Goulart, M.T.; Radanovic, M.; Smid, J.; Herrera, E., Jr.; et al. Comparison between two tests of delayed recall for the diagnosis of dementia. Arq. Neuropsiquiatr. 2006, 64, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sotaniemi, M.; Pulliainen, V.; Hokkanen, L.; Pirttilä, T.; Hallikainen, I.; Soininen, H.; Hänninen, T. CERAD-neuropsychological battery in screening mild Alzheimer’s disease. Acta Neurol. Scand. 2012, 125, 16–23. [Google Scholar] [CrossRef]
- Subramaniam, M.; Chong, S.A.; Vaingankar, J.A.; Abdin, E.; Chua, B.Y.; Chua, H.C.; Eng, G.K.; Heng, D.; Hia, S.B.; Huang, W.; et al. Prevalence of Dementia in People Aged 60 Years and Above: Results from the WiSE Study. J. Alzheimers Dis. 2015, 45, 1127–1138. [Google Scholar] [CrossRef]
- Nozari, N.; Ferri, C.P.; Farin, F.; Noroozian, M.; Salehi, M.; Seyedian, M.; Prince, M. Validation of the 10/66 Dementia Research Group’s 10/66 Dementia diagnosis in Iran. Int. Psychogeriatr. 2009, 21, 604–605. [Google Scholar] [CrossRef] [PubMed]
| Questionnaire | Section | Instruments Used |
|---|---|---|
| 1. Participant | 1.1 Clinical interview | GMS B3 [23] generates hierarchically organised ICD10 [27] and DSM-IV [28] diagnoses. |
| 1.2 Cognitive test battery | CSI-D participant version [24]; CERAD word list memory test (immediate and delayed recall) [25]; CERAD verbal fluency test [25]; Neurological examination—Palm-fist-hand test from the Luria battery of frontal lobe tasks [29]. | |
| 1.3 Sociodemographic status | Sociodemographic and risk factors questionnaire (participant version) [18,20]. | |
| 2. Informant | 2.1 Informant interview | Brief informant history from the CSI-D [24]; Client Service Receipt Inventory or CSRI [30]; Self-reported questionnaire [31,32]; The Zarit Burden Interview [33,34,35]; History and Aetiology Schedule [36]; Neuropsychiatric Inventory Questionnaire or NPI-Q [37]. |
| 2.2 Sociodemographic status | Sociodemographic and risk factors questionnaire (proxy version) * [18,20]. | |
| 3. Household | 3.1 Head of household questionnaire | Questions about house and family income [18,20]. |
| Author, Year | Storey, 2004 [47] | Rowland, 2006 [56] | Ozel-Kizil, 2010 [57] | Goncalves, 2011 b [58] | Sanchez, 2013 [59] | Nielsen, 2013 [60] | Lourenço, 2014 [61] | Cheung, 2015 [51] | Nielsen, 2016 b [55] | Goudsmit, 2020 b [62] |
|---|---|---|---|---|---|---|---|---|---|---|
| Place, ethnic background | Australia Multiple | Australia, Multiple | Turkey, Turkish | Australia, Multiple | Brazil, Brazilian | Denmark, Multiple | Brazil, Brazilian | New Zealand, Multiple | Lebanon, Arabic | The Netherlands, Dutch |
| Reference standard | DSM-IV criteria | DSM-IV criteria | DSM-IV-TR criteria | DSM-IV-TR criteria | DSM-IV and clinical diagnosis | DSM-IV-TR criteria | DSM-IV criteria | Clinical diagnosis in conjunction with MMSE | DSM-IV criteria | DSM-IV-TR criteria |
| Total sample size | 90 | 111 | 220 | 204 | 169 | 142 | 406 | 84 | 225 | 109 |
| RUDAS | ||||||||||
| Cut-off point | <23 | <23 | <21 | <24 | <23 | <23 | <23 | |||
| Sensitivity | 89% | 81% | 66% | 69% | 78.40% | 82% | 80% | |||
| Specificity | 98% | 95.80% | 90% | 80% | 85.10% | 84% | 59% | |||
| IQCODE | ||||||||||
| Cut-off point | >3.4 | >4.1 | >3.51 | >3.26 | >3.34 | >3.7 | ||||
| Sensitivity | 82% | 72% | 83.30% | 89% | 92% | 80% | ||||
| Specificity | 70% | 67% | 80.70% | 72% | 96% | 74% | ||||
| Combined a | ||||||||||
| Sensitivity | 86% | |||||||||
| Specificity | 97% | |||||||||
| Variable | No Clinical Dementia Diagnosis n = 16 (%) | Clinical Dementia Diagnosis n = 13 (%) | p-Value |
|---|---|---|---|
| Mean age (SD) | 72.6 (SD ± 5.0) | 77.8 (SD ± 7.3) | 0.03 |
| Sex (f) | 5 (31.3) | 4 (30.8) | 0.64 a |
| Marital Status | |||
| Married/cohabitating | 13 (81.2) | 9 (69.2) | 0.37 a |
| Other | 3 (18.8) | 4 (30.8) | |
| Education level | |||
| None | 1 (6.2) | 0 (0) | 0.38 |
| Primary not completed | 3 (18.8) | 6 (46.2) | |
| Primary completed | 7 (43.8) | 4 (30.8) | |
| Secondary or above | 5 (31.2) | 3 (23.0) | |
| Informant mean age (SD) | 62.7 (SD ± 16.5) | 58.7 (SD ± 21.6) | 0.57 |
