Impact of Water Regimes and Amendments on Inorganic Arsenic Exposure to Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Soil Sample Collection and Measurement of Soil Properties
2.3. Determination of Soil-Extractable As
2.4. Plant Sample Collection
2.5. Digestion Procedure
2.6. Determination of Inorganic As Species in Rice Grain
2.7. Instruments and Quality Control
2.8. Statistical Analyses
3. Results
3.1. Paddy Soil As
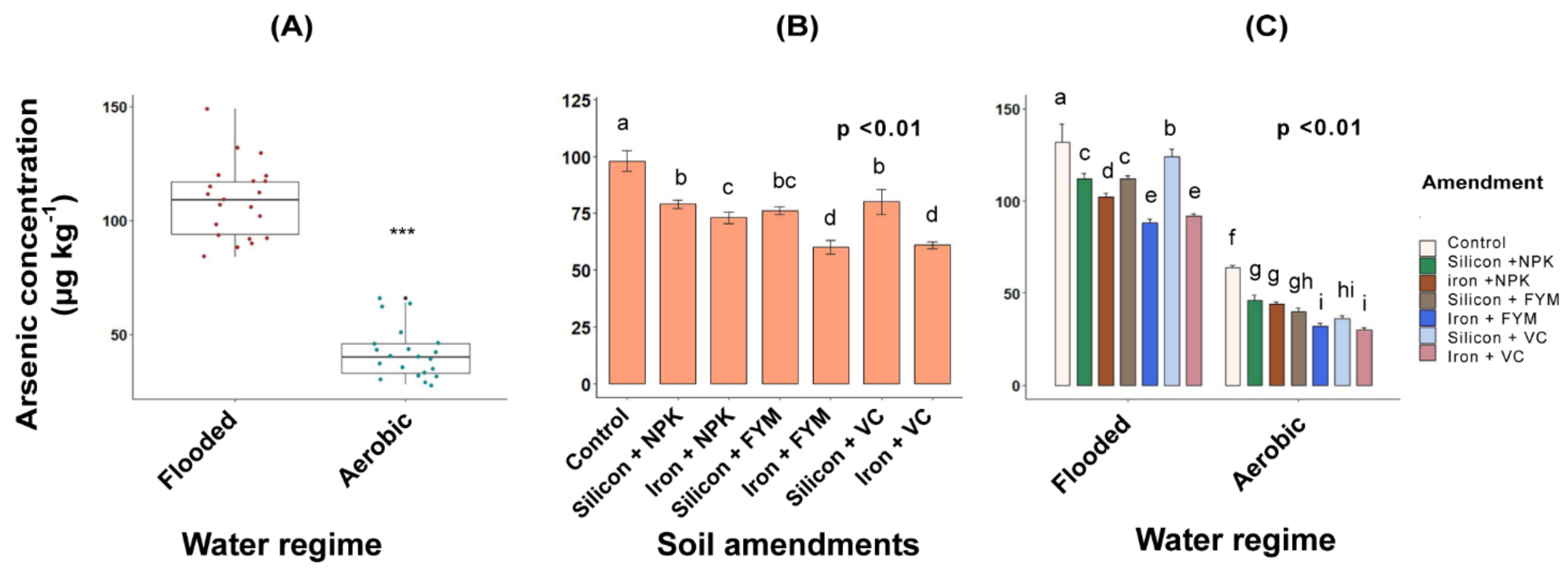
3.2. Total As in Rice
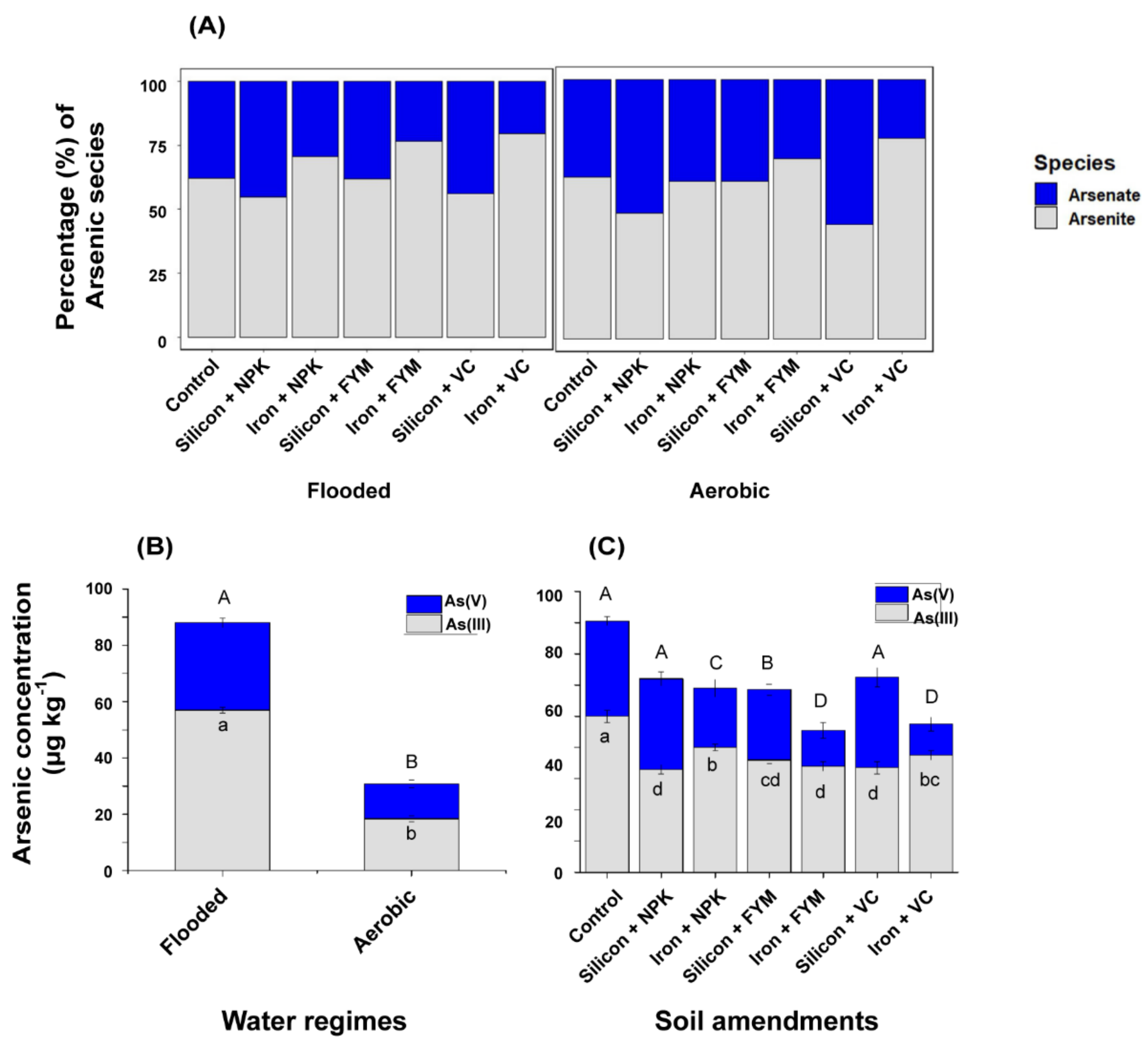
3.3. Grain Inorganic As Speciation
3.4. Correlations of As Concentrations in Rice Grains and Soil Chemical Properties
4. Discussion
4.1. Paddy Soil As Response to Water Regimes and Amendments
4.2. Inorganic As response in Rice to Water Regimes and Amendments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef]
- Kumar, M.; Rahman, M.M.; Ramanathan, A.; Naidu, R. Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: Health risk index. Sci. Total Environ. 2016, 539, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly Enhanced Arsenic Shoot Assimilation in Rice Leads to Elevated Grain Levels Compared to Wheat and Barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef]
- Williams, P.N.; Islam, M.R.; Adomako, E.E.; Raab, A.; Hossain, S.A.; Zhu, Y.G.; Feldmann, J.; Meharg, A.A. Increase in Rice Grain Arsenic for Regions of Bangladesh Irrigating Paddies with Elevated Arsenic in Groundwaters. Environ. Sci. Technol. 2006, 40, 4903–4908. [Google Scholar] [CrossRef]
- Roychowdhury, T. Impact of sedimentary arsenic through irrigated groundwater on soil, plant, crops and human continuum from Bengal delta: Special reference to raw and cooked rice. Food Chem. Toxicol. 2008, 46, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, M.M.; Islam, M.; Naidu, R. Effect of irrigation and genotypes towards reduction in arsenic load in rice. Sci. Total Environ. 2017, 609, 311–318. [Google Scholar] [CrossRef]
- Syu, C.-H.; Huang, C.-C.; Jiang, P.-Y.; Lee, C.-H.; Lee, D.-Y. Arsenic accumulation and speciation in rice grains influenced by arsenic phytotoxicity and rice genotypes grown in arsenic-elevated paddy soils. J. Hazard. Mater. 2015, 286, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sommella, A.; Deacon, C.; Norton, G.; Pigna, M.; Violante, A.; Meharg, A. Total arsenic, inorganic arsenic, and other elements concentrations in Italian rice grain varies with origin and type. Environ. Pollut. 2013, 181, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.J.; Carey, M.; Carbonell-Barrachina, A.A.; Moreno-Jiménez, E.; Green, A.J.; Meharg, A.A. Geographical variation in inorganic arsenic in paddy field samples and commercial rice from the Iberian Peninsula. Food Chem. 2016, 202, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef]
