A Systematic Framework for Collecting Site-Specific Sampling and Survey Data to Support Analyses of Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries
Abstract
1. Introduction
- Small-scale artisanal gold mining (ASGM) predominantly releases mercury (Hg), followed by lead (Pb) and arsenic (As) that are discharged or released to air, soil, and surface water during various phases of the mining process. Hg is transformed to methylmercury (MeHg) in aquatic environments, leading to further pathways of exposure and potential health outcomes.
- Leather tanning is another complex, resource-intensive process that generates a significant number of by-products, solid waste materials, and large amounts of wastewater. These byproducts can contain chromium (Cr), Pb, As, and cadmium (Cd)—metals that will persist in the environment in their original form and are likely to be found in soil, dust, water, and some agricultural products.
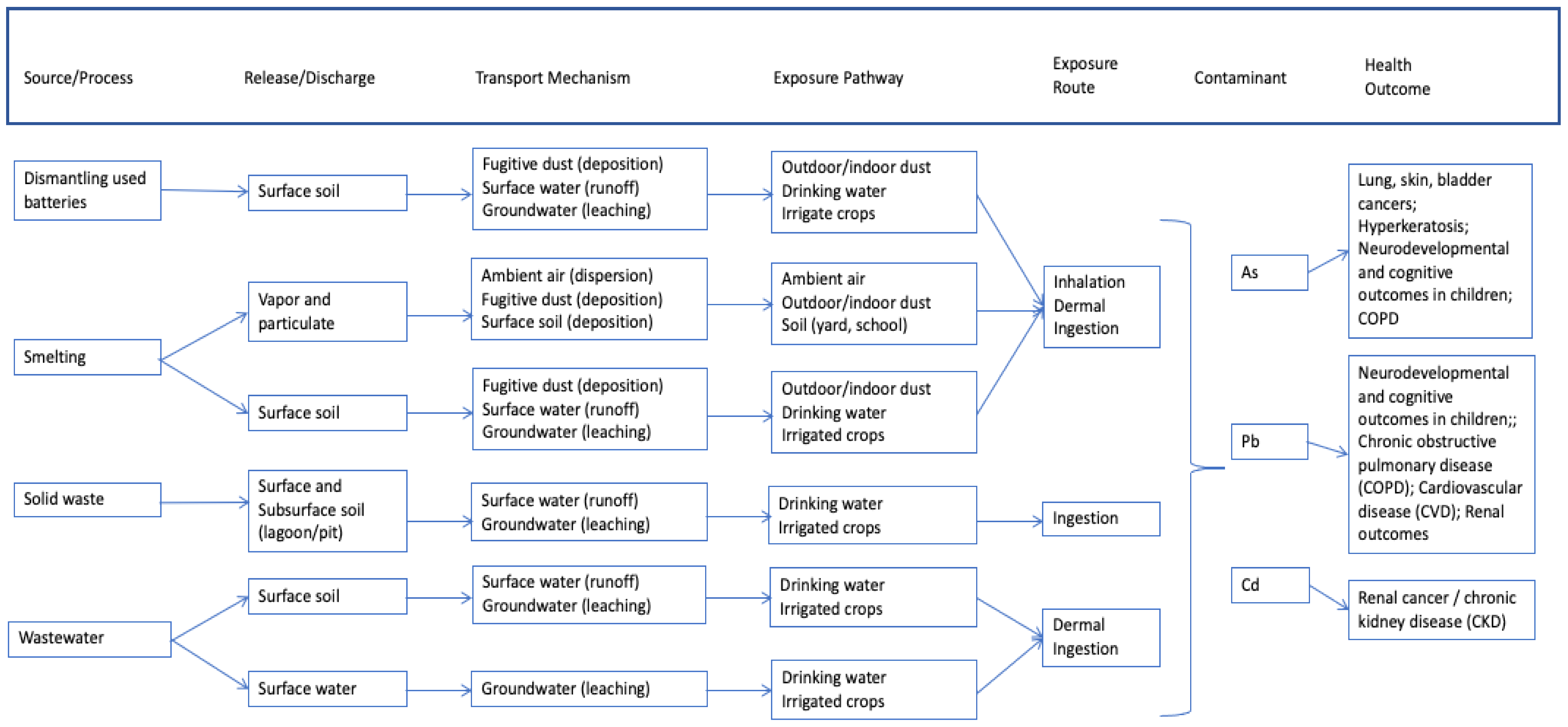
- Used lead-acid battery recycling (ULAB) consists of dismantling and recycling used batteries, usually acquired from motor vehicles. Contaminants encountered during the battery-recycling process primarily arise from the battery components themselves and include Pb, As, and Cd. These metals can be released into the soil after the batteries are dismantled and discharged as solid waste and wastewater during the separation of components in a water bath. Lead is then smelted and refined, which can release toxic vapor and particulate dust.
