Difficulties in Accessing Cancer Care in a Small Island State: A Community-Based Pilot Study of Cancer Survivors in Saint Lucia
Abstract
1. Introduction
2. Materials and Methods
2.1. Community-Based Collaboration
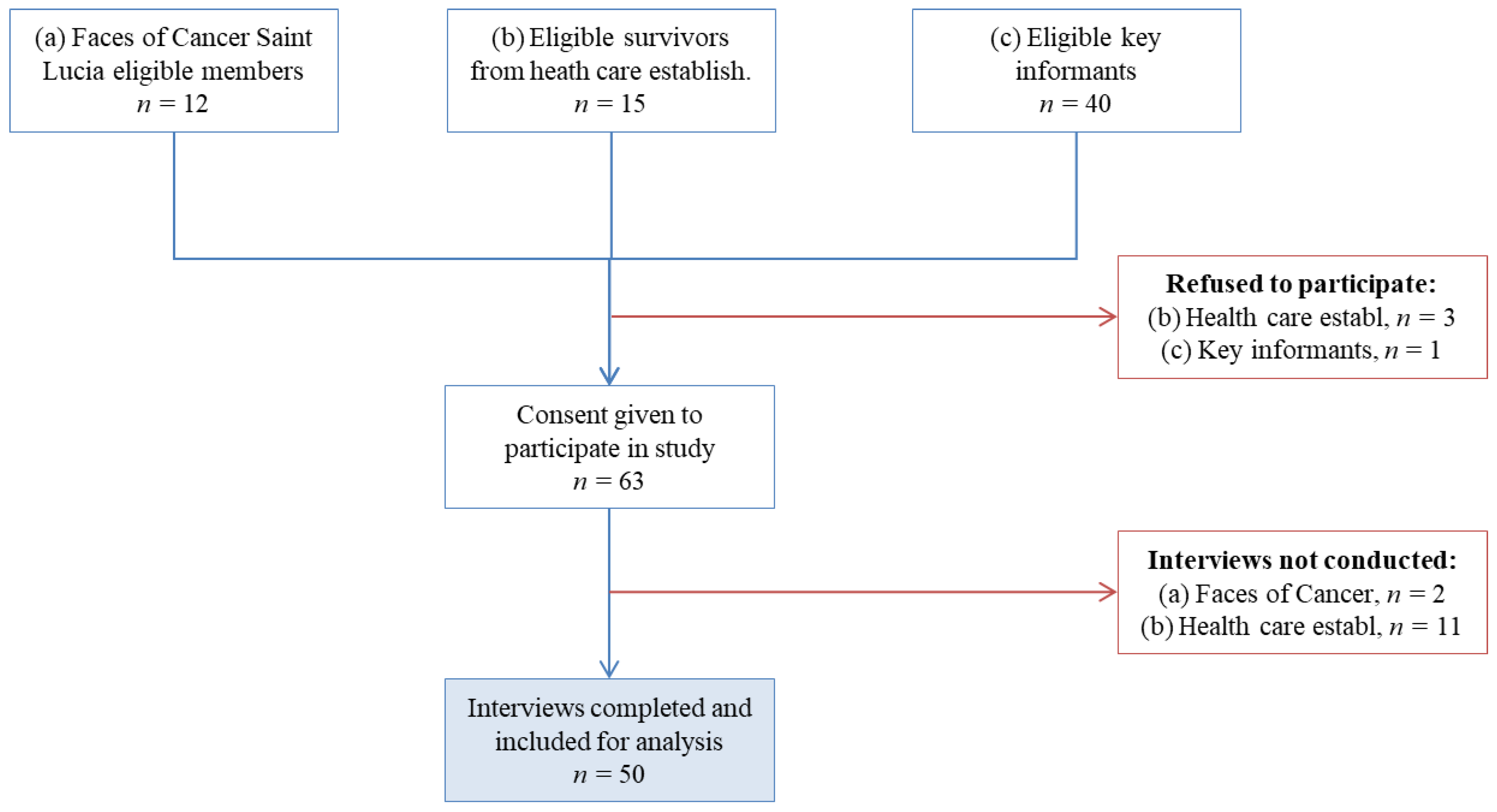
2.2. Patient Recruitment
2.3. Ethics
2.4. Data Collection and Questionnaire
2.5. Variables and Definitions
2.6. Preparation and Implementation
2.7. Data Analysis
3. Results
3.1. Challenges
3.2. Feasibility
3.3. Patient Characteristics
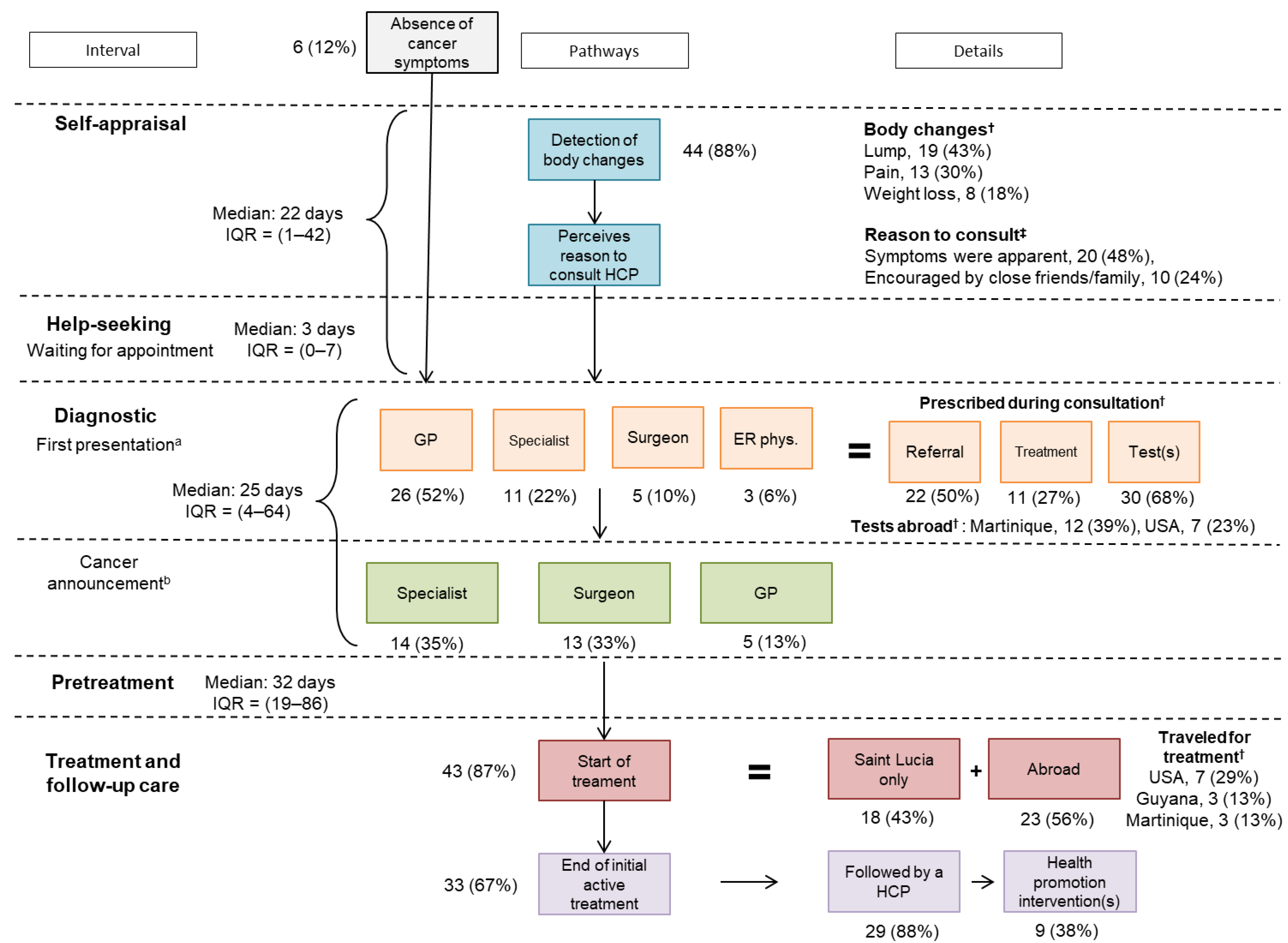
3.4. Cancer Care Pathways
3.5. Perception of Delays and Care Experience
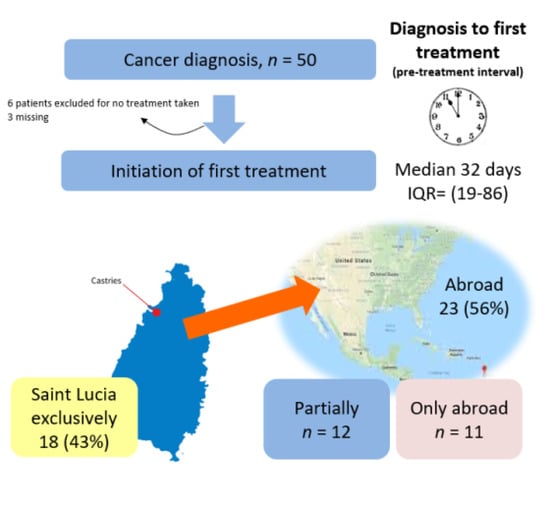
3.6. Funding of Cancer Care
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spence, D.; Dyer, R.; Andall-Brereton, G.; Barton, M.; Stanway, S.; Argentieri, M.A.; Bray, F.; Cawich, S.; Edwards-Bennett, S.; Fosker, C.; et al. Cancer control in the Caribbean island countries and territories: Some progress but the journey continues. Lancet Oncol. 2019, 20, e503–e521. [Google Scholar] [CrossRef]
- Sarfati, D.; Dyer, R.; Vivili, P.; Herman, J.; Spence, D.; Sullivan, R.; Weller, D.; Bray, F.; Hill, S.; Bates, C.; et al. Cancer control in small island nations: From local challenges to global action. Lancet Oncol. 2019, 20, e535–e548. [Google Scholar] [CrossRef]
- Sarfati, D.; Dyer, R.; Sam, F.A.-L.; Barton, M.; Bray, F.; Buadromo, E.; Ekeroma, A.; Foliaki, S.; Fong, J.; Herman, J.; et al. Cancer control in the Pacific: Big challenges facing small island states. Lancet Oncol. 2019, 20, e475–e492. [Google Scholar] [CrossRef]
- Batouli, A.; Jahanshahi, P.; Gross, C.P.; Makarov, D.V.; Yu, J.B. The global cancer divide: Relationships between national healthcare resources and cancer outcomes in high-income vs. middle- and low-income countries. J. Epidemiol. Glob. Health 2013, 4, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Vostakolaei, F.A.; Karim-Kos, H.E.; Janssen-Heijnen, M.L.G.; Visser, O.; Verbeek, A.L.M.; Kiemeney, L.A.L.M. The validity of the mortality to incidence ratio as a proxy for site-specific cancer survival. Eur. J. Public Health 2010, 21, 573–577. [Google Scholar] [CrossRef]
- Suzana, M.; Walls, H.; Smith, R.; Hanefeld, J. Understanding medical travel from a source country perspective: A cross sectional study of the experiences of medical travelers from the Maldives. Glob. Health 2018, 14, 58. [Google Scholar] [CrossRef]