- Meharg, A.A.; Rahman, M.M. Arsenic Contamination of Bangladesh Paddy Field Soils: Implications for Rice Contribution to Arsenic Consumption. Environ. Sci. Technol. 2003, 37, 229–234. [Google Scholar] [CrossRef]
- Halder, D.; Biswas, A.; Šlejkovec, Z.; Chatterjee, D.; Nriagu, J.; Jacks, G.; Bhattacharya, P. Arsenic species in raw and cooked rice: Implications for human health in rural Bengal. Sci. Total Environ. 2014, 497, 200–208. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.-H.; Si, Y.-B.; Wang, R.-F. Release and transformation of arsenic from As-bearing iron minerals by Fe-reducing bacteria. Chem. Eng. J. 2016, 295, 29–38. [Google Scholar] [CrossRef]
- Zecchin, S.; Corsini, A.; Martin, M.; Romani, M.; Beone, G.M.; Zanchi, R.; Zanzo, E.; Tenni, D.; Fontanella, M.C.; Cavalca, L. Rhizospheric iron and arsenic bacteria affected by water regime: Implications for metalloid uptake by rice. Soil Biol. Biochem. 2017, 106, 129–137. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Ohkura, T.; Takahashi, Y.; Maejima, Y.; Arao, T. Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ. Sci. Technol. 2014, 48, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wu, X.; Wang, S.; Yuan, Z.; Xiao, F.; Yang, M.; Jia, Y. Speciation change and redistribution of arsenic in soil under anaerobic microbial activities. J. Hazard. Mater. 2016, 301, 538–546. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, H.; Chen, Z.; Zhu, Y.-G. Arsenic Uptake by Rice Is Influenced by Microbe-Mediated Arsenic Redox Changes in the Rhizosphere. Environ. Sci. Technol. 2014, 48, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Some Drinking-Water Disinfectants and Contaminants, Including Arsenic; IARC: Lyon, France, 2004. [Google Scholar]
- Suriyagoda, L.D.; Dittert, K.; Lambers, H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric. Ecosyst. Environ. 2018, 253, 23–37. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Akbar, N.; Reis, A.F.; Li, C.; Gaudin, A.C.; Parikh, S.J.; Green, P.G.; Linquist, B.A. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics. Field Crop. Res. 2018, 222, 101–110. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Li, C.; Parikh, S.J.; Linquist, B.A. Irrigation management for arsenic mitigation in rice grain: Timing and severity of a single soil drying. Sci. Total Environ. 2019, 649, 300–307. [Google Scholar] [CrossRef]