2. Conceptual Site Models (CSMs) by Activity
2.1. Overview of Processes
2.1.1. Artisanal Scale Gold Mining (ASGM)
- Extraction of ore from alluvial deposits or hard rock via surface excavation and sediment pumping;
- Separating gold from ore using crushing and milling processes;
- Concentrating extracted gold using wet or dry methods;
- Addition of elemental mercury to create mercury-gold alloy (whole ore and concentrate amalgamation);
- Heating of amalgam to vaporize mercury and separate gold (sponge gold);
- Additional refining and heating of sponge gold to remove residual mercury and impurities.
2.1.2. Used Lead Acid Battery Recycling (ULAB)
- Collection and transportation of used batteries to a recycling area;
- Dismantling of used batteries, which can be done by children and not necessarily localized to a central location;
- Separation of component battery parts in a water bath so that Pb sinks to the bottom and plastics rise to the top;
- Air drying of Pb and Pb oxide-containing materials; mixing material with coal, soda ash, and scrap metal; and transferring material to uncovered vessels for heating;
- Smelting and refining of lead components in open kettles, with only rudimentary pollution control equipment or protective gear;
- Washing and shredding or melting of plastic components. These plastic components are then repurposed in the community;
- Purification and treatment of sulfuric acid electrolyte (fluid from the batteries);
- Treatment and disposal of waste products (e.g., plastics, residual lead, water).
2.1.3. Small Scale Leather Processing and Tanning (Tanneries)
- Delivery of raw hides and skins;
- Sorting and trimming of raw hides and skins;
- Curing and storage of skins;
- Soaking of skins involving detergents, enzymes, water, and biocides;
- Unhairing and liming using various deliming agents, surfactants, enzymes, and solvents;
- Pickling and tanning involving chromium (Cr) and aldehydes;
- Retanning and dyeing with dyes, Cr, and vegetable tans;
- Drying and mechanical finishing;
- Coating, which can involve the use of synthetic coating materials and solvents.
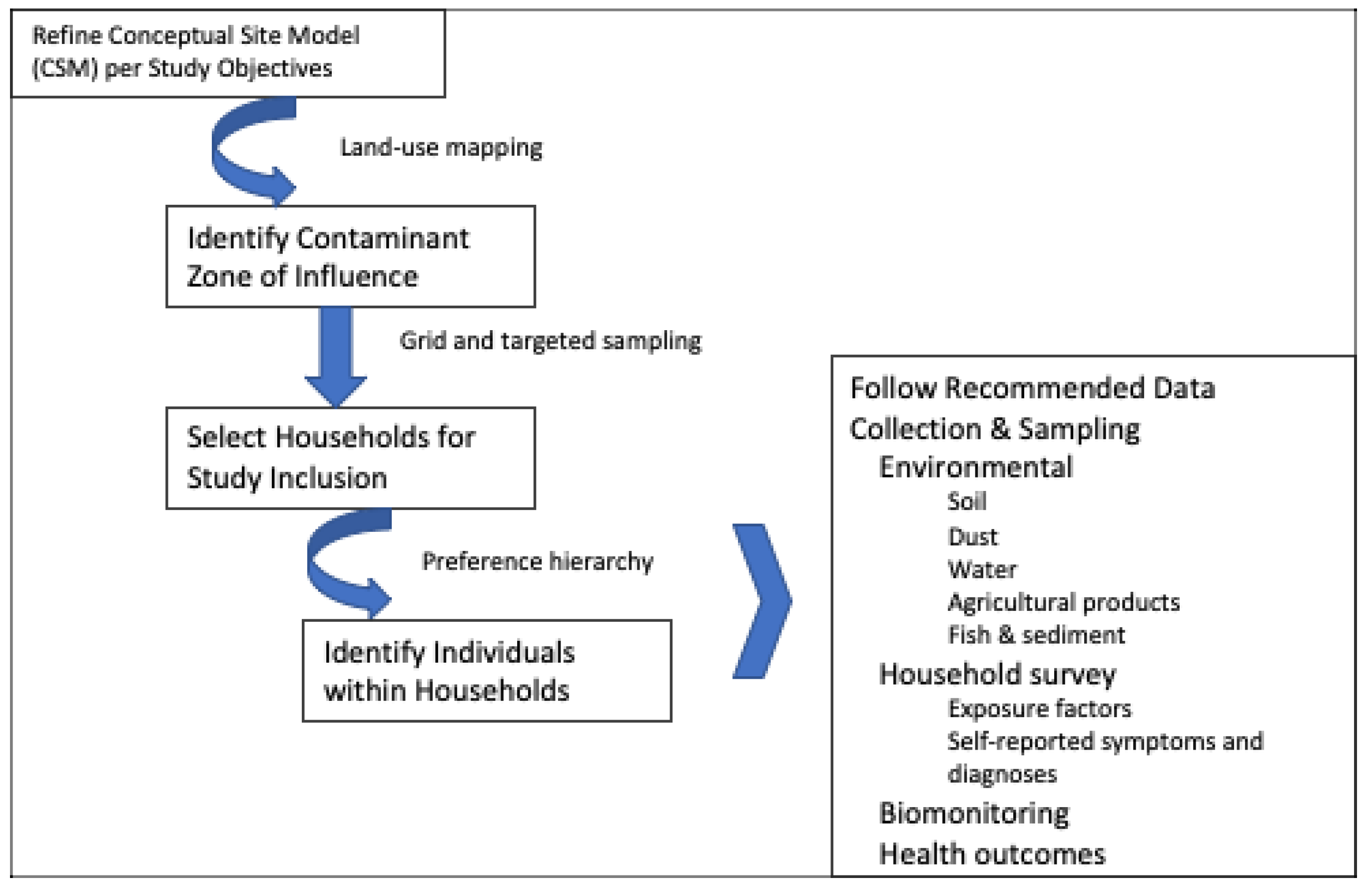
2.2. Identify Contaminant Zone of Influence (Characterize Environmental Setting)
2.3. Select Participating Households and Individuals
3. Data Collection and Sampling Recommendations
3.1. Environmental Sampling
3.1.1. Soil Samples
3.1.2. Dust Samples
3.1.3. Water Samples
3.1.4. Agricultural Product Sampling
- Rice grown in surface water impacted by wastewater from tanning, mining, or smelting activities;
- Chickens foraging directly at processing sites, particularly for tanning activities;
- Root vegetables grown in soils from backyard gardens irrigated with surface water impacted by wastewater;
- Leafy greens grown downwind within a depositional area;
- Beans or other legumes grown in soils (e.g., fertilized with organic solid wastes from tanning operations or irrigated with contaminated surface water);
- The framework recommends that agricultural product samples should be sent to an accredited laboratory for a multimetal screen. The exact biomass required for sampling will need to be determined by the laboratory or laboratories involved in the analyses. Although it is possible to use PXRF for agricultural products, the method does require dried and powdered samples and may not provide an appropriate level of precision [79,80].
3.1.5. Fish/Shellfish and Sediment Samples
3.2. Household Surveys
3.3. Biomonitoring
- “Gold standard”—This biomarker has been well-vetted in the literature with one or more validated, cost-effective laboratory methods with high levels of precision. This is the preferred biomarker given the primary research objectives in this document.