- Suzana, M.; Mills, A.; Tangcharoensathien, V.; Chongsuvivatwong, V. The economic burden of overseas medical treatment: A cross sectional study of Maldivian medical travelers. BMC Health Serv. Res. 2015, 15, 418. [Google Scholar] [CrossRef]
- Suzana, M.; Walls, H.; Smith, R.; Hanefeld, J. Achieving universal health coverage in small island states: Could importing health services provide a solution? BMJ Glob. Health 2018, 3, e000612. [Google Scholar] [CrossRef]
- Spence, D.; Argentieri, M.A.; Andall-Brereton, G.; Anderson, B.O.; Duggan, C.; Bodkyn, C.; Bray, F.; Gibson, T.; Garcia, W.G.; Greaves, N.; et al. Advancing cancer care and prevention in the Caribbean: A survey of strategies for the region. Lancet Oncol. 2019, 20, e522–e534. [Google Scholar] [CrossRef]
- Razzaghi, H.; Quesnel-Crooks, S.; Sherman, R.; Joseph, R.; Kohler, B.; Andall-Brereton, G.; Ivey, M.A.; Edwards, B.K.; Mery, L.; Gawryszewski, V.; et al. Leading Causes of Cancer Mortality—Caribbean Region, 2003–2013. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1395–1400. [Google Scholar] [CrossRef]
- PAHO. Pharmaceutical Situation in Saint Lucia. WHO Assessment of Level II—Health Facilities Survey; Ministry of Health of Saint Lucia: Washington, DC, USA, 2012. [Google Scholar]
- Alleyne-Mike, K.; Sylvester, P.; Henderson-Suite, V.; Mohoyodeen, T. Radiotherapy in the Caribbean: A spotlight on the human resource and equipment challenges among CARICOM nations. Hum. Resour. Health 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Torring, M.L.; Frydenberg, M.; Hansen, R.P.; Olesen, F.; Hamilton, W.J.; Vedsted, P. Time to diagnosis and mortality in colorectal cancer: A cohort study in primary care. Br. J. Cancer 2011, 104, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Tørring, M.L.; Frydenberg, M.; Hansen, R.P.; Olesen, F.; Vedsted, P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: A cohort study in primary care. Eur. J. Cancer 2013, 49, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.S.; Forman, D. Cancer survival in England and the influence of early diagnosis: What can we learn from recent EUROCARE results? Br. J. Cancer 2009, 101, S102–S109. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Anandan, C.; Zalounina, A.; Fourkala, E.O.; Desai, R.; Liston, W.; Jensen, H.; Barisic, A.; Gavin, A.; et al. An investigation of routes to cancer diagnosis in 10 international jurisdictions, as part of the International Cancer Benchmarking Partnership: Survey development and implementation. BMJ Open 2016, 6, e009641. [Google Scholar] [CrossRef]