- Li, C.; Carrijo, D.R.; Nakayama, Y.; Linquist, B.A.; Green, P.G.; Parikh, S.J. Impact of alternate wetting and drying irrigation on arsenic uptake and speciation in flooded rice systems. Agric. Ecosyst. Environ. 2019, 272, 188–198. [Google Scholar] [CrossRef]
- Li, R.; Stroud, J.; Ma, J.; McGrath, S.; Zhao, F. Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ. Sci. Technol. 2009, 43, 3778–3783. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kasuga, J.; Taiki, N.; Makino, T.; Arao, T. Inhibition of arsenic accumulation in Japanese rice by the application of iron and silicate materials. Catena 2015, 135, 328–335. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kasuga, J.; Makino, T.; Arao, T. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environ. Exp. Bot. 2016, 125, 42–51. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Naidu, R. Impact of water and fertilizer management on arsenic bioaccumulation and speciation in rice plants grown under greenhouse conditions. Chemosphere 2019, 214, 606–613. [Google Scholar] [CrossRef]
- Sinha, B.; Bhattacharyya, K.; Giri, P.K.; Sarkar, S. Arsenic contamination in sesame and possible mitigation through organic interventions in the lower Gangetic Plain of West Bengal. India. J. Sci. Food Agric. 2011, 91, 2762–2767. [Google Scholar] [CrossRef]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and arsenate binding to dissolved humic acids: Influence of pH, type of humic acid, and aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Houba, V.; Temminghoff, E.; Gaikhorst, G.; Van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as ex-traction reagent. Commun. Soil Sci. Plan. Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Huang, J.-H.; Ilgen, G.; Fecher, P. Quantitative chemical extraction for arsenic speciation in rice grains. J. Anal. At. Spectrom. 2010, 25, 800–802. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minamikawa, R.; Hattori, K.H.; Kurishima, K.; Kihou, N.; Yuita, K. Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol. 2004, 38, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing Rice Aerobically Markedly Decreases Arsenic Accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef]