- “Screening level”—This biomarker is an appropriate default for low-resource applications. It is the least invasive, lowest cost, typically using in-field XRF. However, only the total metal can be measured with high detection levels, and may not have the precision to evaluate statistical associations with outcomes.
- “Low preference”—This biomarker can be used as a last resort, but is generally not preferred due to limitations with respect to associations (i.e., they are not the best measure of exposure and/or predictive of outcomes based on literature studies).
- “To be avoided”—This biomarker is not recommended as it doesn’t measure the exposure of interest, is expensive, and/or doesn’t have a validated method.
3.4. Measuring Health Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Shaffer, R.M.; Sellers, S.P.; Baker, M.G.; de Buen Kalman, R.; Frostad, J.; Suter, M.K.; Anenberg, S.C.; Balbus, J.; Basu, N.; Bellinger, D.C.; et al. Improving and Expanding Estimates of the Global Burden of Disease Due to Environmental Health Risk Factors. Environ. Health Perspect. 2019, 127, 105001. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.; Caravanos, J.; Grigsby, P.; Rivera, A.; Ericson, B.; Amoyaw-Osei, Y.; Akuffo, B.; Fuller, R. Estimating the Prevalence of Toxic Waste Sites in Low- and Middle-Income Countries. Ann. Glob. Health 2016, 82, 700–710. [Google Scholar] [CrossRef]
- Ericson, B.; Landrigan, P.; Taylor, M.P.; Frostad, J.; Caravanos, J.; Keith, J.; Fuller, R. The Global Burden of Lead Toxicity Attributable to Informal Used Lead-Acid Battery Sites. Ann. Glob. Health 2016, 82, 686–699. [Google Scholar] [CrossRef]
- Gibb, H.; O’Leary, K.G. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: A comprehensive review. Environ. Health Perspect. 2014, 122, 667–672. [Google Scholar] [CrossRef]
- Junaid, M.; Hashmi, M.Z.; Tang, Y.M.; Malik, R.N.; Pei, D.S. Potential health risk of heavy metals in the leather manufacturing industries in Sialkot, Pakistan. Sci. Rep. 2017, 7, 8848. [Google Scholar] [CrossRef]
- Nweke, O.C.; Sanders, W.H. Modern environmental health hazards: A public health issue of increasing significance in Africa. Environ. Health Perspect. 2009, 117, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Chandra, R.; Bharagava, R.N. Environmental Pollution, Toxicity Profile and Treatment Approaches for Tannery Wastewater and Its Chemical Pollutants. Rev. Environ. Contam. Toxicol. 2017, 240, 31–69. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.D.; von Stackelberg, K.; Guerra Lopez, M.G.; Sanchez-Triana, E. Risk Analysis Approaches to Evaluating Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries. Risk Anal. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Goodman, R.A. The CDC Field Epidemiology Manual; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- von Stackelberg, K.; Williams, P.R.D. Evolving Science and Practice of Risk Assessment. Risk Anal. 2020, 41, 571–583. [Google Scholar] [CrossRef]
- Covello, V.T.; Merkhoher, M.W. Risk Assessment Methods: Approaches for Assessing Health and Environmental Risks; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Smolders, E.; Roels, L.; Kuhangana, T.C.; Coorevits, K.; Vassilieva, E.; Nemery, B.; Lubaba Nkulu, C.B. Unprecedentedly High Dust Ingestion Estimates for the General Population in a Mining District of DR Congo. Environ. Sci. Technol. 2019, 53, 7851–7858. [Google Scholar] [CrossRef]
- Utembe, W.; Gulumian, M. Challenges and research needs for risk assessment of pesticides for registration in Africa. Hum. Ecol. Risk Assess. 2015, 21, 1518–1541. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (US EPA). Risk Assessment Guidance for Super-Fund (RAGS). Human Health Evaluation Manual (HHEM), Part A, Interim Final; EPA/540/1-89/002; Office of Emergency and Remedial Response: Washington, DC, USA, 1989; Volume 1.
