Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
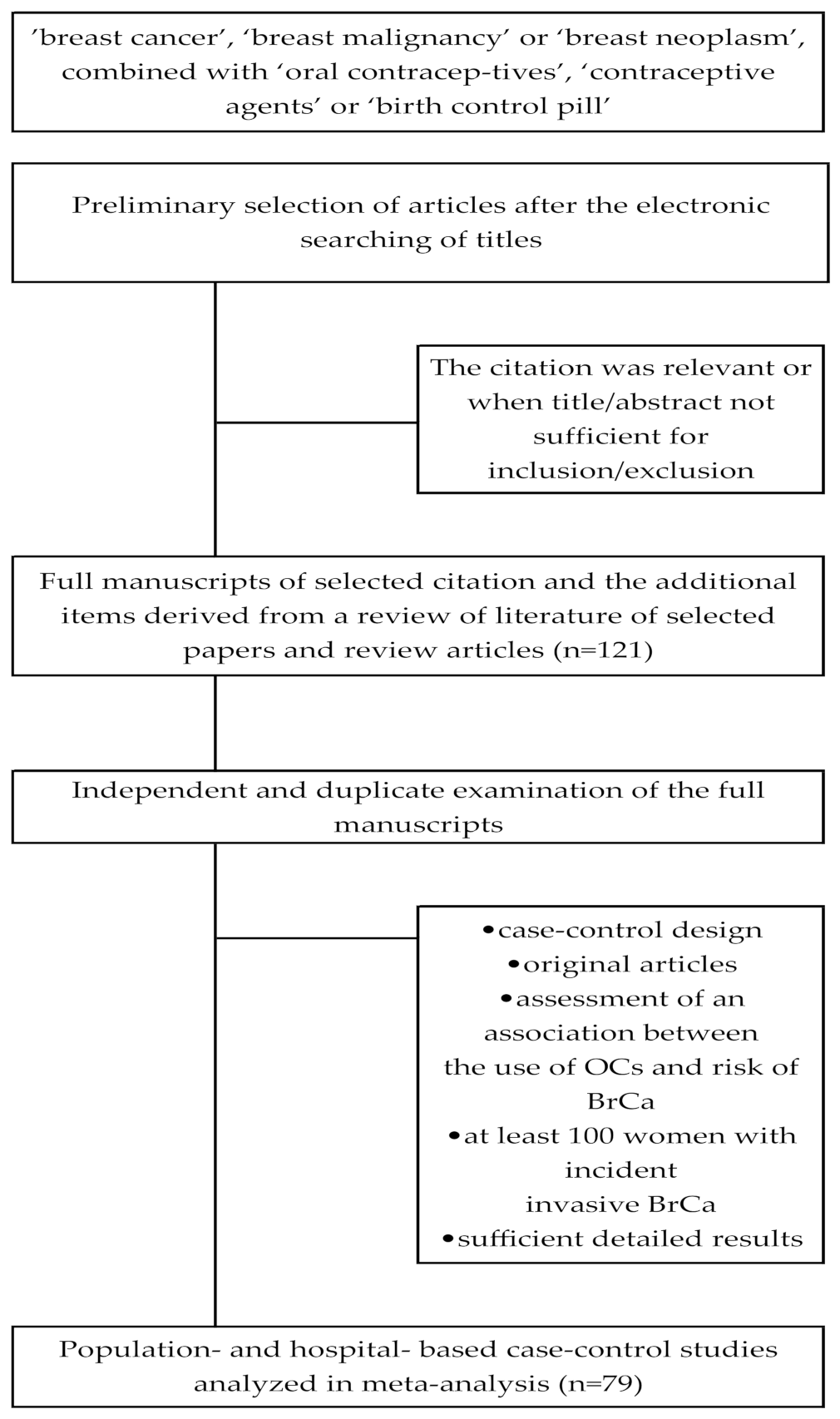
2.2. Study Selection
2.3. Data Extraction
2.4. Assessment of Study Quality
2.5. Statistical Methods
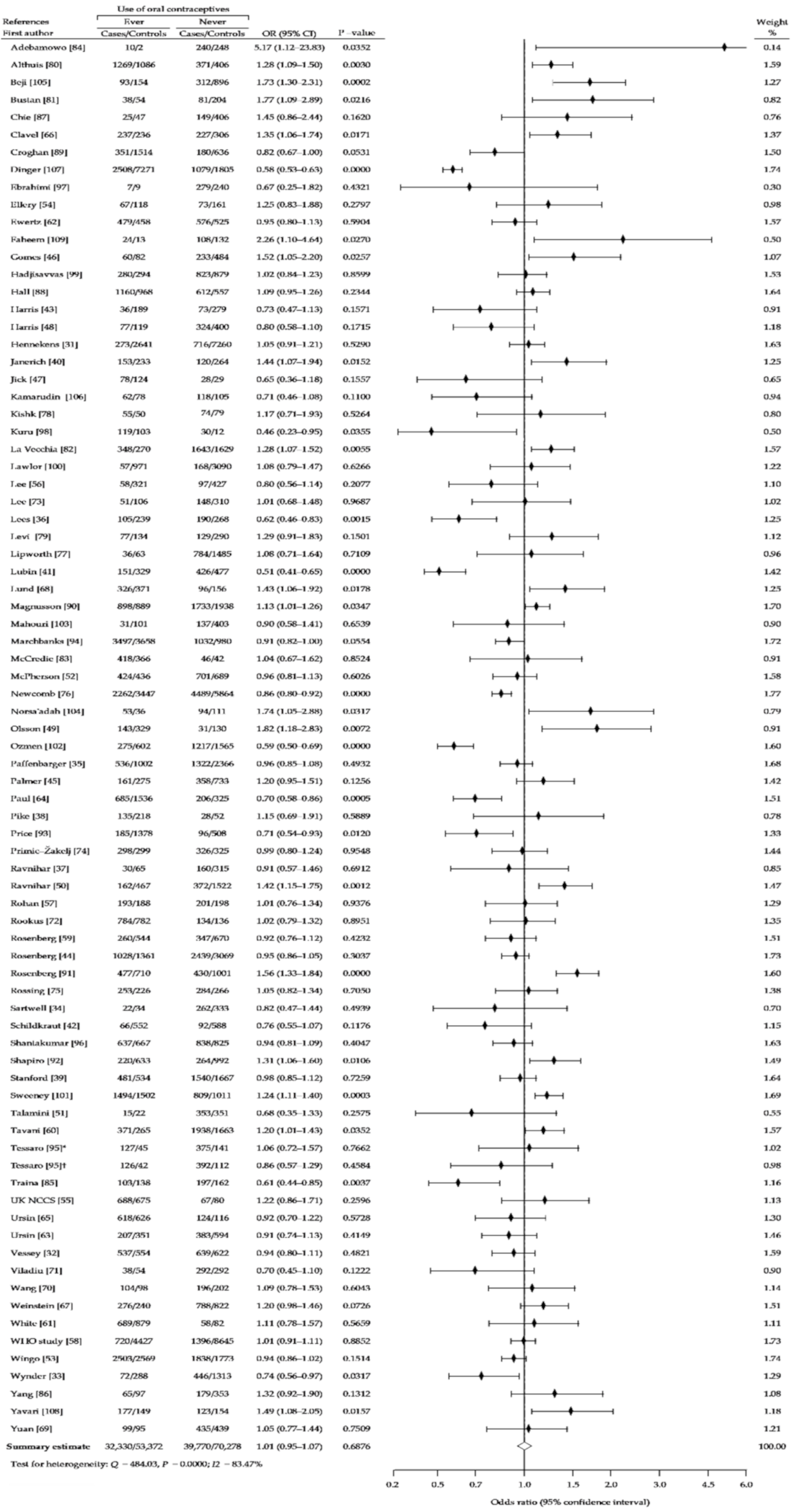
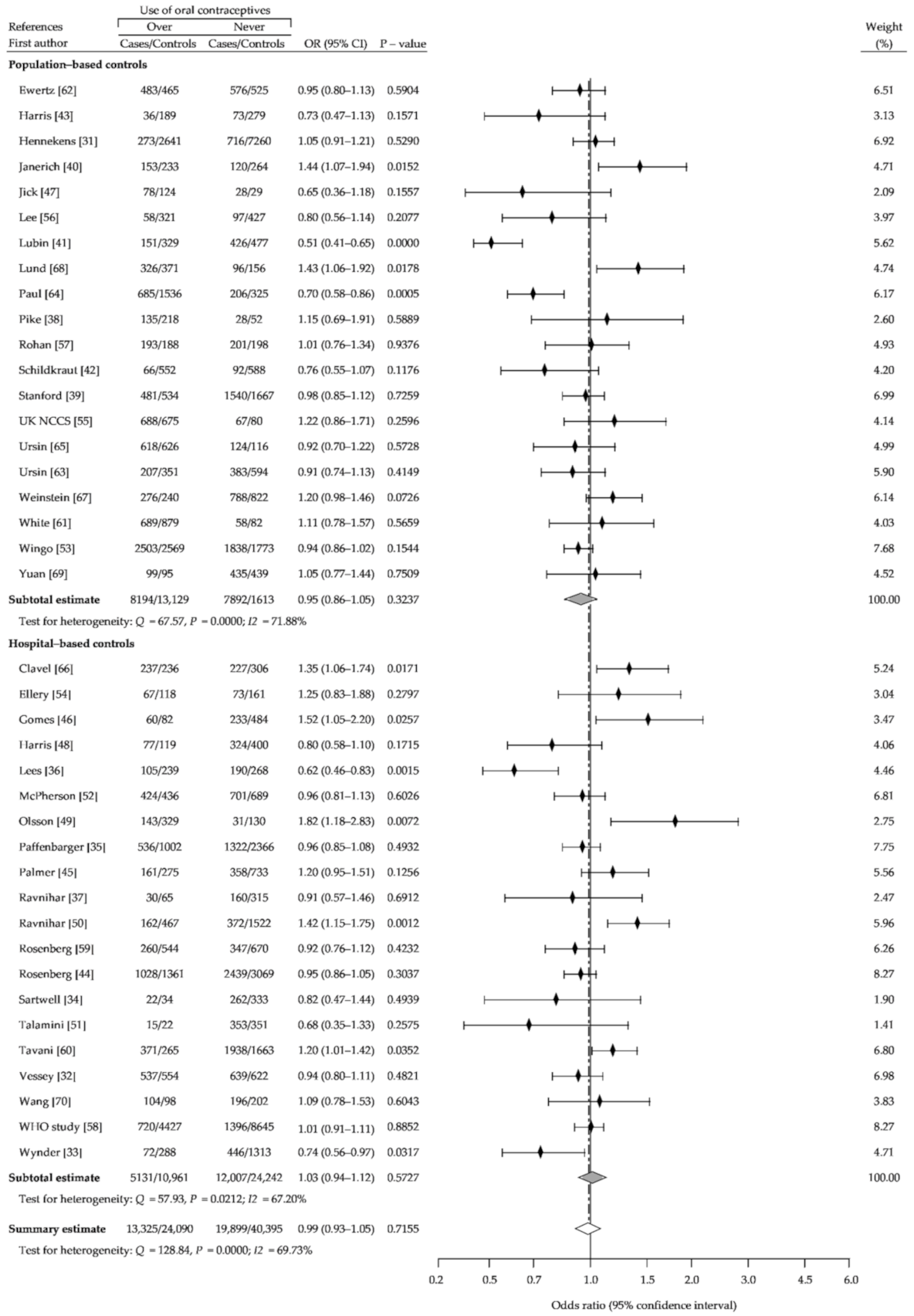
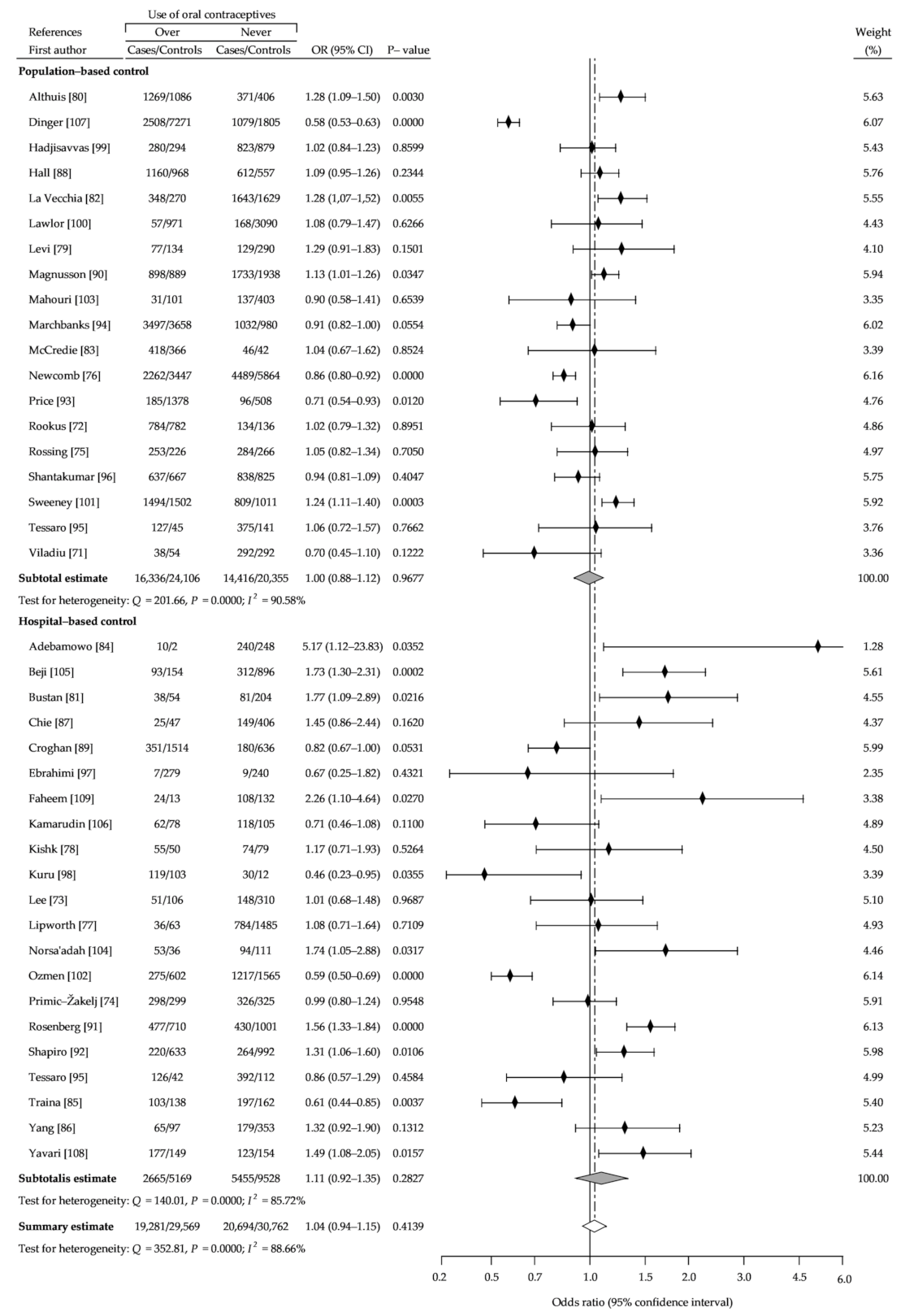
3. Results
3.1. Description of the Studies
3.2. Summary Analysis
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- McPherson, K.; Steel, C.M.; Dixon, J.M. Breast cancer-epidemiology, risk factors, and genetics. BMJ 2000, 321, 624–628. [Google Scholar] [CrossRef]
- Althuis, M.D.; Dozier, J.M.; Anderson, W.F.; Devesa, S.S.; Brinton, L.A. Global trends in breast cancer incidence and mortality 1973–1997. Int. J. Epidemiol. 2005, 34, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Héry, C.; Ferlay, J.; Boniol, M.; Autier, P. Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann. Oncol. 2008, 19, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.G.; Lacey, J.V., Jr.; Carreon, J.D.; Hoover, R.N. Breast cancer incidence, 1980–2006: Combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J. Natl. Cancer Inst. 2007, 99, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ravdin, P.M.; Cronin, K.A.; Howlader, N.; Berg, C.D.; Chlebowski, R.T.; Feuer, E.J.; Edwards, B.K.; Berry, D.A. The decrease in breast-cancer incidence in 2003 in the United States. N. Engl. J. Med. 2007, 356, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Verkooijen, H.M.; Bouchardy, C.; Vinh-Hung, V.; Rapiti, E.; Hartman, M. The