- Fendorf, S.; Herbel, M.J.; Tufano, K.J.; Kocar, B.D. Biogeochemical Processes Controlling the Cycling of Arsenic in Soils and Sediments. Biophys. Chem. Process. Heavy Met. Met. Soil Environ. 2009, 40, 313–338. [Google Scholar] [CrossRef]
- Fendorf, S.; Eick, M.J.; Grossl, P.; Sparks, D.L. Arsenate and Chromate Retention Mechanisms on Goethite. 1. Surface Structure. Environ. Sci. Technol. 1997, 31, 315–320. [Google Scholar] [CrossRef]
- Arai, Y.; Elzinga, E.J.; Sparks, D.L. X-ray Absorption Spectroscopic Investigation of Arsenite and Arsenate Adsorption at the Aluminum Oxide–Water Interface. J. Colloid Interface Sci. 2001, 235, 80–88. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; Fendorf, S. Silicate Mineral Impacts on the Uptake and Storage of Arsenic and Plant Nutrients in Rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 13176–13183. [Google Scholar] [CrossRef]
- Fleck, A.T.; Mattusch, J.; Schenk, M.K. Silicon decreases the arsenic level in rice grain by limiting arsenite transport. J. Plant Nutr. Soil Sci. 2013, 176, 785–794. [Google Scholar] [CrossRef]
- Williams, P.N.; Zhang, H.; Davison, W.; Meharg, A.A.; Hossain, M.; Norton, G.J.; Brammer, H.; Islam, M.R. Organic matter-solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environ. Sci. Technol. 2011, 45, 6080–6087. [Google Scholar] [CrossRef]
- Liu, G.; Fernandez, A.; Cai, Y. Complexation of Arsenite with Humic Acid in the Presence of Ferric Iron. Environ. Sci. Technol. 2011, 45, 3210–3216. [Google Scholar] [CrossRef]
- Das, S.; Chou, M.-L.; Jean, J.-S.; Liu, C.-C.; Yang, H.-J. Water management impacts on arsenic behavior and rhizosphere bacterial communities and activities in a rice agro-ecosystem. Sci. Total Environ. 2016, 542, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Hu, P.; Huang, J.; Ouyang, Y.; Wu, L.; Song, J.; Wang, S.; Li, Z.; Han, C.; Zhou, L.; Huang, Y.; et al. Water management affects arsenic and cadmium accumulation in different rice cultivars. Environ. Geochem. Health 2013, 35, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Meharg, A.A.; Smolders, E.; Manzano, R.; Becerra, D.; Sánchez-Llerena, J.; Albarrán, Á.; López-Piñero, A. Sprinkler irrigation of rice fields reduces grain arsenic but enhances cadmium. Sci. Total Environ. 2014, 485, 468–473. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.G.; Feldmann, J.; et al. Geographical Variation in Total and Inorganic Arsenic Content of Polished (White) Rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef]