- US Environmental Protection Agency (US EPA). Exposure Factors Handbook 2011 Edition (Final Report); EPA/600/R-09/052F; United States Environmental Protection Agency/Office of Research and Development: Washington, DC, USA, 2011.
- US Environmental Protection Agency (US EPA). Guidelines for Human Exposure Assessment; EPA/100/B-19/001; Office of the Science Advisor, Risk Assessment Forum: Washington, DC, USA, 2019.
- Norweigan Pollution Control Authority (NPCA). Guidelines for the Risk Assessment of Contaminated Sites; Report 99:06, TA-1691/1999; Norwegian Pollution Control Authority: Oslo, Norway, 1999. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Considerations for Assessing the Risks of Combined Exposure to Multiple Cchemicals; ENV/JM/MONO(2018)37; Environment, Health and Safety Division, Environment Directorate: Paris, France, 2018.
- World Health Organization (WHO). WHO Human Health Risk Assessment Toolkit: Chemical Hazards; World Health Organization, International Programme on Chemical Safety: Geneva, Switzerland, 2010. [Google Scholar]
- Plumlee, G.S.; Durant, J.T.; Morman, S.A.; Neri, A.; Wolf, R.E.; Dooyema, C.A.; Hageman, P.L.; Lowers, H.A.; Fernette, G.L.; Meeker, G.P.; et al. Linking geological and health sciences to assess childhood lead poisoning from artisanal gold mining in Nigeria. Environ. Health Perspect. 2013, 121, 744–750. [Google Scholar] [CrossRef]
- Rajaee, M.; Long, R.N.; Renne, E.P.; Basu, N. Mercury Exposure Assessment and Spatial Distribution in A Ghanaian Small-Scale Gold Mining Community. Int. J. Environ. Res. Public Health 2015, 12, 10755–10782. [Google Scholar] [CrossRef] [PubMed]
- Boerma, J.T.; Holt, E.; Black, R. Measurement of biomarkers in surveys in developing countries: Opportunities and problems. Popul. Dev. Rev. 2001, 27, 303–314. [Google Scholar] [CrossRef]
- Tan, Y.-M.; Dary, C.C.; Chang, E.M.; Ulrich, J.M.; Van Emon, J.M.; Xue, J.; Pleil, J.D.; Kenneke, J.F.; Sobus, J.; Sheldon, L.S.; et al. Biomonitoring–An Exposure Science Tool for Exposure and Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2012.