incidence of breast cancer and changes in the use of hormone replacement therapy: A review of the evidence. Maturitas 2009, 64, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Casey, P.M.; Cerhan, J.R.; Pruthi, S. Oral contraceptive use and risk of breast cancer. Mayo Clin. Proc. 2008, 83, 86–90. [Google Scholar] [CrossRef]
- Breen, N.; Gentleman, J.F.; Schiller, J.S. Update on mammography trends: Comparisons of rates in 2000, 2005, and 2008. Cancer 2011, 117, 2209–2218. [Google Scholar] [CrossRef]
- Turnbull, C.; Hodgson, S. Genetic predisposition to cancer. Clin. Med. 2005, 5, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Berrino, F.; Key, T.; Rinaldi, S.; Dossus, L.; Biessy, C.; Secreto, G.; Amiano, P.; Bingham, S.; Boeing, H.; et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2005, 97, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Rinaldi, S.; Key, T.J.; Berrino, F.; Peeters, P.M.H.; Biessy, C.; Dossus, L.; Lukanova, A.; Bingham, S.; Khaw, K.-T.; et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: The European prospective investigation into cancer and nutrition. Endocr. Relat. Cancer 2005, 12, 1071–1082. [Google Scholar] [CrossRef]
- Mansour, D.; Inki, P.; Gemzell-Danielsson, K. Efficacy of contraceptive methods: A review of the literature. Eur. J. Contracept. Reprod. Health Care 2010, 15, 4–16. [Google Scholar] [CrossRef]
- IARC Combined estrogen-progestogen contraceptives and combined estrogen-progestogen menopausal therapy. IARC Monogr. Eval. Carcinog. Risks Hum. 2007, 91, 1–528.
