Time Trends in Racial/Ethnic Differences in COVID-19 Infection and Mortality
Abstract
1. Introduction
2. Materials & Methods
2.1. Data and Sample
2.2. Measures
2.3. Analysis
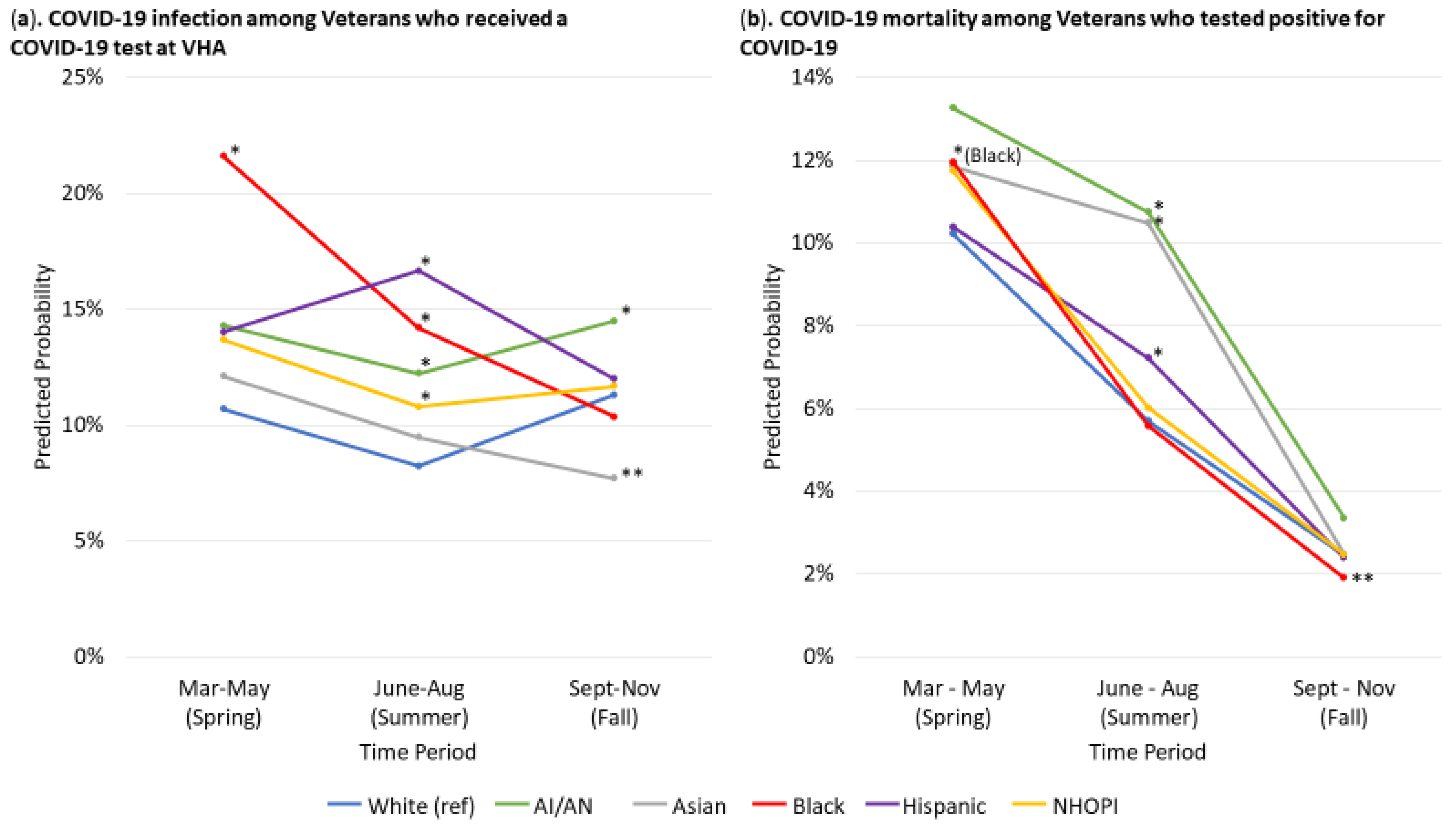
3. Results
3.1. COVID-19 Infection
3.2. Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mackey, K.; Ayers, C.K.; Kondo, K.K.; Saha, S.; Advani, S.M.; Young, S.; Spencer, H.; Rusek, M.; Anderson, J.; Veazie, S.; et al. Racial and Ethnic Disparities in COVID-19–Related Infections, Hospitalizations, and Deaths. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Zylla, E.; Bernard, S. Advances in States’ Reporting of COVID-19 Health Equity Data. 2020. Available online: https://www.shvs.org/advances-in-states-reporting-of-covid-19-health-equity-data/ (accessed on 3 March 2021).