- Santonen, T.A.A.; Fowler, B.A.; Nordberg, M. Biological monitoring and biomarkers. In Handbook on the Toxicology of Metals; Nordberg, G.F.F., Nordberg, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 151–171. [Google Scholar]
- Bentler, P.M.; Stein, J. Structural equation models in medical research. Stat. Methods Med. Res. 1992, 1, 159–181. [Google Scholar] [CrossRef] [PubMed]
- Buncher, C.R.; Succop, P.A.; Dietrich, K.N. Structural equation modeling in environmental risk assessment. Environ. Health Perspect. 1991, 90, 209–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, Y.M.; Chan, M.; Chukwudebe, A.; Domoradzki, J.; Fisher, J.; Hack, C.E.; Hinderliter, P.; Hirasawa, K.; Leonard, J.; Lumen, A.; et al. PBPK model reporting template for chemical risk assessment applications. Regul. Toxicol. Pharmacol. 2020, 115, 104691. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.; Taylor, H.; Lister, T.; Smith, B.; Drasch, G.; Boese-O’Reilly, S. Final Report for an Assessment of the Environment and Health in the Rwamagasa Area, Tanzania. UNIDO Project EG. GLO/01; British Geological Survey—Natural Environment Research Council: Nottingham, UK, 2004. [Google Scholar]
- Nemery, B.; Banza Lubaba Nkulu, C. Assessing exposure to metals using biomonitoring: Achievements and challenges experienced through surveys in low- and middle-income countries. Toxicol. Lett. 2018, 298, 13–18. [Google Scholar] [CrossRef]
- Van Straaten, P. Mercury contamination associated with small-scale gold mining in Tanzania and Zimbabwe. Sci. Total Environ. 2000, 259, 105–113. [Google Scholar] [CrossRef]
- Basu, N.; Clarke, E.; Green, A.; Calys-Tagoe, B.; Chan, L.; Dzodzomenyo, M.; Fobil, J.; Long, R.N.; Neitzel, R.L.; Obiri, S.; et al. Integrated assessment of artisanal and small-scale gold mining in Ghana—Part 1: Human health review. Int. J. Environ. Res. Public Health 2015, 12, 5143–5176. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health assessment of artisanal gold miners in Tanzania. Sci. Total Environ. 2010, 408, 796–805. [Google Scholar] [CrossRef]
- Caravanos, J.; Ericson, B.; Ponce-Canchihuamán, J.; Hanrahan, D.; Block, M.; Susilorini, B.; Fuller, R. Rapid assessment of environmental health risks posed by mining operations in low- and middle-income countries: Selected case studies. Environ. Sci. Pollut. Res. Int. 2013, 20, 7711–7718. [Google Scholar] [CrossRef] [PubMed]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chemistry 2018, 24, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Bose-O’Reilly, S.; Lettmeier, B.; Gothe, R.M.; Beinhoff, C.; Siebert, U.; Drasch, G. Mercury as a serious health hazard for children in gold mining areas. Environ. Res. 2008, 107, 89–97. [Google Scholar] [CrossRef]
- Secretariat of the Basel Convention. Technical Guidelines for the Environmentally Sound Management of Waste Lead-acid Batteries; Basel Convention Series/SBC No. 2003/9; UNEP—UN Environment Programme: Nairobi, Kenya, 2003. [Google Scholar]
- World Health Organization (WHO). Recycling Used Lead-Acid Batteries: Health Considerations; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- European Commission (EC). Reference Document on Best Available Techniques for the Tanning of Hides and Skins; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Bari, M.L.; Simol, H.A.; Khandoker, N.; Begum, R.; Sultana, U.N. Potential human health risks of tannery waste-contaminated poultry feed. J. Health Pollut. 2015, 5, 68–77. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Chromium levels in vegetables and grains grown on tannery effluent irrigated area of Jajmau, Kanpur, India: Influence on dietary intake. Bull. Environ. Contam. Toxicol. 2006, 77, 658–664. [Google Scholar] [CrossRef]
- Chen, H.; Arocena, J.M.; Li, J.; Thring, R.W.; Zhou, J. Mobility and storage sinks for chromium and other metals in soils impacted by leather tannery wastes. J. Environ. Monit. 2012, 14, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Arocena, J.M.; Li, J.; Thring, R.W.; Zhou, J. Assessments of chromium (and other metals) in vegetables and potential bio-accumulations in humans living in areas affected by tannery wastes. Chemosphere 2014, 112, 412–419. [Google Scholar] [CrossRef]
- Onakpa, M.M.; Njan, A.A.; Kalu, O.C. A Review of Heavy Metal Contamination of Food Crops in Nigeria. Ann. Glob. Health 2018, 84, 488–494. [Google Scholar] [CrossRef]
- Qader, S.H.; Lefebvre, V.; Tatem, A.J.; Pape, U.; Jochem, W.; Himelein, K.; Ninneman, A.; Wolburg, P.; Nunez-Chaim, G.; Bengtsson, L.; et al. Using gridded population and quadtree sampling units to support survey sample design in low-income settings. Int. J. Health Geogr. 2020, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.R.; Stevens, F.R.; Ruktanonchai, N.W.; Tatem, A.J.; Castro, M.C. GridSample: An R package to generate household survey primary sampling units (PSUs) from gridded population data. Int. J. Health Geogr. 2017, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Grafström, A.; Schelin, L. How to select representative samples. Scand. J. Stat. 2014, 41, 277–290. [Google Scholar] [CrossRef]
- Mattuck, R.; Blanchet, R.; Wait, A.D. Data representativeness for risk assessment. Environ. Forensics 2005, 6, 65–70. [Google Scholar] [CrossRef]
- Ramsey, C.A.; Hewitt, A.D. A methodology for assessing sample representativeness. Environ. Forensics 2005, 6, 71–75. [Google Scholar] [CrossRef]
- Zhu, A.; Liu, J.; Du, F.; Zhang, S.; Qin, C.; Burt, J.; Behrens, T.; Scholten, T. Predictive soil mapping with limited sample data. Eur. J. Soil Sci. 2015, 66, 535–547. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Pulsipher, B.A. Role of sampling designs in obtaining representative data. Environ. Forensics 2005, 6, 27–33. [Google Scholar] [CrossRef]
- Wagenaar, B.H.; Augusto, O.; Ásbjörnsdóttir, K.; Akullian, A.; Manaca, N.; Chale, F.; Muanido, A.; Covele, A.; Michel, C.; Gimbel, S.; et al. Developing a representative community health survey sampling frame using open-source remote satellite imagery in Mozambique. Int. J. Health Geogr. 2018, 17, 37. [Google Scholar] [CrossRef]
- Lwanga, S.K.; Lemeshow, S. ; World Health Organization. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Thomas, D.C.; Siemiatycki, J.; Dewar, R.; Robins, J.; Goldberg, M.; Armstrong, B.G. The problem of multiple inference in studies designed to generate hypotheses. Am. J. Epidemiol. 1985, 122, 1080–1095. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency (US EPA). Guidance on Choosing a Sampling Design for Environmental Data Collection for Use in Developing a Quality Assurance Project Plan; EPA/240/R-02/005; Office of Environmental Information: Washington, DC, USA, 2002.