- Cibula, D.; Gompel, A.; Mueck, A.O.; La Vecchia, C.; Hannaford, P.C.; Skouby, S.O.; Zikan, M.; Dusek, L. Hormonal contraception and risk of cancer. Hum. Reprod. Update 2010, 16, 631–650. [Google Scholar] [CrossRef]
- Kahlenborn, C.; Modugno, F.; Severs, W.B. Oral contraceptives and breast cancer. Mayo Clin. Proc. 2008, 83, 849–851. [Google Scholar] [CrossRef]
- Hawley, W.; Nuovo, J.; DeNeef, C.P.; Carter, P. Do oral contraceptive agents affect the risk of breast cancer? A meta-analysis of the case-control reports. J. Am. Board Fam. Pract. 1993, 6, 123–135. [Google Scholar]
- Rushton, L.; Jones, D.R. Oral contraceptive use and breast cancer risk: A meta-analysis of variations with age at diagnosis, parity and total duration of oral contraceptive use. Br. J. Obs. Gynaecol. 1992, 99, 239–246. [Google Scholar] [CrossRef]
- Delgado-Rodriguez, M.; Sillero-Arenas, M.; Rodriguez-Contreras, R.; López Gigosos, R.; Gàlvez Vargas, R. Oral contraceptives and breast cancer. A meta-analysis. Rev. Epidemiol. Sante Publique 1991, 39, 165–181. [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727. [Google Scholar] [CrossRef]
- Kahlenborn, C.; Modugno, F.; Potter, D.M.; Severs, W.B. Oral contraceptive use as a risk factor for premenopausal breast cancer: A meta-analysis. Mayo Clin. Proc. 2006, 81, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Gerstman, B.B.; Gross, T.P.; Kennedy, D.L.; Bennett, R.C.; Tomita, D.K.; Stadel, B.V. Trends in the content and use of oral contraceptives in the United States, 1964–1988. Am. J. Public Health 1991, 81, 90–96. [Google Scholar] [CrossRef][Green Version]
- Westhoff, C. Trends in oral contraceptive development and utilization: Looking to the future. Contracept. Rep. 1997, 7, 4–13. [Google Scholar]
- Heinemann, K.; Moehner, S.; Lewis, M.; Assmann, A.; Garbe, E.; Heinemann, L.A. Trend der OC-Nutzung in einer Deutschen Frauen-Kohorte 1980-1999. Ergebnisse der Deutschen Kohortenstudie zur Frauengesundheit. Zent. Gynakol 2002, 124, 128–131. [Google Scholar] [CrossRef]
- Thorogood, M.; Vessey, M.P. Trends in use of oral contraceptives in Britain. Brit. J. Fam. Plan. 1990, 16, 11–53. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in metaanalyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Speizer, F.E.; Lipnick, R.J.; Rosner, B.; Bain, C.; Belanger, C.; Stampfer, M.J.; Willett, W.; Peto, R. A case-control study of oral contraceptive use and breast cancer. J. Natl. Cancer Inst. 1984, 72, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Vessey, M.; Baron, J.; Doll, R.; McPherson, K.; Yeates, D. Oral contraceptives and breast cancer: Final report of an epidemiological study. Br. J. Cancer 1983, 47, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Wynder, E.L.; MacCornack, F.A.; Stellman, S.D. The epidemiology of breast cancer in 785 United States Caucasian women. Cancer 1978, 41, 2341–2354. [Google Scholar] [CrossRef]
- Sartwell, P.E.; Arthes, F.G.; Tonascia, J.A. Exogenous hormones, reproductive history, and breast cancer. J. Natl. Cancer Inst. 1977, 59, 1589–1592. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Kampert, J.B.; Chang, H.G. Characteristics that predict risk of breast cancer before and after the menopause. Am. J. Epidemiol. 1980, 112, 258–268. [Google Scholar] [CrossRef]
- Lees, A.W.; Burns, P.E.; Grace, M. Oral contraceptives and breast disease in premenopausal northern Albertan women. Int. J. Cancer 1978, 22, 700–707. [Google Scholar] [CrossRef]
- Ravnihar, B.; Seigel, D.G.; Lindtner, J. An epidemiologic study of breast cancer and benign breast neoplasia in relation to the oral contraceptive and estrogen use. Eur. J. Cancer 1979, 15, 395–405. [Google Scholar] [CrossRef]
- Pike, M.C.; Henderson, B.E.; Casagrande, J.T.; Rosario, I.; Gray, G.E. Oral contraceptive use and early abortion as risk factors for breast cancer in young women. Br. J. Cancer 1981, 43, 72–76. [Google Scholar] [CrossRef]
- Stanford, J.L.; Brinton, L.A.; Hoover, R.N. Oral contraceptives and breast cancer: Results from an expanded case-control study. Br. J. Cancer 1989, 60, 375–381. [Google Scholar] [CrossRef]
- Janerich, D.T.; Polednak, A.P.; Glebatis, D.M.; Lawrence, C.E. Breast cancer and oral contraceptive use: A case-control study. J. Chronic Dis. 1983, 36, 639–646. [Google Scholar] [CrossRef]
- Lubin, J.H.; Burns, P.E.; Blot, W.J.; Lees, A.W.; May, C.; Morris, L.E.; Fraumeni, J.F., Jr. Risk factors for breast cancer in women in northern Alberta, Canada, as related to age at diagnosis. J. Natl. Cancer Inst. 1982, 68, 211–217. [Google Scholar]
- Schildkraut, J.M.; Hulka, B.S.; Wilkinson, W.E. Oral contraceptives and breast cancer: A case-control study with hospital and community controls. Obs. Gynecol. 1990, 76, 395–402. [Google Scholar] [CrossRef]
- Harris, N.V.; Weiss, N.S.; Francis, A.M.; Polissar, L. Breast cancer in relation to patterns of oral contraceptive use. Am. J. Epidemiol. 1982, 116, 643–651. [Google Scholar] [CrossRef]
- Rosenberg, L.; Palmer, J.R.; Rao, R.S.; Zauber, A.G.; Strom, B.L.; Warshauer, M.E.; Harlap, S.; Shapiro, S. Case-control study of oral contraceptive use and risk of breast cancer. Am. J. Epidemiol. 1996, 143, 25–37. [Google Scholar] [CrossRef]
- Palmer, J.R.; Rosenberg, L.; Rao, R.S.; Strom, B.L.; Warshauer, M.E.; Harlap, S.; Zauber, A.; Shapiro, S. Oral contraceptive use and breast cancer risk among African-American women. Cancer Causes Control 1995, 6, 321–331. [Google Scholar] [CrossRef]