- Centers for Disease Control and Prevention (CDC). CDC COVID-19 Response Health Equity Strategy: Accelerating Progress Towards Reducing COVID-19 Disparities and Achieving Health Equity. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/downloads/community/CDC-Strategy.pdf (accessed on 4 March 2021).
- Peretz, P.J.; Islam, N.; Matiz, L.A. Community Health Workers and Covid-19—Addressing Social Determinants of Health in Times of Crisis and Beyond. N. Engl. J. Med. 2020, 383, e108. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.I.; Castañeda, H. Addressing Health Disparities in the Rural United States: Advocacy as Caregiving among Community Health Workers and Promotores de Salud. Int. J. Environ. Res. Public Health 2020, 17, 9223. [Google Scholar] [CrossRef]
- Stracqualursi, V. Birx Warns US in “in a New Phase” of Coronavirus Pandemic with More Widepsread Cases. CNN Politics 2020. Available online: https://www.cnn.com/2020/08/02/politics/birx-coronavirus-new-phase-cnntv/index.html (accessed on 8 January 2021).
- The Lancet Infectious Diseases. Curing COVID-19. Lancet Infect. Dis. 2020, 20, 1101. [Google Scholar] [CrossRef]
- Harris, R. How Will the Limited Supply of Antibody Drugs for COVID-19 be Allocated. NPR 2020. Available online: https://www.npr.org/sections/health-shots/2020/10/21/926376342/how-will-the-limited-supply-of-antibody-drugs-for-covid-19-be-allocated (accessed on 8 March 2021).
- Rivera, D.R.; Peters, S.; A Panagiotou, O.; Shah, D.P.; Kuderer, N.M.; Hsu, C.-Y.; Rubinstein, S.M.; Lee, B.J.; Choueiri, T.K.; de Lima Lopes, G., Jr.; et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: A COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov. 2020, 10, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, C.; Garuccio, J.; Le, A.; Pinkston, J.; Yelowitz, A. Strong Social Distancing Measures in The United States Reduced The COVID-19 Growth Rate. Health Aff. 2020, 39, 1237–1246. [Google Scholar] [CrossRef]
- Williams, J.C.; Anderson, N.; Holloway, T.; Samford, E., III; Eugene, J.; Isom, J. Reopening the United States: Black and Hispanic Workers Are Essential and Expendable Again. Am. J. Public Health 2020, 110, 1506–1508. [Google Scholar] [CrossRef]
- Garnier, R.; Benetka, J.R.; Kraemer, J.; Bansal, S. Socio-economic disparities in social distancing during the COVID-19 pandemic in the United States. medRxiv Prepr. Serv. Health Sci. 2020. [Google Scholar] [CrossRef]
- The New York Times. Coronavirus in the U.S.: Latest Map and Case Count. The New York Times. New York 2021. Available online: https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html (accessed on 26 February 2021).
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.B.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019): People Who Need to Take Extra Precautions. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html (accessed on 1 July 2020).