- Weller, D.; Menon, U.; Falborg, A.Z.; Jensen, H.; Barisic, A.; Knudsen, A.K.; Bergin, R.J.; Brewster, D.H.; Cairnduff, V.; Gavin, A.T.; et al. Diagnostic routes and time intervals for patients with colorectal cancer in 10 international jurisdictions; findings from a cross-sectional study from the International Cancer Benchmarking Partnership (ICBP). BMJ Open 2018, 8, e023870. [Google Scholar] [CrossRef]
- Menon, U.; Vedsted, P.; Falborg, A.Z.; Jensen, H.; Harrison, S.; Reguilon, I.; Barisic, A.; Bergin, R.J.; Brewster, D.H.; Butler, J.; et al. Time intervals and routes to diagnosis for lung cancer in 10 jurisdictions: Cross-sectional study findings from the International Cancer Benchmarking Partnership (ICBP). BMJ Open 2019, 9, e025895. [Google Scholar] [CrossRef]
- Rose, P.W.; Rubin, G.; Perera-Salazar, R.; Almberg, S.S.; Barisic, A.; Dawes, M.; Grunfeld, E.; Hart, N.; Neal, R.D.; Pirotta, M.; et al. Explaining variation in cancer survival between 11 jurisdictions in the International Cancer Benchmarking Partnership: A primary care vignette survey. BMJ Open 2015, 5, e007212. [Google Scholar] [CrossRef]
- Parsonage, R.K.; Hiscock, J.; Law, R.-J.; Neal, R.D. Patient perspectives on delays in diagnosis and treatment of cancer: A qualitative analysis of free-text data. Br. J. Gen. Pract. 2016, 67, e49–e56. [Google Scholar] [CrossRef]
- Allgar, V.L.; Neal, R.D. Delays in the diagnosis of six cancers: Analysis of data from the National Survey of NHS Patients: Cancer. Br. J. Cancer 2005, 92, 1959–1970. [Google Scholar] [CrossRef]
- Barrett, J.; Sharp, D.; Stapley, S.; Stabb, C.; Hamilton, W. Pathways to the diagnosis of ovarian cancer in the UK: A cohort study in primary care. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Hamilton, W. Pathways to the diagnosis of lung cancer in the UK: A cohort study. BMC Fam. Pract. 2008, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.; Jiwa, M.; Rose, P.; Hamilton, W. Pathways to the diagnosis of colorectal cancer: An observational study in three UK cities. Fam. Pract. 