- Gomes, A.L.; Guimarães, M.D.; Gomes, C.C.; Chaves, I.G.; Gobbi, H.; Camargos, A.F. A case-control study of risk factors for breast cancer in Brazil, 1978–1987. Int. J. Epidemiol. 1995, 24, 292–299. [Google Scholar] [CrossRef]
- Jick, S.S.; Walker, A.M.; Stergachis, A.; Jick, H. Oral contraceptives and breast cancer. Br. J. Cancer 1989, 59, 618–621. [Google Scholar] [CrossRef]
- Harris, R.E.; Zang, E.A.; Wynder, E.L. Oral contraceptives and breast cancer risk: A case-control study. Int. J. Epidemiol. 1990, 19, 240–246. [Google Scholar] [CrossRef]
- Olsson, H.; Möller, T.R.; Ranstam, J. Early oral contraceptive use and breast cancer among premenopausal women: Final report from a study in southern Sweden. J. Natl. Cancer Inst. 1989, 81, 1000–1004. [Google Scholar] [CrossRef]
- Ravnihar, B.; Primic-Žakelj, M.; Košmelj, K.; Stare, J. A case-control study of breast cancer in relation to oral contraceptive use in Slovenia. Neoplasia 1988, 35, 109–121. [Google Scholar]
- Talamini, R.; La Vecchia, C.; Franceschi, S.; Colombo, F.; Decarli, A.; Grattoni, E.; Grigoletto, E.; Tognoni, G. Reproductive and hormonal factors and breast cancer in a Northern Italian population. Int. J. Epidemiol. 1985, 14, 70–74. [Google Scholar] [CrossRef] [PubMed]
- McPherson, K.; Vessey, M.P.; Neil, A.; Doll, R.; Jones, L.; Roberts, M. Early oral contraceptive use and breast cancer: Results of another case-control study. Br. J. Cancer 1987, 56, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Wingo, P.A.; Lee, N.C.; Ory, H.W.; Beral, V.; Peterson, H.B.; Rhodes, P. Age specific differences in the relationship between oral contraceptive use and breast cancer. Obs. Gynecol. 1991, 78, 161–170. [Google Scholar] [CrossRef]
- Ellery, C.; MacLennan, R.; Berry, G.; Shearman, R.P. A case-control study of breast cancer in relation to the use of steroid contraceptive agents. Med. J. Aust. 1986, 144, 173–176. [Google Scholar] [CrossRef]
- UK National Case-control Study Group. Oral contraceptive use and breast cancer risk in young women. Lancet 1989, 1, 973–982. [Google Scholar]
- Lee, N.C.; Rosero-Bixby, L.; Oberle, M.W.; Grimaldo, C.; Whatley, A.S.; Rovira, E.Z. A case-control study of breast cancer and hormonal contraception in Costa Rica. J. Natl. Cancer Inst. 1987, 79, 1247–1254. [Google Scholar]
- Rohan, T.E.; McMichael, A.J. Oral contraceptive agents and breast cancer: A population-based case-control study. Med. J. Aust. 1988, 149, 520–526. [Google Scholar] [CrossRef]
- WHO Collaborative Study of Neoplasia and Steroid Contraceptives. Breast cancer and combined oral contraceptives: Results from a multinational study. Br. J. Cancer 1990, 61, 110–119. [Google Scholar] [CrossRef][Green Version]
- Rosenberg, L.; Palmer, J.R.; Clarke, E.A.; Shapiro, S. A case-control study of the risk of breast cancer in relation to oral contraceptive use. Am. J. Epidemiol. 1992, 136, 1437–1444. [Google Scholar] [CrossRef]
- Tavani, A.; Negri, E.; Franceschi, S.; Parazzini, F.; La Vecchia, C. Oral contraceptives and breast cancer in North Italy. Final report from a case-control study. Br. J. Cancer 1993, 68, 568–571. [Google Scholar] [CrossRef]
- White, E.; Malone, K.E.; Weiss, N.S.; Daling, J.R. Breast cancer among young U.S. women in relation to oral contraceptive use. J. Natl. Cancer Inst. 1994, 86, 505–514. [Google Scholar] [CrossRef]
- Ewertz, M. Oral contraceptives and breast cancer risk in Denmark. Eur. J. Cancer 1992, 28A, 1176–1781. [Google Scholar] [CrossRef]
- Ursin, G.; Wu, A.H.; Hoover, R.N.; West, D.W.; Nomura, A.M.; Kolonel, L.N.; Pike, M.C.; Ziegler, R.G. Breast cancer and oral contraceptive use in Asian-American women. Am. J. Epidemiol. 1999, 150, 561–567. [Google Scholar] [CrossRef]
- Paul, C.; Skegg, D.C.G. Spears GF. Oral contraceptive use and risk of breast cancer in older women (New Zealand). Cancer Causes Control 1995, 6, 485–591. [Google Scholar] [CrossRef]
- Ursin, G.; Ross, R.K.; Sullivan-Halley, J.; Hanisch, R.; Henderson, B.; Bernstein, L. Use of oral contraceptives and risk of breast cancer in young women. Breast Cancer Res. Treat. 1998, 50, 175–184. [Google Scholar] [CrossRef]
- Clavel, F.; Andrieu, N.; Gairard, B.; Brémond, A.; Piana, L.; Lansac, J.; Bréart, G.; Rumeau-Rouquette, C.; Flamant, R.; Renaud, R. Oral contraceptives and breast cancer: A French case-control study. Int. J. Epidemiol. 1991, 20, 32–38. [Google Scholar] [CrossRef]
- Weinstein, A.L.; Mahoney, M.C.; Nasca, P.C.; Leske, M.C.; Varma, A.O. Breast cancer risk and oral contraceptive use: Results from a large case-control study. Epidemiology 1991, 2, 353–358. [Google Scholar] [CrossRef]
- Lund, E.; Meirik, O.; Adami, H.O.; Bergstrøm, R.; Christoffersen, T.; Bergsjø, P. Oral contraceptive use and premenopausal breast cancer in Sweden and Norway: Possible effects of different pattern of use. Int. J. Epidemiol. 1989, 18, 527–532. [Google Scholar] [CrossRef]
- Yuan, J.M.; Yu, M.C.; Ross, R.K.; Gao, Y.T.; Henderson, B.E. Risk factors for breast cancer in Chinese women in Shanghai. Cancer Res. 1988, 48, 1949–1953. [Google Scholar]
- Wang, Q.S.; Ross, R.K.; Yu, M.C.; Ning, J.P.; Henderson, B.E.; Kimm, H.T. A case-control study of breast cancer in Tianjin, China. Cancer Epidemiol. Biomark. Prev. 1992, 1, 435–439. [Google Scholar]
- Viladiu, P.; Izquierdo, A.; de Sanjosé, S.; Bosch, F.X. A breast cancer case-control study in Girona, Spain. Endocrine, familial and lifestyle factors. Eur. J. Cancer Prev. 1996, 5, 329–335. [Google Scholar] [CrossRef]