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988; p. 567. [Google Scholar]
- Greenland, S. Methods for epidemiologic analyses of multiple exposures: A review and comparative study of maximum-likelihood, preliminary-testing, and empirical-Bayes regression. Stat. Med. 1993, 12, 717–736. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Phillips, L. A review of soil and dust ingestion studies for children. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Ravansari, R.; Wilson, S.C.; Tighe, M. Portable X-ray fluorescence for environmental assessment of soils: Not just a point and shoot method. Environ. Int. 2020, 134, 105250. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, E.; Korhonen, M.; Viskari, E.-L.; Haapamäki, S.; Järvinen, M.; Lu, L. Comparison of XRF and FAAS methods in analysing CCA contaminated soils. Water Air Soil Pollut. 2006, 171, 95–110. [Google Scholar] [CrossRef]
- Wu, C.-M.; Tsai, H.-T.; Yang, K.-H.; Wen, J.-C. How reliable is X-ray fluorescence (XRF) measurement for different metals in soil contamination? Environ. Forensics 2012, 13, 110–121. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (US EPA). Environmental Technology Verification Report: Field Portable X-ray Fluorescence Analyzer-Spectrace TN 9000 and TN Pb Field Portable X-ray Fluorescence Analyzers; EPA 600-R-97-145; Office of Research and Development: Washington, DC, USA, 1998; p. 119.
- Bougnom, B.P.; Piddock, L.J.V. Wastewater for Urban Agriculture: A Significant Factor in Dissemination of Antibiotic Resistance. Environ. Sci. Technol. 2017, 51, 5863–5864. [Google Scholar] [CrossRef]
- Gupta, N.; Khan, D.K.; Santra, S.C. Heavy metal accumulation in vegetables grown in a long-term wastewater-irrigated agricultural land of tropical India. Environ. Monit. Assess. 2012, 184, 6673–6682. [Google Scholar] [CrossRef]
- Kumar Sharma, R.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef]
- Mateo-Sagasta, J.M.K.; Qadir, M.; Raschid-Sally, L.; Drechsel, P.; Liebe, J. Proceedings of the UN-Water Project on the Safe Use of Wastewater in Agriculture; UN-Water Decade Programme on Capacity Development (UNW-DPC): Bonn, Germany, 2013; p. 82. [Google Scholar]
- Aranda, P.R.; Moyano, S.; Martinez, L.D.; De Vito, I.E. Determination of trace chromium (VI) in drinking water using X-ray fluorescence spectrometry after solid-phase extraction. Anal. Bioanal. Chem. 2010, 398, 1043–1048. [Google Scholar] [CrossRef]
- Marguí, E.; Hidalgo, M.; Queralt, I.; Van Meel, K.; Fontàs, C. Analytical capabilities of laboratory, benchtop and handheld X-ray fluorescence systems for detection of metals in aqueous samples pre-concentrated with solid-phase extraction disks. Spectrochim. Acta Part B At. Spectrosc. 2012, 67, 17–23. [Google Scholar] [CrossRef]
- Marguí, E.; Tapias, J.C.; Casas, A.; Hidalgo, M.; Queralt, I. Analysis of inlet and outlet industrial wastewater effluents by means of benchtop total reflection X-ray fluorescence spectrometry. Chemosphere 2010, 80, 263–270. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Environ. Pollut. 2018, 243, 1325–1333. [Google Scholar] [CrossRef]
- Pearson, D.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Koch, J.; Van Deventer, P.; de Wet, J.; Kusi, N.Y. Analysis of metal-laden water via portable X-ray fluorescence spectrometry. J. Hydrol. 2018, 561, 267–276. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mottalib, M.A.; Zilani, G.; Suman, T.I.; Ahmed, T.; Islam, S. Assessment of Trace Metals in Consumer Chickens in Bangladesh. J. Health Pollut. 2018, 8, 181208. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Hill, J.; Phillips, C.J. The accumulation of potentially-toxic metals by grazing ruminants. Proc. Nutr. Soc. 2003, 62, 267–277. [Google Scholar] [CrossRef] [PubMed]
- McGladdery, C.; Weindorf, D.C.; Chakraborty, S.; Li, B.; Paulette, L.; Podar, D.; Pearson, D.; Kusi, N.Y.O.; Duda, B. Elemental assessment of vegetation via portable X-ray fluorescence (PXRF) spectrometry. J. Environ. Manag. 2018, 210, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Weindorf, D.C.; Cheng, Q.; Yang, B.; Yuan, Z.; Chakraborty, S. Elemental assessment of vegetation via portable XRF: Sample preparation and methodological considerations. Spectrochim. Acta Part B At. Spectrosc. 2020, 174, 105999. [Google Scholar] [CrossRef]