2005, 23, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Keeble, S.; Abel, G.A.; Saunders, C.L.; McPhail, S.; Walter, F.M.; Neal, R.D.; Rubin, G.P.; Lyratzopoulos, G. Variation in promptness of presentation among 10,297 patients subsequently diagnosed with one of 18 cancers: Evidence from a National Audit of Cancer Diagnosis in Primary Care. Int. J. Cancer 2014, 135, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.; Stuckler, D.; Basu, S. Where There Is No Health Research: What Can Be Done to Fill the Global Gaps in Health Research? PLoS Med. 2012, 9, e1001209. [Google Scholar] [CrossRef]
- Sy, A.U.; Hernandez, B.Y.; Tareg, A.; Reichhardt, M.; Buenconsejo-Lum, L. Acceptability and feasibility of a community based participatory research project comparing cytology and urine HPV DNA testing for cervical cancer screening in Yap, Federated States of Micronesia. Cancer Epidemiol. 2017, 50, 283–288. [Google Scholar] [CrossRef]
- Neupane, G.; Acharya, S.; Bhattarai, M.; Upadhyay, A.; Belbase, B.; Bhandari, M.; Pandeya, D.; Pokharel, S.; Ghimire, S.; Thapa, G.; et al. Study, Design, and Rationale of Noncommunicable Diseases in Nepal (NCD Nepal) Study: A Community-Based Prospective Epidemiological and Implementation Study in Rural Nepal. Glob. Adv. Health Med. 2020, 9. [Google Scholar] [CrossRef]
- Tillyard, G.; Surena, G.; Cornely, J.R.; Mondestin, M.J.; Senatus, D.; DeGennaro, V. A mixed methods, community-based investigation on women’s cancer awareness in Haiti. Health Soc. Care Community 2019, 27, 1458–1468. [Google Scholar] [CrossRef]
- Palinkas, L.A.; Horwitz, S.M.; Green, C.A.; Wisdom, J.P.; Duan, N.; Hoagwood, K. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015, 42, 533–544. [Google Scholar] [CrossRef]
- Cohen, N.; Arieli, T. Field research in conflict environments: Methodological challenges and snowball sampling. J. Peace Res. 2011, 48, 423–435. [Google Scholar] [CrossRef]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.E.; Campbell, C.E.; Andersen, R.S.; Hamilton, W.O.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef]
- Walter, F.; Webster, A.; Scott, S.; Emery, J. The Andersen Model of Total Patient Delay: A Systematic Review of Its Application in Cancer Diagnosis. J. Health Serv. Res. Policy 2012, 17, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.; Dugas, J.; Menvielle, G.; Barul, C.; Richard, J.-B.; Luce, D. Social distribution of tobacco smoking, alcohol drinking and obesity in the French West Indies. BMC Public Health 2019, 19, 1424. [Google Scholar] [CrossRef] [PubMed]
- Lansang, M.A.; Dennis, R. Building capacity in health research in the developing world. Bull. World Health Organ. 2004, 82, 764–770. [Google Scholar]