- Rookus, M.A.; van Leeuwen, F.E. Netherlands Oral Contraceptives and Breast Cancer Study Group. Oral contraceptives and risk of breast cancer in women aged 20-54 years. Lancet 1994, 344, 844–851. [Google Scholar] [CrossRef]
- Lee, H.P.; Gourley, L.; Duffy, S.W.; Estève, J.; Lee, J.; Day, N.E. Risk factors for breast cancer by age and menopausal status: A case-control study in Singapore. Cancer Causes Control 1992, 3, 313–322. [Google Scholar] [CrossRef]
- Primic-Žakelj, M.; Evstifeeva, T.; Ravnihar, B.; Boyle, P. Breast-cancer risk and oral contraceptive use in Slovenian women aged 25 to 54. Int. J. Cancer 1995, 62, 414–420. [Google Scholar] [CrossRef]
- Rossing, M.A.; Stanford, J.L.; Weiss, N.S.; Habel, L.A. Oral contraceptive use and risk of breast cancer in middle-aged women. Am. J. Epidemiol. 1996, 144, 161–164. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Longnecker, M.P.; Storer, B.E.; Mittendorf, R.; Baron, J.; Clapp, R.W.; Trentham-Dietz, A.; Willett, W.C. Recent oral contraceptive use and risk of breast cancer (United States). Cancer Causes Control 1996, 7, 525–532. [Google Scholar] [CrossRef]
- Lipworth, L.; Katsouyanni, K.; Stuver, S.; Samoli, E.; Hankinson, S.E.; Trichopoulos, D. Oral contraceptives, menopausal estrogens, and the risk of breast cancer: A case-control study in Greece. Int. J. Cancer 1995, 62, 548–551. [Google Scholar] [CrossRef]
- Kishk, N.A. Breast cancer in relation to some reproductive factors. J. Egypt Public Health Assoc. 1999, 74, 547–566. [Google Scholar]
- Levi, F.; Lucchini, F.; Pasche, C.; La Vecchia, C. Oral contraceptives, menopausal hormone replacement treatment and breast cancer risk. Eur. J. Cancer Prev. 1996, 5, 259–266. [Google Scholar] [CrossRef]
- Althuis, M.D.; Brogan, D.R.; Coates, R.J.; Daling, J.R.; Gammon, M.D.; Malone, K.E.; Schoenberg, J.B.; Brinton, L.A. Hormonal content and potency of oral contraceptives and breast cancer risk among young women. Br. J. Cancer 2003, 88, 50–57. [Google Scholar] [CrossRef]
- Bustan, M.N.; Coker, A.L.; Addy, C.L.; Macera, C.A.; Greene, F.; Sampoerno, D. Oral contraceptive use and breast cancer in Indonesia. Contraception 1993, 47, 241–249. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; Franceschi, S.; Talamini, R.; Amadori, D.; Filiberti, R.; Conti, E.; Montella, M.; Veronesi, A.; Parazzini, F.; et al. Oral contraceptives and breast cancer: A cooperative Italian study. Int. J. Cancer 1995, 60, 163–167. [Google Scholar] [CrossRef] [PubMed]
- McCredie, M.R.; Dite, G.S.; Giles, G.G.; Hopper, J.L. Breast cancer in Australian women under the age of 40. Cancer Causes Control 1998, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Adebamowo, C.A.; Adekunle, O.O. Case-controlled study of the epidemiological risk factors for breast cancer in Nigeria. Br. J. Surg. 1999, 86, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Traina, A.; Cusimano, R.; Liquori, M.; Calabria, C.; Biglia, N.; Ponzone, R.; Sgrò, L.; Sismondi, P. Oral contraceptive use and breast cancer risk in areas with different incidence: A case-control study among young women. Ann. N. Y. Acad. Sci. 1996, 784, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.S.; Yang, T.L.; Liu, C.L.; Wu, C.W.; Shen, C.Y. A case-control study of breast cancer in Taiwan–a low-incidence area. Br. J. Cancer 1997, 75, 752–756. [Google Scholar] [CrossRef]
- Chie, W.C.; Li, C.Y.; Huang, C.S.; Chang, K.J.; Yen, M.L.; Lin, R.S. Oral contraceptives and breast cancer risk in Taiwan, a country of low incidence of breast cancer and low use of oral contraceptives. Int. J. Cancer 1998, 77, 219–223. [Google Scholar] [CrossRef]
- Hall, I.J.; Moorman, P.G.; Millikan, R.C.; Newman, B. Comparative analysis of breast cancer risk factors among African-American women and White women. Am. J. Epidemiol. 2005, 161, 40–51. [Google Scholar] [CrossRef]
- Croghan, I.T.; Pruthi, S.; Hays, J.T.; Cha, S.; Johnson, R.E.; Kosel, M.; Morris, R.; Hurt, R.D. The role of smoking in breast cancer development: An analysis of a Mayo Clinic cohort. Breast J. 2009, 15, 489–495. [Google Scholar] [CrossRef]
- Magnusson, C.M.; Persson, I.R.; Baron, J.A.; Ekbom, A.; Bergström, R.; Adami, H.O. The role of reproductive factors and use of oral contraceptives in the aetiology of breast cancer in women aged 50 to 74 years. Int. J. Cancer 1999, 80, 231–236. [Google Scholar] [CrossRef]
- Rosenberg, L.; Zhang, Y.; Coogan, P.F.; Strom, B.L.; Palmer, J.R. A case-control study of oral contraceptive use and incident breast cancer. Am. J. Epidemiol. 2009, 169, 473–479. [Google Scholar] [CrossRef]
- Shapiro, S.; Rosenberg, L.; Hoffman, M.; Truter, H.; Cooper, D.; Rao, S.; Dent, D.; Gudgeon, A.; van Zyl, J.; Katzenellenbogen, J.; et al. Risk of breast cancer in relation to the use of injectable progestogen contraceptives and combined estrogen/progestogen contraceptives. Am. J. Epidemiol. 2000, 151, 396–403. [Google Scholar] [CrossRef]
- Price, M.A.; Tennant, C.C.; Smith, R.C.; Kennedy, S.J.; Butow, P.N.; Kossoff, M.B.; Dunn, S.M. Predictors of breast cancer in women recalled following screening. Aust. N. Z. J. Surg. 1999, 69, 639–646. [Google Scholar] [CrossRef]
- Marchbanks, P.A.; McDonald, J.A.; Wilson, H.G.; Folger, S.G.; Mandel, M.G.; Daling, J.R.; Bernstein, L.; Malone, K.E.; Ursin, G.; Strom, B.L.; et al. Oral contraceptives and the risk of breast cancer. N. Engl. J. Med. 2002, 346, 2025–2032. [Google Scholar] [CrossRef]
- Tessaro, S.; Béria, J.U.; Tomasi, E.; Barros, A.J.D. Contraceptivos orais e cancer de mama: Estudo de casos e controles. Rev. Saúde Pública 2001, 35, 32–38. [Google Scholar] [CrossRef]