- Aday, L.A.; Cornelius, L.J. Designing and Conducting Health Surveys: A Comprehensive Guide; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Creswell, J.W.; Creswell, J.D. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches; Sage Publications: Newbury Park, CA, USA, 2017; p. 304. [Google Scholar]
- Rea, L.M.; Parker, R.A. Designing and Conducting Survey Research: A Comprehensive Guide; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 352. [Google Scholar]
- National Research Council (NRC). Human Biomonitoring for Environmental Chemicals; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Sexton, K.L.; Needham, L.L. Pirkle, J. Human Biomonitoring of Environmental Chemicals: Measuring chemicals in human tissues is the “gold standard” for assessing people’s exposure to pollution. Am. Sci. 2004, 92, 38–45. [Google Scholar] [CrossRef]
- National Research Council (NRC). Exposure Science in the 21st Century: A Vision and a Strategy; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Bernard, A. Biomarkers of metal toxicity in population studies: Research potential and interpretation issues. J. Toxicol. Environ. Health Part A 2008, 71, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Jaffery, F.N. Biological markers for metal toxicity. Environ. Toxicol. Pharmacol. 2005, 19, 335–349. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (US EPA). Framework for Human Health Risk Assessment to Inform Decision Making; EPA/100/R-14/001; Office of the Science Advisor, Risk Assessment Forum: Washington, DC, USA, 2014.
- Cindi, M.D.; Mbonane, T.P.; Naicker, N. Study protocol to examine the relationship between environmental exposure to lead and blood lead levels among children from day-care centres in Ekurhuleni Metropolitan Municipality. BMJ Open 2020, 10, e036687. [Google Scholar] [CrossRef]
- National Research Council (NRC). Science and Decisions: Advancing Risk Assessment; Committee on Improving Risk Analysis Approaches Used by the US EPA: Washington DC, USA, 2009. [Google Scholar]
- Filippelli, G.; Anenberg, S.; Taylor, M.; van Geen, A.; Khreis, H. New Approaches to Identifying and Reducing the Global Burden of Disease From Pollution. GeoHealth 2020, 4, e2018GH000167. [Google Scholar] [CrossRef]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER Statement. PLoS Med. 2016, 13, e1002056. [Google Scholar] [CrossRef]
- Orach, C.G. Health equity: Challenges in low income countries. Afr. Health Sci. 2009, 9 (Suppl 2), S49–S51. [Google Scholar]
- Wyber, R.; Vaillancourt, S.; Perry, W.; Mannava, P.; Folaranmi, T.; Celi, L.A. Big data in global health: Improving health in low- and middle-income countries. Bull. World Health Organ. 2015, 93, 203–208. [Google Scholar] [CrossRef]
- Eshetu, E.B.; Woldesenbet, S.A. Are there particular social determinants of health for the world’s poorest countries? Afr. Health Sci. 2011, 11, 108–115. [Google Scholar] [PubMed]
- Coughlin, S.S. Toward a road map for global -omics: A primer on -omic technologies. Am. J. Epidemiol. 2014, 180, 1188–1195. [Google Scholar] [CrossRef]
- García-Sevillano, M.; García-Barrera, T.; Gómez-Ariza, J.L. Environmental metabolomics: Biological markers for metal toxicity. Electrophoresis 2015, 36, 2348–2365. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Hwang, S.Y. Multi-omics approaches for understanding environmental exposure and human health. Mol. Cell. Toxicol. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Vineis, P.; van Veldhoven, K.; Chadeau-Hyam, M.; Athersuch, T.J. Advancing the application of omics-based biomarkers in environmental epidemiology. Environ. Mol. Mutagen 2013, 54, 461–467. [Google Scholar] [CrossRef]
- Vlaanderen, J.; Moore, L.E.; Smith, M.T.; Lan, Q.; Zhang, L.; Skibola, C.F.; Rothman, N.; Vermeulen, R. Application of OMICS technologies in occupational and environmental health research; current status and projections. Occup. Environ. Med. 2010, 67, 136–143. [Google Scholar] [CrossRef]
- Marsit, C.J. Influence of environmental exposure on human epigenetic regulation. J. Exp. Biol. 2015, 218, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Early life nutrition, epigenetics and programming of later life disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, N.; Vahter, M.; Broberg, K. The Epigenetic Effects of Prenatal Cadmium Exposure. Curr. Environ. Health Rep. 2015, 2, 195–203. [Google Scholar] [CrossRef]
- DeBord, D.G.; Carreón, T.; Lentz, T.J.; Middendorf, P.J.; Hoover, M.D.; Schulte, P.A. Use of the “Exposome” in the Practice of Epidemiology: A Primer on-Omic Technologies. Am. J. Epidemiol. 2016, 184, 302–314. [Google Scholar] [CrossRef]