- Killerby, M.E.; Link-Gelles, R.; Haight, S.C.; Schrodt, C.A.; England, L.; Gomes, D.J.; Shamout, M.; Pettrone, K.; O’Laughlin, K.; Kimball, A.; et al. Characteristics Associated with Hospitalization Among Patients with COVID-19—Metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 790–794. [Google Scholar] [CrossRef]
- Breland, J.Y.; Wong, M.S.; Steers, W.N.; Yuan, A.H.; Haderlein, T.P.; Washington, D.L. BMI and Risk for Severe COVID-19 Among veterans Health Administration Patients. Obesity 2021. [Google Scholar] [CrossRef]
- Muller, C.J.; MacLehose, R.F. Estimating predicted probabilities from logistic regression: Different methods correspond to different target populations. Int. J. Epidemiol. 2014, 43, 962–970. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Kidwai-Khan, F.; Tate, J.P.; Park, L.S.; King, J.T., Jr.; Skanderson, M.; Hauser, R.G.; Schultze, A.; Jarvis, C.I.; Holodniy, M.; et al. Covid-19 by Race and Ethnicity: A National Cohort Study of 6 Million United States veterans. medRxiv 2020. [Google Scholar] [CrossRef]
- Haderlein, T.P.; Wong, M.S.; Yuan, A.; Llorente, M.D.; Washington, D.L. Association of PTSD with COVID-19 testing and infection in the veterans Health Administration. J. Psychiatr. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Hoggatt, K.J.; Steers, W.N.; Frayne, S.M.; Huynh, A.K.; Yano, E.M.; Saechao, F.S.; Ziaeian, B.; Washington, D.L. Racial/Ethnic Disparities in Mortality Across the veterans Health Administration. Health Equity 2019, 3, 99–108. [Google Scholar] [CrossRef]
- Peterson, K.; Anderson, J.; Boundy, E.; Ferguson, L.; McCleery, E.; Waldrip, K. Mortality Disparities in Racial/Ethnic Minority Groups in the veterans Health Administration: An Evidence Review and Map. Am. J. Public Health 2018, 108, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Washington, D.L.; Villa, V.; Brown, A.; Damron-Rodriguez, J.; Harada, N. Racial/ethnic variations in veterans’ ambulatory care use. Am. J. Public Health 2005, 95, 2231–2237. [Google Scholar] [CrossRef]
- Rose, D.E.; Farmer, M.M.; Yano, E.M.; Washington, D.L. Racial/ethnic differences in cardiovascular risk factors among women veterans. J. Gen. Intern. Med. 2013, 28 (Suppl. 2), S524–S528. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, S.M.; Agnew-Brune, C.; Anderson, M.; Zambrano, L.D.; Rose, C.E.; Jim, M.A.; Baugher, A.; Liu, G.S.; Patel, S.V.; Evans, M.E.; et al. COVID-19 Among American Indian and Alaska Native Persons—23 States, January 31–July 3, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1166–1169. [Google Scholar] [CrossRef]
- Curtice, K.; Choo, E. Indigenous populations: Left behind in the COVID-19 response. Lancet 2020, 395, 1753. [Google Scholar] [CrossRef]
- Kakol, M.; Upson, D.; Sood, A. Susceptibility of Southwestern American Indian Tribes to Coronavirus Disease 2019 (COVID-19). J. Rural Health 2020. [Google Scholar] [CrossRef]
- Wong, M.S.; Steers, W.N.; Hoggatt, K.J.; Ziaeian, B.; Washington, D.L. Relationship of neighborhood social determinants of health on racial/ethnic mortality disparities in US veterans—Mediation and moderating effects. Health Serv. Res. 2020, 55, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gee, G.C.; Bahiru, E.; Yang, E.H.; Hsu, J.J. Asian-Americans and Pacific Islanders in COVID-19: Emerging Disparities Amid Discrimination. J. Gen. Intern. Med. 2020, 35, 3685–3688. [Google Scholar] [CrossRef] [PubMed]
- Louis-Jean, J.; Cenat, K.; Njoku, C.V.; Angelo, J.; Sanon, D. Coronavirus (COVID-19) and Racial Disparities: A Perspective Analysis. J. Racial Ethn. Health Disparities 2020, 7, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Veterans Health Administration (VHA). Coronavirus Disease 2019 (COVID-19) Response Report. 2020. Available online: https://www.va.gov/HEALTH/docs/VHA_COVID-19_Response_Report.pdf (accessed on 15 February 2021).