- Shantakumar, S.; Terry, M.B.; Paykin, A.; Teitelbaum, S.L.; Britton, J.A.; Moorman, P.G.; Kritchevsky, S.B.; Neugut, A.I.; Gammon, M.D. Age and menopausal effects of hormonal birth control and hormone replacement therapy in relation to breast cancer risk. Am. J. Epidemiol. 2007, 165, 1187–1198. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Vahdaninia, M.; Montazeri, A. Risk factors for breast cancer in Iran: A case-control study. Breast Cancer Res. 2002, 4, R10. [Google Scholar] [CrossRef]
- Kuru, B.; Ozaslan, C.; Ozdemir, P.; Dinç, S.; Camlibel, M.; Alagöl, H. Risk factors for breast cancer in Turkish women with early pregnancies and long-lasting lactation—a case-control study. Acta Oncol. 2002, 41, 556–561. [Google Scholar] [CrossRef]
- Hadjisavvas, A.; Loizidou, M.A.; Middleton, N.; Michael, T.; Papachristoforou, R.; Kakouri, E.; Daniel, M.; Papadopoulos, P.; Malas, S.; Marcou, Y.; et al. An investigation of breast cancer risk factors in Cyprus: A case control study. BMC Cancer 2010, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.A.; Ebrahim, S.; Smith, G.D. Smoking before the birth of a first child is not associated with increased risk of breast cancer: Findings from the British Women’s Heart and Health Cohort Study and a meta-analysis. Br. J. Cancer 2004, 91, 512–518. [Google Scholar] [CrossRef][Green Version]
- Sweeney, C.; Giuliano, A.R.; Baumgartner, K.B.; Byers, T.; Herrick, J.S.; Edwards, S.L.; Slattery, M.L. Oral injected implanted contraceptives breast cancer risk among, U.S. Hispanic and non-Hispanic white women. Int. J. Cancer 2007, 121, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, V.; Ozcinar, B.; Karanlik, H.; Cabioglu, N.; Tukenmez, M.; Disci, R.; Ozmen, T.; Igci, A.; Muslumanoglu, M.; Kecer, M.; et al. Breast cancer risk factors in Turkish women—a University Hospital based nested case control study. World J. Surg. Oncol. 2009, 7, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahouri, K.; Dehghani Zahedani, M.; Zare, S. Breast cancer risk factors in south of Islamic Republic of Iran: A case-control study. East. Mediterr. Health J. 2007, 13, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Norsa’adah, B.; Rusli, B.N.; Imran, A.K.; Naing, I.; Winn, T. Risk factors of breast cancer in women in Kelantan, Malaysia. Singap. Med. J. 2005, 46, 698–705. [Google Scholar]
- Beji, N.K.; Reis, N. Risk factors for breast cancer in Turkish women: A hospital-based case-control study. Eur. J. Cancer Care 2007, 16, 178–184. [Google Scholar] [CrossRef]
- Kamarudin, R.; Shah, S.A.; Hidayach, N. Lifestyle factors and breat cancer: A case-control study in Kuala Lumpur, Malaysia. Asian Pac. J. Cancer Prev. 2006, 7, 51–54. [Google Scholar]
- Dinger, J.C.; Heinemann, L.A.J.; Möhner, S.; Thai, D.M.; Assman, A. Breast cancer risk associated with different HRT formulations: A register-based case-control study. BMC Women’s Health 2006, 6, 13. [Google Scholar] [CrossRef][Green Version]
- Yavari, P.; Mosavizadeh, M.; Sadrol-Hefazi, B.; Mehrabi, Y. Reproductive characteristics and the risk of breast cancer-a case-control study in Iran. Asian Pac. J. Cancer Prev. 2005, 6, 370–375. [Google Scholar]
- Faheem, M.; Khurram, M.; Jafri, I.A.; Mehmood, H.; Hasan, Z.; Iqbal, G.S.; Maqsood, F.; Jafri, S.R.A. Risk factors for breast cancer in patients treated at NORI Hospital, Islamabad. J. Pak. Med. Assoc. 2007, 57, 242–245. [Google Scholar]
- Romieu, I.; Berlin, J.A.; Colditz, G. Oral contraceptives and breast cancer. Review and meta-analysis. Cancer 1990, 66, 2253–2263. [Google Scholar] [CrossRef]
- Thomas, D.B. Oral contraceptives and breast cancer: Review of the epidemiologic literature. Contraception 1991, 43, 597–642. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Further results. Contraception 1996, 54, 1S–106S. [Google Scholar] [CrossRef]
- Brohet, R.M.; Goldgar, D.E.; Easton, D.F.; Antoniou, A.C.; Andrieu, N.; Chang-Claude, J.; Peock, S.; Eeles, R.A.; Cook, M.; Chu, C.; et al. Oral Contraceptives and Breast Cancer Risk in the International BRCA1/2 Carrier Cohort Study: A Report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J. Clin. Oncol. 2007, 25, 3831–3836. [Google Scholar] [CrossRef]
- Lee, E.; Ma, H.; McKean-Cowdin, R.; Van Den Berg, D.; Bernstein, L.; Henderson, B.E.; Ursin, G. Effect of Reproductive Factors and Oral Contraceptives on Breast Cancer Risk in BRCA1/2 Mutation Carriers and Noncarriers: Results from a Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3170–3178. [Google Scholar] [CrossRef]
- Milne, R.L.; Knight, J.A.; John, E.M.; Dite, G.S.; Balbuena, R.; Ziogas, A.; Andrulis, I.L.; West, D.W.; Li, P.F.; Southey, M.C.; et al. Oral Contraceptive Use and Risk of Early-Onset Breast Cancer in Carriers and Noncarriers of BRCA1 and BRCA2 Mutations. Cancer Epidemiol. Biomark. Prev. 2005, 14, 350–356. [Google Scholar] [CrossRef][Green Version]
- Petitti, D. Meta-Analysis, Decision, Analysis, and Cost-Effectiveness Analysis; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
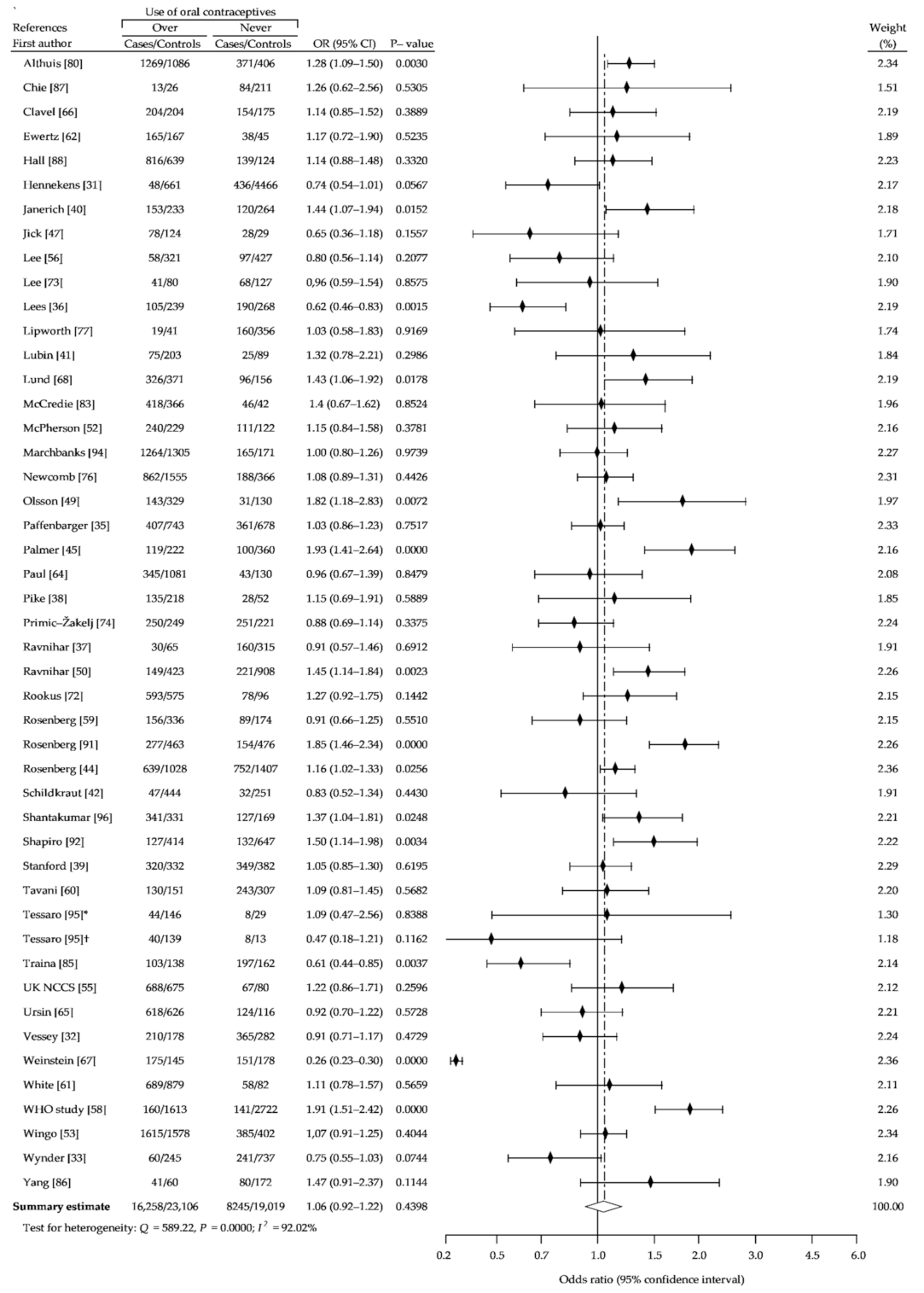
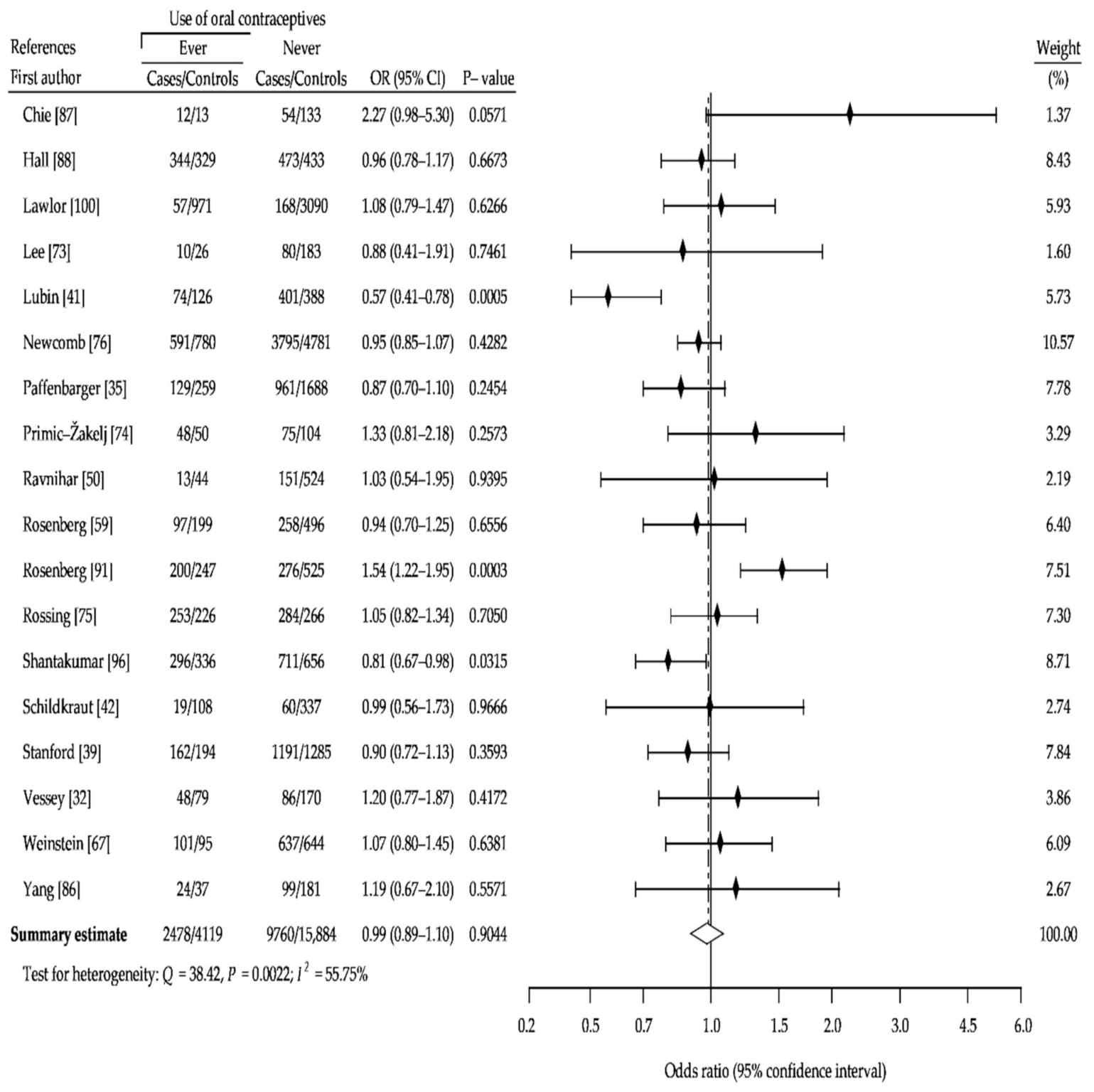
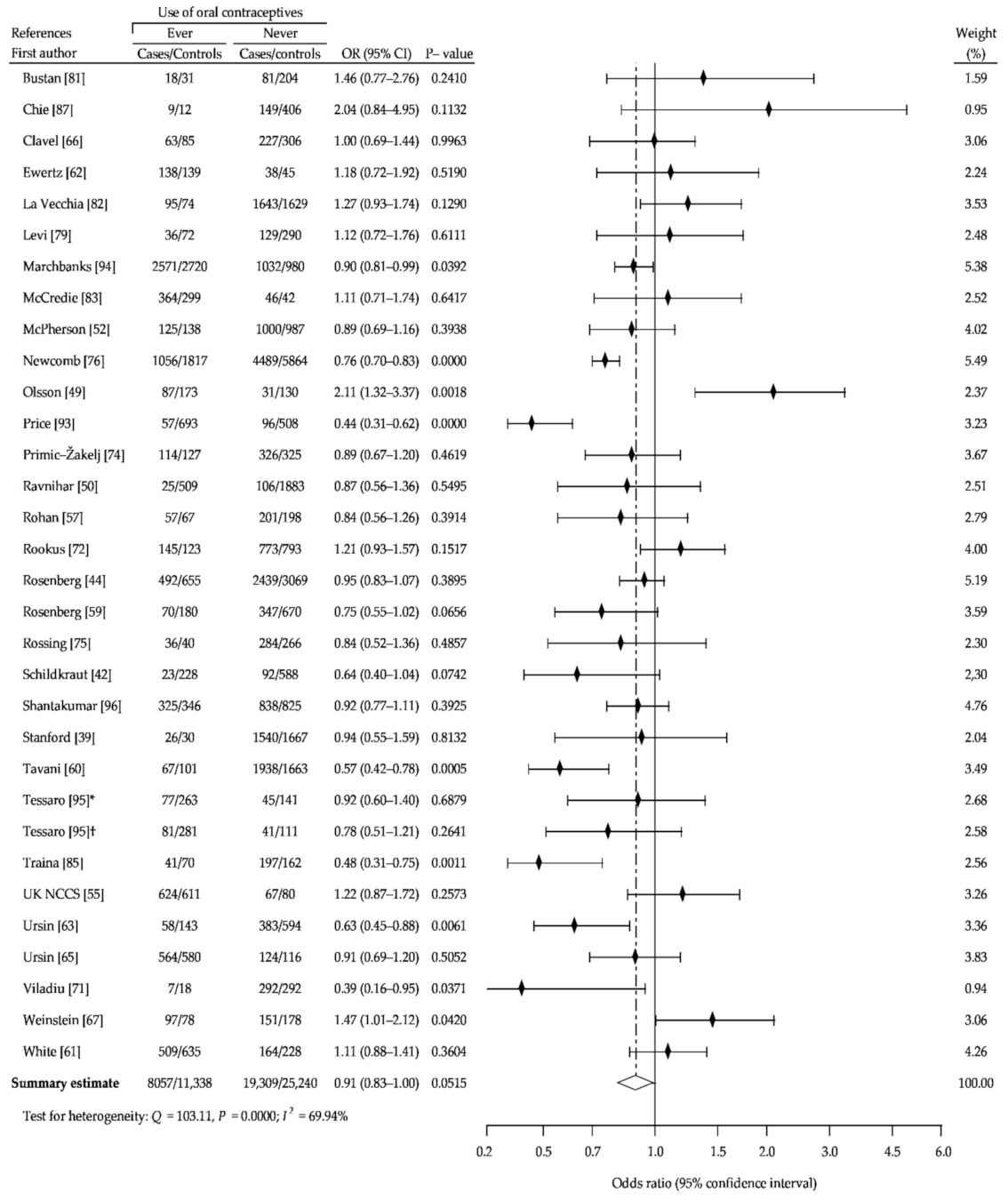
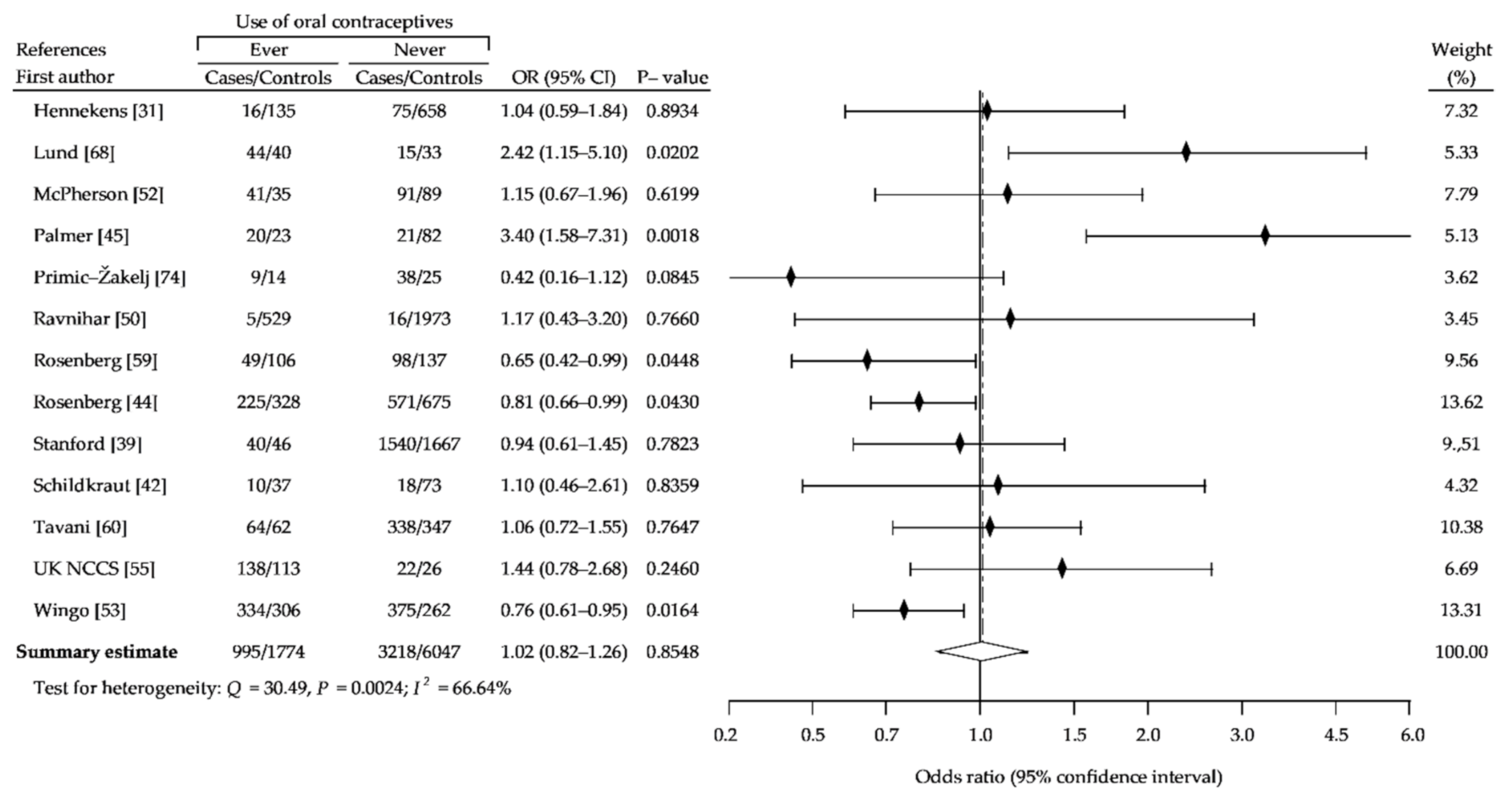
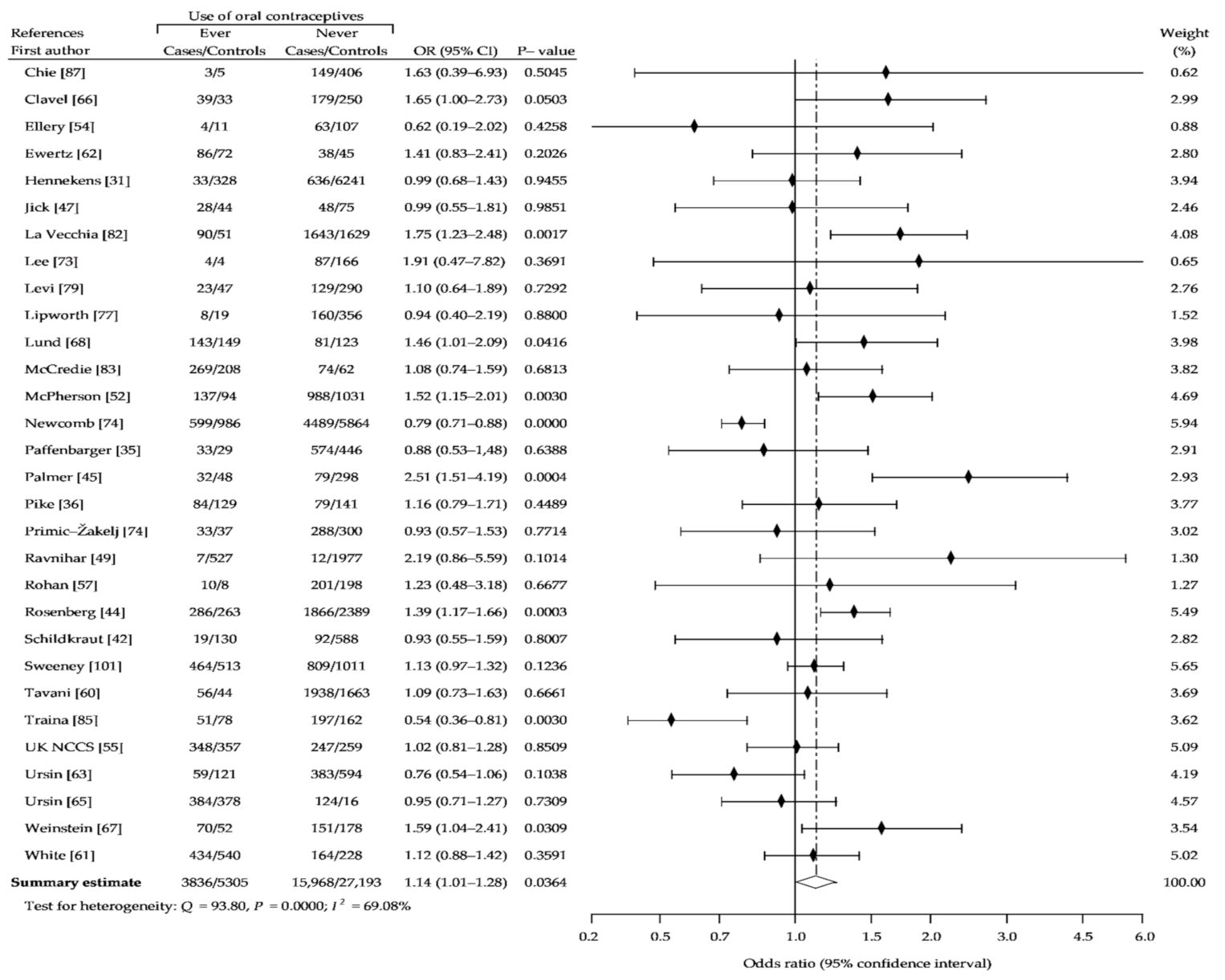
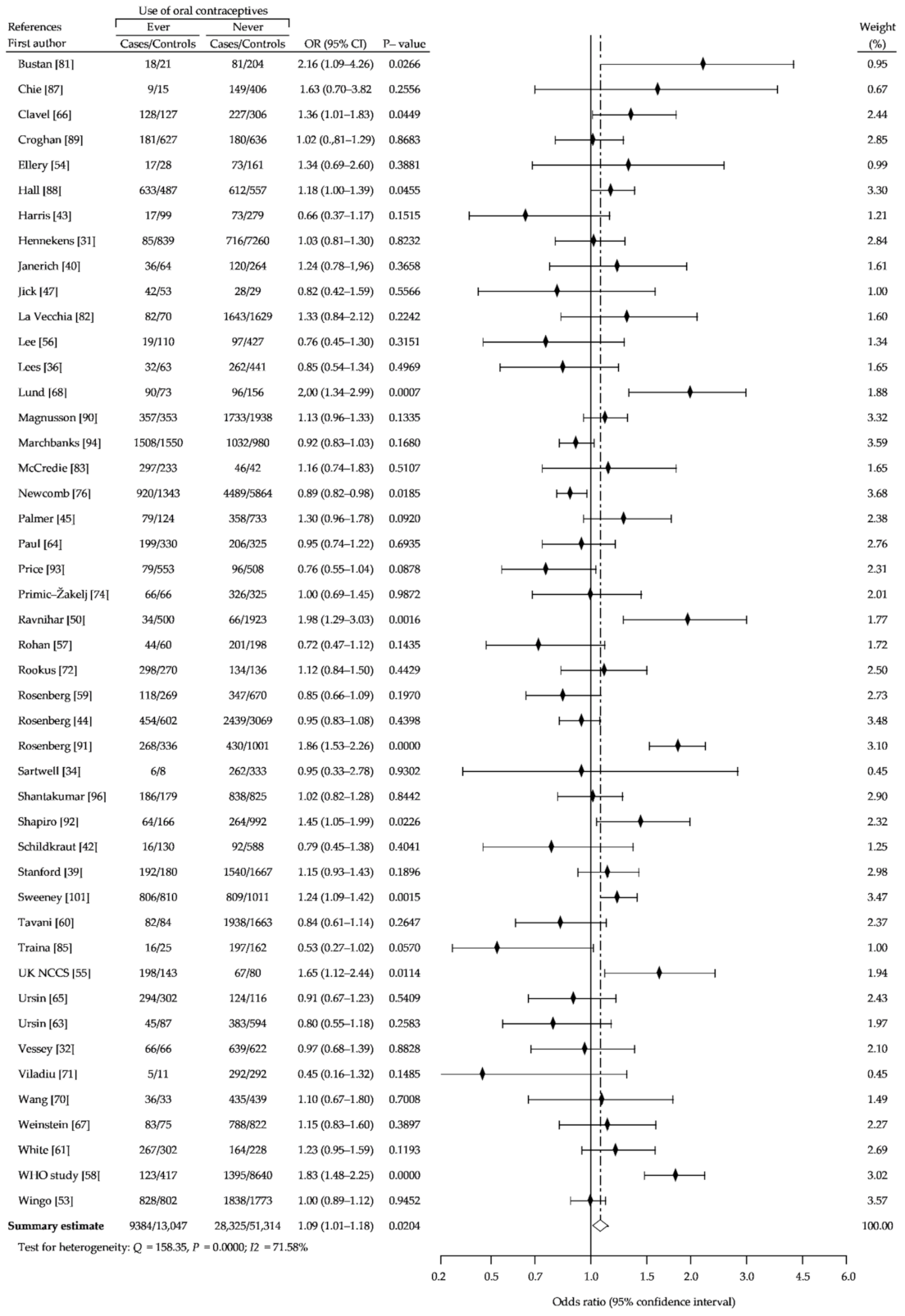
| Study Name | Recruitment Period | Number of Case Subjects | Percent of OCs Users | Age Range | Source of Cases | Number of Controls Subjects | Percent of OCs Users | Source of Controls | NOS Sc. |
|---|---|---|---|---|---|---|---|---|---|