- Rappaport, S.M. Implications of the exposome for exposure science. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 5–9. [Google Scholar] [CrossRef]
- Vrijheid, M.; Slama, R.; Robinson, O.; Chatzi, L.; Coen, M.; van den Hazel, P.; Thomsen, C.; Wright, J.; Athersuch, T.J.; Avellana, N.; et al. The human early-life exposome (HELIX): Project rationale and design. Environ. Health Perspect. 2014, 122, 535–544. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef] [PubMed]


| ASGM | ULAB | Tanning |
|---|---|---|
|
|
|
| Media | ASGM | Tannery | ULAB |
|---|---|---|---|
| Soil | If Pb-based ores are used and resources allow, 25% of randomly selected household samples and 50% of targeted samples undergo bioavailability testing for Pb | 25% of randomly selected household samples and 50% of targeted samples undergo laboratory analysis for CrVI If resources allow, 25% of randomly selected household samples and 50% of targeted samples undergo bioavailability testing for As, Pb | If resources allow, 50% of randomly selected household samples and 100% of targeted samples undergo bioavailability testing for Pb |
| Dust | No additional specific recommendations | 25% of randomly selected household samples and 50% of targeted samples undergo laboratory analysis for CrVI | No additional specific recommendations |
| Water | 25% of randomly selected samples and 100% of community drinking water samples undergo laboratory analysis for MeHg | 25% of randomly selected household samples and 100% of community drinking water samples undergo laboratory analysis for CrVI | No additional specific recommendations |
| Agricultural Products | Contaminated water used as irrigation water and/or airborne or soil deposition are the most common pathways by which agricultural products can become contaminated | In addition to contaminated water used as irrigation water and deposition, tanning activities lead to large amounts of organic wastes, which may be used as fertilizer with little additional processing | Contaminated water used as irrigation water and/or airborne or soil deposition are the most common pathways by which agricultural products can become contaminated |
| Fish & Sediment | See text | Not required for tanning sites | Not required for ULAB sites |
| CoC | Biomonitoring (Exposure) | Health Outcomes |
|---|---|---|
| As | Gold standard is metabolite monomethylarsonic acid (%MMA) obtained from a speciated creatinine-adjusted urine sample |
|
| Cd | International consensus on use of creatine-adjusted urine |
|
| Cr (CrVI) | Red blood cells or urine; can speciate. Recommend hair given non-occupational exposures |
|
| Hg | In-field PXRF toenails |
|
| MeHg | Hair | Conduct age-specific, culturally-relevant cognitive testing for each child |
| Pb | Venous blood is the gold standard; dried capillary blood spot also used, allows in-field LeadCare Analyzer |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Stackelberg, K.; Williams, P.R.D.; Sánchez-Triana, E. A Systematic Framework for Collecting Site-Specific Sampling and Survey Data to Support Analyses of Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2021, 18, 4676. https://doi.org/10.3390/ijerph18094676
von Stackelberg K, Williams PRD, Sánchez-Triana E. A Systematic Framework for Collecting Site-Specific Sampling and Survey Data to Support Analyses of Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries. International Journal of Environmental Research and Public Health. 2021; 18(9):4676. https://doi.org/10.3390/ijerph18094676
Chicago/Turabian Stylevon Stackelberg, Katherine, Pamela R.D. Williams, and Ernesto Sánchez-Triana. 2021. "A Systematic Framework for Collecting Site-Specific Sampling and Survey Data to Support Analyses of Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries" International Journal of Environmental Research and Public Health 18, no. 9: 4676. https://doi.org/10.3390/ijerph18094676
APA Stylevon Stackelberg, K., Williams, P. R. D., & Sánchez-Triana, E. (2021). A Systematic Framework for Collecting Site-Specific Sampling and Survey Data to Support Analyses of Health Impacts from Land-Based Pollution in Low- and Middle-Income Countries. International Journal of Environmental Research and Public Health, 18(9), 4676. https://doi.org/10.3390/ijerph18094676