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
| Tested (n = 705,715) | Tested Positive (n = 83,542) | |
|---|---|---|
| Race/Ethnicity, % | ||
| Non-Hispanic White | 61.8 | 56.1 |
| AI/AN | 0.7 | 0.8 |
| Asian | 1.1 | 0.8 |
| Non-Hispanic Black | 22.1 | 25.2 |
| Hispanic | 8.5 | 10.9 |
| NHOPI | 0.7 | 0.7 |
| Age, % | ||
| <60 years | 39 | 43.7 |
| 60–64 years | 11.7 | 10.1 |
| 65–69 years | 12 | 10.2 |
| 70–74 years | 19.4 | 17.1 |
| 75–79 years | 9 | 8.3 |
| 80+ years | 8.9 | 10.6 |
| Gender, % | ||
| Female | 10.9 | 10.1 |
| Male | 89.1 | 89.9 |
| CDC Co-morbidities, % | ||
| Asthma | 7.1 | 6.7 |
| Chronic Kidney Disease | 2 | 1.9 |
| Chronic pulmonary disease | 25 | 20.6 |
| Types 2 Diabetes | 33.5 | 35.6 |
| Heart Disease | 35.9 | 32.4 |
| Immunocompromised | 9.7 | 7.6 |
| Liver Disease | 10.5 | 8 |
| Obesity | 50.2 | 58 |
| Time Period of Visit, % | ||
| Spring (3/1/20–5/31/20) | 8.1 | 13.5 |
| Summer (6/1/20–8/31/20) | 36.2 | 33.1 |
| Fall (9/1/20–11/25/20) | 55.7 | 53.4 |
| Race/Ethnicity | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| White (n = 436,022) | AI/AN (n = 4860) | Asian (n = 7635) | Black (n = 155,787) | Hispanic (n = 59,740) | NHOPI (n = 5159) | ||
| Tested | |||||||
| Age categories, % | |||||||
| <60 years | 34.6 | 44.3 | 66.2 | 42.9 | 54.2 | 46 | <0.001 |
| 60–64 years | 10.2 | 12.8 | 7.6 | 17 | 9.8 | 11.1 | |
| 65–69 years | 11.7 | 11.9 | 8.3 | 14.4 | 9.3 | 12 | |
| 70–74 years | 22.5 | 18.1 | 8.6 | 14.1 | 13.3 | 16.5 | |
| 75–79 years | 10.7 | 7 | 4.4 | 6.1 | 5.9 | 7.5 | |
| 80+ years | 10.3 | 5.9 | 4.9 | 5.6 | 7.5 | 6.9 | |
| Gender, % | |||||||
| Female | 9 | 15.2 | 14.2 | 15.4 | 11.5 | 13.7 | <0.001 |
| Male | 91 | 84.8 | 85.8 | 84.6 | 88.5 | 86.3 | |
| CDC comorbidities, % | |||||||
| Asthma | 6.5 | 7.4 | 8.8 | 8.1 | 8.8 | 7.8 | <0.001 |
| Chronic kidney disease | 1.3 | 2.1 | 2.1 | 3.8 | 2.1 | 2.4 | <0.001 |
| Chronic pulmonary disease | 27.5 | 24.1 | 13.9 | 22.4 | 16.9 | 21 | <0.001 |
| Type 2 diabetes | 32.4 | 36.6 | 28.7 | 37.5 | 32.9 | 35.5 | <0.001 |
| Heart disease | 39.8 | 31.5 | 19.4 | 31.3 | 24.9 | 31.4 | <0.001 |
| Immunocompromised | 9.5 | 8.4 | 5.5 | 11.5 | 8 | 8.3 | <0.001 |
| Liver disease | 10.1 | 11.5 | 8.7 | 11.7 | 12.1 | 9.5 | <0.001 |
| Obesity | 50.4 | 53 | 34.3 | 49.9 | 52.4 | 52.6 | <0.001 |
| Time Period, % | |||||||