| Setting [Reference] | (Years) | ||||||||
| USA—Nationwide [31] | 1960—1976 | 989 | 27.6 | 30–55 | Population | 9901 | 26.7 | Population | 5 |
| UK—London, Oxford [32] | December 1968–September 1980 | 1176 | 45.7 | 16–50 | Hospital | 1176 | 47.1 | Hospital | 5 |
| USA—New York City [33] | January 1969–December 1975 | 518 | 13.9 | 30< | Hospital | 1601 | 18.0 | Hospital | 5 |
| USA—Baltimore [34] | 1969–1972 | 284 | 7.7 | 20–74 | Hospital | 367 | 9.3 | Hospital | 5 |
| USA—San Francisco Bay Area [35] | January 1970–December 1972 | 1868 | 28.7 | 15–49+ | Hospital | 3391 | 29.6 | Hospital | 5 |
| Canada—northern Alberta [36] | January 1971–December 1974 | 295 | 35.6 | 30–49 | Hospital | 507 | 47.1 | Clinic | 5 |
| former Yugoslavia—Slovenia [37] | May 1972–November 1974 | 190 | 15.8 | 20–49 | Hospital | 380 | 17.1 | Clinic | 5 |
| USA—Los Angeles County [38] | July 1972–December 1978 | 163 | 82.8 | <33 | Population | 270 | 80.7 | Population | 5 |
| Breast Cancer Detection Demonstration Project USA–Nationwide [39] | July 1973–November 1980 | 2022 | 23.8 | <40–60+ | Population | 2183 | 24.5 | Population | 5 |
| USA—New York State [40] | January 1974–August 1976 | 253 | 52.6 | ≤45 | Population | 497 | 46.9 | Population | 5 |
| Canada—northern Alberta [41] | 1976–1977 | 577 | 26.2 | 30–80 | Population | 826 | 42.3 | Population | 5 |
| USA—North Carolina [42] | April 1977–December 1978 | 158 | 41.8 | 25–59 | Hospital | 1140 | 48.4 | Population | 5 |
| USA—King County [43] | July 1977–August 1978 | 112 | 34.8 | 35–54 | Population | 466 | 39.7 | Population | 5 |
| Case-Control Surveillance Study USA—Philadelphia. Baltimore, New York City [44,45] | 1977–1992 | 3540 | 31.1 | 25–59 | Hospital | 4488 | 31.6 | Hospital | 7 |
| 519 | 31.0 | 25–59 | Hospital | 1008 | 27.3 | Hospital | 5 | ||
| Brazil—Belo Horizonte [46] | January 1978–December 1987 | 293 | 20.5 | 25–75 | Hospital | 566 | 14.5 | Hospital | 5 |
| USA—Seattle [47] | July 1978–December 1983 | 127 | 61.4 | <43 | Population | 174 | 71.3 | Population | 5 |
| USA—New York City [48] | June 1979–February 1981 | 401 | 19.2 | <30–70+ | Hospital | 519 | 22.9 | Hospital | 5 |
| Sweden—southern region [49] | 1979–1985 | 174 | 82.2 | <45 | Population | 459 | 71.7 | Population | 5 |
| former Yugoslavia—Slovenia [50] | January 1980–September 1983 | 534 | 30.3 | 24–54 | Hospital | 1989 | 23.9 | Hospital | 5 |
| Italy—Pordenone [51] | January 1980–March 1983 | 368 | 4.1 | 27–79 | Hospital | 373 | 5.9 | Hospital | 5 |
| UK—London. Oxford [52] | September 1980–1984 | 1125 | 37.7 | 16–64 | Hospital | 1125 | 38.8 | Hospital | 5 |
| Cancer and Steroid Hormone Study USA—Atlanta. Connecticut, Detroit. Iowa, New Mexico. San Francisco. Seattle, Utach [53] | December 1980–December 1982 | 4341 | 57.7 | 20–54 | Population | 4343 | 59.2 | Population | 5 |
| Australia—New South Wales [54] | 1980–1982 | 141 | 48.2 | 25–64 | Hospital | 279 | 42.3 | Hospital | 5 |
| UK National Case-Control Study UK—England and Scotland [55] | January 1982–December 1985 | 755 | 91.1 | <35 | Population | 755 | 89.3 | Clinic | 6 |
| Costa Rica [56] | January 1982–March1984 | 155 | 37.4 | 20–49 | Population | 748 | 42.9 | Population | 5 |
| Australia—Adelaide [57] | April 1982–July 1984 | 395 | 48.9 | 20–69 | Population | 386 | 48.7 | Population | 5 |
| WHO Collaborative Study of Neoplasia and Steroid Contraceptives * [58] | November 1982–February 1986 | 2116 | 34.0 | 20–59 | Hospital | 13072 | 33.9 | Hospital | 7 |
| Canada—Toronto [59] | 1982–1986 | 607 | 42.8 | <69 | Hospital | 1214 | 44.8 | Population | 5 |
| Italy—greater Milan area [60] | January 1983–December 1991 | 2309 | 16.1 | 22–59 | Hospital | 1928 | 13.7 | Hospital | 5 |
| USA—Seattle metropolitan area [61] | January 1983–April 1990 | 747 | 92.2 | 21–45 | Population | 961 | 91.5 | Population | 6 |
| Denmark [62] | March 1983–August 1984 | 1059 | 45.6 | <59 | Population | 990 | 47.0 | Population | 5 |
| USA—San Francisco-Oakland, Los Angeles, Oahu [63] | April 1983–June 1987 | 590 | 35.1 | 20–55 | Population | 945 | 37.1 | Population | 6 |
| Auckland Breast Cancer Study New Zealand [64] | July 1983–June 1987 | 891 | 76.9 | 25–54 | Population | 1861 | 82.5 | Population | 6 |
| USA—Los Angeles County [65] | July 1983–January 1989 | 744 | 83.1 | <40 | Population | 744 | 84.1 | Population | 6 |
| France–Strasburg, Lyons, Tours, Marseilles [66] | 1983–1987 | 464 | 51.1 | 20–55 | Hospital | 542 | 43.5 | Hospital | 5 |
| USA—Nassau and Suffolk County [67] | January 1984–December 1986 | 1420 | 25.9 | 20–79 | Population | 1420 | 22.6 | Population | 5 |
| Sweden and Norway [68] | May 1984–May 1985 | 422 | 77.3 | 20–49 | Population | 527 | 70.4 | Population | 5 |
| China—Shanghai [69] | June 1984–May 1985 | 534 | 18.5 | 20–69 | Population | 534 | 17.8 | Population | 5 |
| China—Tiajin [70] | January 1985–November 1986 | 300 | 34.7 | 20–55 | Hospital | 300 | 32.7 | Population | 5 |
| Spain—Girona [71] | July 1986–June 1993 | 330 | 11.5 | <75 | Population | 346 | 18.5 | Population | 6 |
| Netherlands Oral Contraceptives and Breast Cancer Study The Netherlands [72] | October 1986–June 1989 | 918 | 85.4 | 20–54 | Population | 918 | 85.2 | Population | 6 |
| Singapore [73] | 1986–1988 | 200 | 25.5 | <40–70+ | Hospital | 420 | 25.2 | Hospital | 5 |
| Slovenia [74] | January 1988–December 1990 | 624 | 47.8 | 25–54 | Population | 624 | 47.9 | Population | 6 |
| USA—King County [75] | January 1988–June 1990 | 537 | 47.1 | 50–64 | Population | 492 | 45.9 | Population | 6 |