- Adewole, I.; Martin, D.N.; Williams, M.J.; Adebamowo, C.; Bhatia, K.; Berling, C.; Casper, C.; ElShamy, K.; Elzawawy, A.; Lawlor, R.T.; et al. Building capacity for sustainable research programmes for cancer in Africa. Nat. Rev. Clin. Oncol. 2014, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.; Almont, T.; Drame, M.; Contaret, C.; Vestris, M.; Najioullah, F.; Aline-Fardin, A.; Escarmant, P.; LeDuc, N.; Grossat, N.; et al. International cooperation in public health in Martinique: Geostrategic utility for cancer surveillance in the Caribbean. Glob. Health 2020, 16, 20–29. [Google Scholar] [CrossRef]
- Friedman, G.D.; Skilling, J.S.; Udaltsova, N.V.; Smith, L.H. Early symptoms of ovarian cancer: A case–control study without recall bias. Fam. Pract. 2005, 22, 548–553. [Google Scholar] [CrossRef]
- Astin, M.; Griffin, T.; Neal, R.D.; Rose, P.; Hamilton, W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br. J. Gen. Pract. 2011, 61, e231–e243. [Google Scholar] [CrossRef]
- Hamilton, W.; Peters, T.J.; Bankhead, C.; Sharp, D. Risk of ovarian cancer in women with symptoms in primary care: Population based case-control study. BMJ 2009, 339, b2998. [Google Scholar] [CrossRef] [PubMed]
- Zachary, I.; Boren, S.A.; Simoes, E.; Jackson-Thompson, J.; Davis, J.W.; Hicks, L. Information Management in Cancer Registries: Evaluating the Needs for Cancer Data Collection and Cancer Research. Online J. Public Health Inform. 2015, 7, e213. [Google Scholar] [CrossRef]
- Coxon, D.; Campbell, C.; Walter, F.M.; Scott, S.E.; Neal, R.D.; Vedsted, P.; Emery, J.; Rubin, G.; Hamilton, W.; Weller, D. The Aarhus statement on cancer diagnostic research: Turning recommendations into new survey instruments. BMC Health Serv. Res. 2018, 18, 677. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall | Breast | Female Pelvis a | Prostate | Other b | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 50 | % | n = 26 | % | n = 10 | % | n = 9 | % | n = 5 | % | ||
| Sex | <0.0001 | ||||||||||
| Male | 13 | 26 | 0 | 0 | 0 | 0 | 9 | 100 | 4 | 80 | |
| Female | 37 | 74 | 26 | 100 | 10 | 100 | 0 | 0 | 1 | 20 | |
| Age at diagnosis | 0.05 | ||||||||||
| <50 | 15 | 30 | 10 | 38.5 | 3 | 30 | 0 | 0 | 2 | 40 | |
| 50–65 | 26 | 52 | 14 | 53.8 | 6 | 60 | 4 | 44.4 | 2 | 40 | |
| >65 | 9 | 18 | 2 | 7.7 | 1 | 10 | 5 | 55.6 | 1 | 20 | |