- Finnegan, P.; Chen, W. Arsenic Toxicity: The Effects on Plant Metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Zhao, F.-J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Xu, X.-Y.; Su, Y.-H.; McGrath, S.P.; Zhao, F.-J. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA 2008, 105, 9931–9935. [Google Scholar] [CrossRef]


| Parameters | Values |
|---|---|
| Sand (%) | 25.2 |
| Silt (%) | 34.9 |
| Clay (%) | 39.9 |
| Texture | Clay loam |
| pH | 7.59 |
| Organic carbon (%) | 0.81 |
| Amorphous Fe (g·kg−1) DCB-extracted Fe (g·kg−1) | 1.92 5.43 |
| Total As (mg·kg−1) | 11.62 |
| Factors | Soil As (mg·kg−1) | |
|---|---|---|
| Water regime | ||
| Flooded | 0.33 ± 0.06 a | |
| Aerobic | 0.21 ± 0.08 b | |
| Soil amendments | ||
| Control | 0.31 ± 0.035 a | |
| Silicon + NPK | 0.29 ± 0.045 ab | |
| Iron + NPK | 0.27 ± 0.051 c | |
| Silicon + FYM | 0.29 ± 0.028 ab | |
| Iron + FYM | 0.23 ± 0.019 d | |
| Silicon + VC | 0.30 ± 0.058 ab | |
| Iron + VC | 0.26 ± 0.028 c | |
| p-Value | Water regime | 0.0005 |
| Soil amendments | <0.0001 | |
| Water regime × soil amendments | <0.0001 | |
| Rice Grain | Soil Properties | |||
|---|---|---|---|---|
| Soil-Extractable As | Available Si | Amorphous Fe | DCB-Extracted Fe | |
| Arsenite | 0.80 *** | −0.64 *** | −0.86 *** | −61 ** |
| Arsenate | 0.79 *** | −0.38 * | −0.88 *** | −86 *** |
| Sum of As species | 0.84 *** | −0.55 ** | −0.92 *** | −83 *** |
| Total As | 0.87 *** | −0.65 ** | −0.92 *** | −64 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumder, S.; Biswas, P.K.; Banik, P. Impact of Water Regimes and Amendments on Inorganic Arsenic Exposure to Rice. Int. J. Environ. Res. Public Health 2021, 18, 4643. https://doi.org/10.3390/ijerph18094643
Majumder S, Biswas PK, Banik P. Impact of Water Regimes and Amendments on Inorganic Arsenic Exposure to Rice. International Journal of Environmental Research and Public Health. 2021; 18(9):4643. https://doi.org/10.3390/ijerph18094643
Chicago/Turabian StyleMajumder, Supriya, Pabitra Kumar Biswas, and Pabitra Banik. 2021. "Impact of Water Regimes and Amendments on Inorganic Arsenic Exposure to Rice" International Journal of Environmental Research and Public Health 18, no. 9: 4643. https://doi.org/10.3390/ijerph18094643
APA StyleMajumder, S., Biswas, P. K., & Banik, P. (2021). Impact of Water Regimes and Amendments on Inorganic Arsenic Exposure to Rice. International Journal of Environmental Research and Public Health, 18(9), 4643. https://doi.org/10.3390/ijerph18094643