| USA—Wisconsin, Maine, Massachusetts (excluding Boston), New Hampshire [76] | April 1988–December 1991 | 6751 | 35.5 | <75 | Population | 9311 | 37.0 | Population | 5 |
| Greece—Athens, Piareus [77] | January 1989–December 1991 | 820 | 4.4 | 56.4 † | Hospital | 1548 | 4.1 | Hospital | 6 |
| Egypt [78] | n.a. | 129 | 57.4 | 44.5 † | Hospital | 129 | 61.2 | Hospital | 5 |
| Switzerland—Canton of Vaud [79] | January 1990–August 1995 | 206 | 37.4 | 27–75 | Population | 424 | 31.6 | Hospital | 6 |
| USA—Atlanta, Seattle, New Jersey [80] | May 1990–December 1992 | 1640 | 77.4 | 20–44 | Population | 1492 | 72.8 | Population | 5 |
| Indonesia [81] | 1990–1991 | 119 | 31.9 | 25–55 | Hospital | 258 | 20.9 | Hospital | 5 |
| Italy—Milan, Genoa, Naples, the provinces of Pordenone, Gorizia. Forli, Latina (near Rome) [82] | June 1991–February 1994 | 1991 | 17.5 | 23–64 | Population | 1899 | 14.2 | Population | 6 |
| Australian Breast Cancer Family Study Australia—Melbourne, Sydney [83] | January 1992–July 1995 | 467 | 90.2 | <40 | Population | 408 | 89.7 | Population | 5 |
| Nigeria [84] | April 1992–December 1995 | 250 | 4.0 | 43 † | Hospital | 250 | 0.8 | Hospital | 5 |
| Italy—Palermo, Turin [85] | 1992–1994 | 300 | 34.3 | <46 | Hospital | 300 | 46.0 | Hospital | 5 |
| Taiwan—Taipei [86] | January 1993–December 1994 | 224 | 29.0 | 20–80 | Hospital | 450 | 21.6 | Clinic | 5 |
| Taiwan—Taipei [87] | February 1993–June 1994 | 174 | 14.4 | 47.7 † | Hospital | 453 | 10.8 | Hospital | 5 |
| Carolina Breast Cancer Study USA—North Carolina [88] | May 1993–December 2000 | 1778 | 65.6 | 20–74 | Population | 1535 | 63.7 | Population | 5 |
| USA–Rochester [89] | August 1993–November 2003 | 531 | 66.1 | 58 † | Hospital | 2150 | 70.4 | Clinic | 6 |
| Sweden [90] | October 1993–March 1995 | 3008 | 35.5 | 50–74 | Population | 3248 | 33.0 | Population | 5 |
| Case-Control Surveillance Study USA—Philadelphia, Baltimore, New York [91] | 1993–2007 | 907 | 52.6 | 25–69 | Hospital | 1711 | 41.5 | Hospital | 7 |
| South Africa—Cape Town [92] | January 1994–October 1997 | 484 | 45.5 | 20–54 | Hospital | 1625 | 39.0 | Hospital | 7 |
| Australia—New South Wales [93] | April 1994–April 1997 | 298 | 62.1 | 40–87 | Population | 1926 | 71.5 | Population | 5 |
| Women’s Contraceptive and Reproductive Experiences Study USA—Atlanta, Detroit, Philadelphia, Los Angeles, Seattle [94] | July 1994–April 1998 | 4575 | 76.1 | 35–64 | Population | 4682 | 78.1 | Population | 5 |
| Brazil–Pelotas [95] | March 1995–July 1998 | 172 | 73.8 | 20–60 | Hospital | 516 | 72.7 | Hospital | 6 |
| 168 | 75.0 | 504 | 77.8 | Population | |||||
| Long Island Breast Cancer Study Project USA—Long Island [96] | August 1996–July 1997 | 1475 | 43.2 | 20–98 | Population | 1492 | 44.7 | Population | 6 |
| Iran—Tehran [97] | April 1997–April 1998 | 286 | 2.4 | 24–81 | Hospital | 249 | 3.6 | Hospital | 5 |
| Turkey—Ankara [98] | January 1998–September 1999 | 504 | 23.6 | 49.4 † | Hospital | 610 | 16.9 | Hospital | 5 |
| Cyprus [99] | January 1999–December 2005 | 1109 | 25.3 | 40–70 | Population | 1177 | 25.0 | Population | 6 |
| British Women’s Heart and Health Cohort Study UK—Nationwide [100] | April 1999–March 2001 | 225 | 25.4 | 60–79 | Population | 4061 | 23.9 | Population | 5 |
| USA—Arizona, Colorado, Utah, New Mexico [101] | October 1999–May 2004 | 2303 | 64.9 | <64 | Population | 2513 | 59.8 | Population | 7 |
| Turkey–Istanbul [102] | January 2000–December 2006 | 1492 | 18.4 | 35–70 | Hospital | 2167 | 27.8 | Clinic | 7 |
| Iran—Bandar Abbas [103] | April 2000–March 2002 | 168 | 18.5 | 27–92 | Population | 504 | 20.0 | Population | 6 |
| Malaysia–Kelantan [104] | July 2000–June 2001 | 147 | 36.1 | 26–70 | Hospital | 147 | 24.5 | Hospital | 5 |
| Turkey—Istanbul [105] | September 2002–October 2003 | 405 | 23.0 | 28–72 | Hospital | 1050 | 14.7 | Hospital | 7 |
| Malaysia—Kuala Lumper [106] | July 2004–September 2004 | 200 | 34.4 | 48.7 † | Hospital | 183 | 42.6 | Clinic | 6 |
| Germany [107] | 2004–2005 | 3593 | 69.8 | n.a. | Population | 9098 | 79.8 | Population | 5 |
| Iran—Teheran [108] | 2004 | 300 | 59.0 | 24–84 | Hospital | 303 | 49.2 | Hospital | 6 |
| Pakistan–Islamabad [109] | January 2005–July 2005 | 132 | 18.2 | 42.4 † | Hospital | 145 | 9.0 | Hospital | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanadys, W.; Barańska, A.; Malm, M.; Błaszczuk, A.; Polz-Dacewicz, M.; Janiszewska, M.; Jędrych, M. Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010. Int. J. Environ. Res. Public Health 2021, 18, 4638. https://doi.org/10.3390/ijerph18094638
Kanadys W, Barańska A, Malm M, Błaszczuk A, Polz-Dacewicz M, Janiszewska M, Jędrych M. Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010. International Journal of Environmental Research and Public Health. 2021; 18(9):4638. https://doi.org/10.3390/ijerph18094638
Chicago/Turabian StyleKanadys, Wiesław, Agnieszka Barańska, Maria Malm, Agata Błaszczuk, Małgorzata Polz-Dacewicz, Mariola Janiszewska, and Marian Jędrych. 2021. "Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010" International Journal of Environmental Research and Public Health 18, no. 9: 4638. https://doi.org/10.3390/ijerph18094638
APA StyleKanadys, W., Barańska, A., Malm, M., Błaszczuk, A., Polz-Dacewicz, M., Janiszewska, M., & Jędrych, M. (2021). Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010. International Journal of Environmental Research and Public Health, 18(9), 4638. https://doi.org/10.3390/ijerph18094638