| Spring (3/1/2020–5/31/2020) | 8 | 9.2 | 7.2 | 9 | 7.3 | 7.2 | <0.001 |
| Summer (6/1/2020–8/31/2020) | 35.8 | 36.4 | 38.4 | 36.5 | 36.9 | 37.7 | |
| Fall (9/1/2020–11/25/2020) | 56.2 | 54.4 | 54.4 | 54.5 | 55.8 | 55.1 | |
| Tested positive | |||||||
| Age categories, % | |||||||
| <60 years | 37 | 47.6 | 72.5 | 48.3 | 63.7 | 48.4 | <0.001 |
| 60–64 years | 8.3 | 10.8 | 7.3 | 15 | 8.5 | 10 | |
| 65–69 years | 9.9 | 12.1 | 7.1 | 12.4 | 7.3 | 12.8 | |
| 70–74 years | 20.8 | 17.8 | 6.4 | 12.6 | 10.4 | 15.1 | |
| 75–79 years | 10.5 | 5.3 | 2.2 | 5.5 | 4.3 | 5.9 | |
| 80+ years | 13.4 | 6.3 | 4.5 | 6.4 | 5.8 | 7.9 | |
| Gender, % | |||||||
| Female | 8 | 15 | 13.7 | 14.7 | 10.3 | 11.1 | <0.001 |
| Male | 92 | 85 | 86.3 | 85.3 | 89.7 | 88.9 | |
| CDC comorbidities, % | |||||||
| Asthma | 6.2 | 7.7 | 7.9 | 7.7 | 7.2 | 7.3 | <0.001 |
| Chronic kidney disease | 1.2 | 2 | 0.9 | 3.6 | 1.5 | 2.4 | <0.001 |
| Chronic pulmonary disease | 23 | 20.9 | 12.7 | 19.2 | 12.8 | 16.5 | <0.001 |
| Type 2 diabetes | 34.6 | 36.8 | 25 | 40.2 | 32.5 | 39.9 | <0.001 |
| Heart disease | 37 | 28.3 | 19 | 28.6 | 19.7 | 27.8 | <0.001 |
| Immunocompromised | 7.1 | 6.2 | 5.1 | 9.9 | 5.7 | 5.5 | <0.001 |
| Liver disease | 7.3 | 8.7 | 8.8 | 9 | 9.8 | 5.3 | <0.001 |
| Obesity | 57.3 | 59 | 46.9 | 58.7 | 61.6 | 59.4 | <0.001 |
| Time Period, % | |||||||
| Spring (3/1/2020–5/31/2020) | 11.1 | 11.5 | 14.1 | 20.5 | 10.7 | 10.6 | <0.001 |
| Summer (6/1/2020–8/31/2020) | 28.5 | 33.2 | 37.8 | 38.6 | 43.2 | 34.4 | |
| Fall (9/1/2020–11/25/2020) | 60.4 | 55.3 | 48.2 | 40.9 | 46.1 | 55 | |
| COVID-19 Infection Proportion | Mortality Proportion | |||||||
|---|---|---|---|---|---|---|---|---|
| Time Period | Total | Time Period | Total | |||||
| Spring 3/1/20–5/31/20 | Summer 6/1/20–8/31/20 | Fall 9/1/20–11/25/20 | Spring 3/1/20–5/31/20 | Summer 6/1/20–8/31/20 | Fall 9/1/20–11/25/20 | |||
| Non-Hispanic White (ref) (n = 436,023) | 11.6 | 8.9 | 12.0 | 10.8 | 16.3 | 6.5 | 2.9 | 5.4 |
| AI/AN (n = 4860) | 14.1 | 13.7 ** | 15.2 ** | 14.6 ** | 16.7 | 9.9 * | 2.7 | 6.7 |
| Asian (n = 7635) | 13.1 | 9.4 | 8.5 ** | 9.2 ** | 7.8 * | 4.4 | 1.1 | 3.3 * |
| Non-Hispanic Black (n = 155,740) | 22.1 ** | 14.8 ** | 10.5 ** | 13.1 ** | 12.0 ** | 4.1 ** | 1.6 ** | 4.7 ** |
| Hispanic (n = 59,740) | 15.6 ** | 18.6 ** | 13.1 ** | 15.3 ** | 8.8 ** | 4.4 ** | 1.6 ** | 3.6 ** |