| Survivorship | 0.39 | ||||||||||
| 0–4 months | 4 | 8.2 | 1 | 4 | 3 | 30 | 0 | 0 | 0 | 0 | |
| 5 months–1 y | 11 | 22.4 | 5 | 20 | 2 | 20 | 2 | 22.2 | 2 | 40 | |
| 2–3 y | 11 | 22.4 | 6 | 24 | 1 | 10 | 4 | 44.4 | 0 | 0 | |
| 4–5 y | 7 | 14.3 | 2 | 8 | 2 | 20 | 2 | 22.2 | 1 | 20 | |
| 6–9 y | 11 | 22.4 | 7 | 28 | 2 | 20 | 1 | 11.1 | 1 | 20 | |
| 10 + y | 5 | 10.2 | 4 | 16 | 0 | 0 | 0 | 0 | 1 | 20 | |
| Missing | 1 | 1 | 0 | 0 | 0 | ||||||
| Stage at diagnosis | 1 | ||||||||||
| Early (I/II) | 26 | 60.5 | 16 | 61.5 | 5 | 55.6 | 4 | 66.7 | 1 | 50 | |
| Advanced (III/IV) | 17 | 39.5 | 10 | 38.5 | 4 | 44.4 | 2 | 33.3 | 1 | 50 | |
| Missing | 7 | 0 | 1 | 3 | 3 | ||||||
| Category | n | % |
|---|---|---|
| Interviewee | ||
| Patient | 46 | 92 |
| Next-of-kin | 4 | 8 |
| Vital status | ||
| Alive | 48 | 96 |
| Deceased | 2 | 4 |
| Time between diagnosis and interview | ||
| Median (y), IQR | 3.4 | 1.5–7 |
| Treatment status | ||
| Finished initial active treatment | 33 | 67.4 |
| Still on treatment | 10 | 20.4 |
| No treatment taken | 6 | 12.2 |
| Missing | 1 | |
| Interview length | ||
| Mean (SD). Min Max | 1:24 (0:35) | 0:37–2:49 |
| Interview quality rating † | ||
| Poor/Mediocre | 0 | 0 |
| Good | 22 | 48.9 |
| Very Good | 15 | 33.3 |
| Excellent | 8 | 17.8 |
| Missing | 5 |
| Characteristic | Faces of Cancer | Key Informants † | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | 0.17 | ||||
| Male | 1 | 10 | 12 | 30 | |
| Female | 9 | 90 | 28 | 70 | |
| Cancer stage | 0.16 | ||||
| I | 2 | 20 | 10 | 30.3 | |
| II | 2 | 20 | 12 | 36.4 | |
| III | 6 | 60 | 7 | 21.2 | |
| IV | 0 | 0 | 4 | 12.1 | |
| Missing | 0 | 7 | |||
| Age at diagnosis | 0.32 | ||||
| <50 | 4 | 40 | 11 | 27.5 | |
| 50–65 | 6 | 60 | 20 | 50 | |
| >65 | 0 | 0 | 9 | 22.5 | |
| Survivorship (y) | 0 | ||||
| <7 | 2 | 22.2 | 33 | 82.5 | |
| 7–10 | 5 | 56 | 4 | 10 | |
| >10 | 2 | 22.2 | 3 | 7.5 | |
| Missing | 1 | 0 | |||
| Marital status | 1 | ||||
| Single | 5 | 50 | 18 | 46.2 | |
| Married | 3 | 30 | 13 | 33.3 | |
| Divorced/Separated | 1 | 10 | 3 | 7.7 | |
| Widowed | 1 | 10 | 5 | 12.8 | |
| Missing | 0 | 1 | |||
| Education level | 0.02 | ||||
| Primary | 1 | 10 | 15 | 38.5 | |
| Secondary | 7 | 70 | 8 | 20.5 | |
| Tertiary | 2 | 20 | 16 | 41 | |
| Missing | 0 | 1 | |||
| Private medical insurance | 0.46 | ||||
| Yes | 5 | 50 | 13 | 32.5 | |
| No | 5 | 50 | 27 | 67.5 | |
| Hot water at home | 0.50 | ||||