| Informant sex (f) | 10 (62.5) | 10 (76.9) | 0.33 a |
| Informant relationship with participant | |||
| Spouse/partner | 12 (75) | 10 (76.9) | 0.62 a |
| Other | 4 (25) | 3 (23.1) |
| (±SD) | p Value | ||
|---|---|---|---|
| CSI-D COGSCORE | 30.0 (±4.0) | 21.0 (±9.7) | 0.002 |
| CSI-D DFSCORE | 0.17 (±0.18) | 0.64 (±0.35) | 0 |
| CERAD delayed recall | 5.19 (±2.3) | 0.92 (±1.3) | 0 |
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | LR+ (95% CI) | LR− (95% CI) | Youden’s Index | AUROC (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| 10/66 global assessment | 92.3 | 93.8 | 92.3 | 93.8 | 14.7 | 0.08 | 0.86 | |
| (70.3–99.5) | (75.3–99.6) | (70.3–99.5) | (75.3–99.6) | (2.2–99.1) | (0.01–0.54) | |||
| Sub-assessments | ||||||||
| CSI-D COGSCORE | 92.3 | 81.3 | 80 | 92.9 | 4.9 | 0.09 | 0.73 | 0.89 |
| (70.3–99.5) | (58.3–95.0) | (56.0–94.6) | (72.1–99.6) | (1.7–13.8) | (0.01–0.63) | (0.77–0.99) | ||
| CSI-D DFSCORE | 100 | 93.8 | 92.9 | 100 | 16 | 0 | 0.93 | 0.95 |
| (75.3–99.6) | (72.1–99.6) | (2.3–106.7) | (0.85–0.99) | |||||
| CERAD | 76.9 | 87.5 | 83.3 | 82.4 | 6.1 | 0.26 | 0.64 | 0.93 |
| delayed recall | (50.5–93.7) | (66.2–97.8) | (56.9–97.0) | (60.4–95.3) | (1.6–23.2) | (0.09–0.72) | (0.83–0.99) |
| Author, Year | Present Study | Khan, 2020 [22] | Phung, 2015 [21] | Subramaniam, 2015 [70] | Nozari, 2009 a [71] | Prince, 2008 [26] | Prince, 2003 [20] |
|---|---|---|---|---|---|---|---|
| Population | New Zealand | Pakistan | Lebanon | Singapore | Iran | Cuba | India, China, Taiwan, Nigeria, Latin America |
| Language | Fijian Indian | Urdu | Arabic | English, Chinese, Malay, and Tamil | Farsi | Spanish | Translations into all local languages |
| Reference standard | Clinical diagnosis | Clinical diagnosis | Clinical diagnosis | Clinical | Clinical diagnosis | Clinical diagnosis | Clinical |
| diagnosis | diagnosis | ||||||
| Sample Size (n) | 29 | 257 | 244 | 2421 | 120 | 1887 | 2885 |
| 10/66 global assessment | |||||||
| Sensitivity | 92.30% | 70.30% | 92% | 95.60% | 98.30% | 93.20% | 94% |
| Specificity | 93.80% | 91.70% | 95.10% | 81.80% | 98.30% | 96.80% | 94% b |
| CSI-D COGSCORE | |||||||
| Sensitivity | 92.30% | 86.70% | 98% | 98.30% | 92% | ||
| Specificity | 81.30% | 72.10% | 49.30% | 81.70% | |||
| CSI-D DFSCORE | |||||||
| Sensitivity | 100% | 71.10% | 92% | 96.70% | 95% | ||
| Specificity | 93.80% | 96.10% | 90.30% | 96.70% | |||
| CERAD delayed recall | |||||||
| Sensitivity | 76.90% | 85.90% | 91% | 93.3% b | |||
| Specificity | 87.50% | 62.20% | 67.40% | 90.0% b | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Ruiz, A.; Krishnamurthi, R.; Dahiya, E.S.; Rai-Bala, R.; Naicker, S.; Yates, S.; Rivera Rodriguez, C.; Cheung, G.; Dudley, M.; Kerse, N.; et al. Diagnostic Accuracy of 10/66 Dementia Protocol in Fijian-Indian Elders Living in New Zealand. Int. J. Environ. Res. Public Health 2021, 18, 4870. https://doi.org/10.3390/ijerph18094870
Martinez-Ruiz A, Krishnamurthi R, Dahiya ES, Rai-Bala R, Naicker S, Yates S, Rivera Rodriguez C, Cheung G, Dudley M, Kerse N, et al. Diagnostic Accuracy of 10/66 Dementia Protocol in Fijian-Indian Elders Living in New Zealand. International Journal of Environmental Research and Public Health. 2021; 18(9):4870. https://doi.org/10.3390/ijerph18094870
Chicago/Turabian StyleMartinez-Ruiz, Adrian, Rita Krishnamurthi, Ekta Singh Dahiya, Reshmi Rai-Bala, Sanjalin Naicker, Susan Yates, Claudia Rivera Rodriguez, Gary Cheung, Makarena Dudley, Ngaire Kerse, and et al. 2021. "Diagnostic Accuracy of 10/66 Dementia Protocol in Fijian-Indian Elders Living in New Zealand" International Journal of Environmental Research and Public Health 18, no. 9: 4870. https://doi.org/10.3390/ijerph18094870
APA StyleMartinez-Ruiz, A., Krishnamurthi, R., Dahiya, E. S., Rai-Bala, R., Naicker, S., Yates, S., Rivera Rodriguez, C., Cheung, G., Dudley, M., Kerse, N., & Cullum, S. (2021). Diagnostic Accuracy of 10/66 Dementia Protocol in Fijian-Indian Elders Living in New Zealand. International Journal of Environmental Research and Public Health, 18(9), 4870. https://doi.org/10.3390/ijerph18094870