| NHOPI (n = 5159) | 13.6 | 11.5 ** | 12.6 | 12.2 * | 10.2 | 4.5 * | 2.8 | 4.2 |
| COVID-19 Infection Proportion | Mortality Proportion | |||||
|---|---|---|---|---|---|---|
| Spring 3/1/20–5/31/20 | Summer 6/1/20–8/31/20 | Fall 9/1/20–11/25/20 | Spring 3/1/20–5/31/20 | Summer 6/1/20–8/31/20 | Fall 9/1/20–11/25/20 | |
| Non-Hispanic White (ref) (n = 436,023) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| AI/AN (n = 4860) | 1.40 (0.90, 2.16) | 1.56 (1.31, 1.85) * | 1.33 (1.08, 1.64) * | 1.41 (0.70, 2.82) | 2.16 (1.17, 3.97) * | 1.40 (0.70, 2.81) |
| Asian (n = 7635) | 1.15 (0.76, 1.74) | 1.17 (0.89, 1.53) | 0.65 (0.48, 0.89) ** | 1.21 (0.57, 2.58) | 2.09 (1.09, 4.01) * | 1.01 (0.25, 4.09) |
| Non-Hispanic Black (n = 155,787) | 2.32 (1.95, 2.76) * | 1.85 (1.66, 2.07) * | 0.91 (0.81, 1.01) | 1.23 (1.04, 1.45) * | 0.98 (0.84, 1.14) | 0.77 (0.60, 0.98) ** |
| Hispanic (n = 59,740) | 1.37 (0.93, 2.02) | 2.24 (1.51, 3.32) * | 1.07 (0.86, 1.34) | 1.03 (0.70, 1.49) | 1.32 (1.06, 1.64) * | 0.97 (0.69, 1.36) |
| NHOPI (n = 5159) | 1.33 (0.93, 1.89) | 1.35 (1.10, 1.66) * | 1.04 (0.85, 1.27) | 1.20 (0.41, 3.52) | 1.07 (0.54, 2.09) | 1.00 (0.46, 2.20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, M.S.; Haderlein, T.P.; Yuan, A.H.; Moy, E.; Jones, K.T.; Washington, D.L. Time Trends in Racial/Ethnic Differences in COVID-19 Infection and Mortality. Int. J. Environ. Res. Public Health 2021, 18, 4848. https://doi.org/10.3390/ijerph18094848
Wong MS, Haderlein TP, Yuan AH, Moy E, Jones KT, Washington DL. Time Trends in Racial/Ethnic Differences in COVID-19 Infection and Mortality. International Journal of Environmental Research and Public Health. 2021; 18(9):4848. https://doi.org/10.3390/ijerph18094848
Chicago/Turabian StyleWong, Michelle S., Taona P. Haderlein, Anita H. Yuan, Ernest Moy, Kenneth T. Jones, and Donna L. Washington. 2021. "Time Trends in Racial/Ethnic Differences in COVID-19 Infection and Mortality" International Journal of Environmental Research and Public Health 18, no. 9: 4848. https://doi.org/10.3390/ijerph18094848
APA StyleWong, M. S., Haderlein, T. P., Yuan, A. H., Moy, E., Jones, K. T., & Washington, D. L. (2021). Time Trends in Racial/Ethnic Differences in COVID-19 Infection and Mortality. International Journal of Environmental Research and Public Health, 18(9), 4848. https://doi.org/10.3390/ijerph18094848