| Yes | 6 | 60 | 18 | 46.2 | |
| No | 4 | 40 | 21 | 53.9 | |
| Missing | 0 | 1 | |||
| History of medical condition(s) | 0.29 | ||||
| Yes | 7 | 70 | 18 | 45 | |
| No | 3 | 30 | 22 | 55 | |
| Professional status | 0.52 | ||||
| Still working | 4 | 44.4 | 20 | 51.3 | |
| Retirement/Volunteer | 5 | 55.6 | 12 | 30.8 | |
| Unemployed | 0 | 0 | 4 | 10.2 | |
| Invalidity due to sickness | 0 | 0 | 3 | 7.7 | |
| Missing | 1 | 1 | |||
| Motive for Choice of Country of Care | Diagnostic Test | Treatment | ||
|---|---|---|---|---|
| n = 26 | % | n = 22 | % | |
| Attracted by the price | 6 | 23.1 | 8 | 36.7 |
| Personal preference for location | 10 | 38.5 | 13 | 59.1 |
| Recommended by someone a | 5 | 19.2 | 6 | 27.3 |
| Referral b by HCP | 9 | 34.6 | 8 | 36.4 |
| Location of specific lab/hospital | 1 | 4 | 0 | 0 |
| Proximity to family/close friend(s) | 9 | 34.6 | 9 | 40.9 |
| The service accessed was not available in Saint Lucia * | 8 | 30.8 | 8 | 36.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auguste, A.; Jones, G.; Phillip, D.; St. Catherine, J.; Dos Santos, E.; Gabriel, O.; Radix, C. Difficulties in Accessing Cancer Care in a Small Island State: A Community-Based Pilot Study of Cancer Survivors in Saint Lucia. Int. J. Environ. Res. Public Health 2021, 18, 4770. https://doi.org/10.3390/ijerph18094770
Auguste A, Jones G, Phillip D, St. Catherine J, Dos Santos E, Gabriel O, Radix C. Difficulties in Accessing Cancer Care in a Small Island State: A Community-Based Pilot Study of Cancer Survivors in Saint Lucia. International Journal of Environmental Research and Public Health. 2021; 18(9):4770. https://doi.org/10.3390/ijerph18094770
Chicago/Turabian StyleAuguste, Aviane, Glenn Jones, Dorothy Phillip, James St. Catherine, Elizabeth Dos Santos, Owen Gabriel, and Carlene Radix. 2021. "Difficulties in Accessing Cancer Care in a Small Island State: A Community-Based Pilot Study of Cancer Survivors in Saint Lucia" International Journal of Environmental Research and Public Health 18, no. 9: 4770. https://doi.org/10.3390/ijerph18094770
APA StyleAuguste, A., Jones, G., Phillip, D., St. Catherine, J., Dos Santos, E., Gabriel, O., & Radix, C. (2021). Difficulties in Accessing Cancer Care in a Small Island State: A Community-Based Pilot Study of Cancer Survivors in Saint Lucia. International Journal of Environmental Research and Public Health, 18(9), 4770. https://doi.org/10.3390/ijerph18094770