Effect of Seawater Temperature Increase on the Occurrence of Coastal Vibrio vulnificus Cases: Korean National Surveillance Data from 2003 to 2016
Abstract
1. Introduction
2. Materials and Methods
2.1. V. vulnificus Cases in South Korea
2.2. Seawater Temperatures
2.3. Statistical Analysis
3. Results
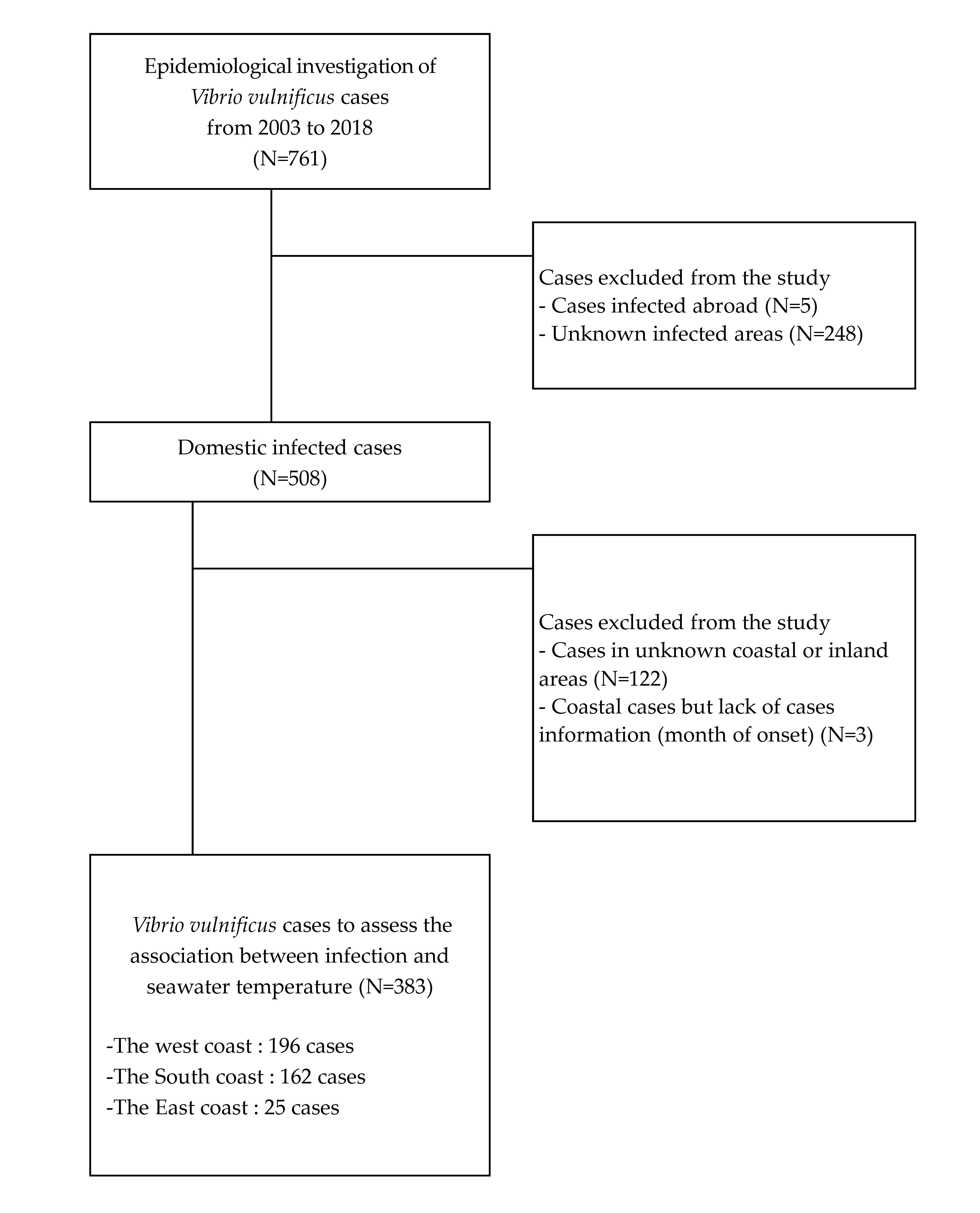
3.1. Research Subjects
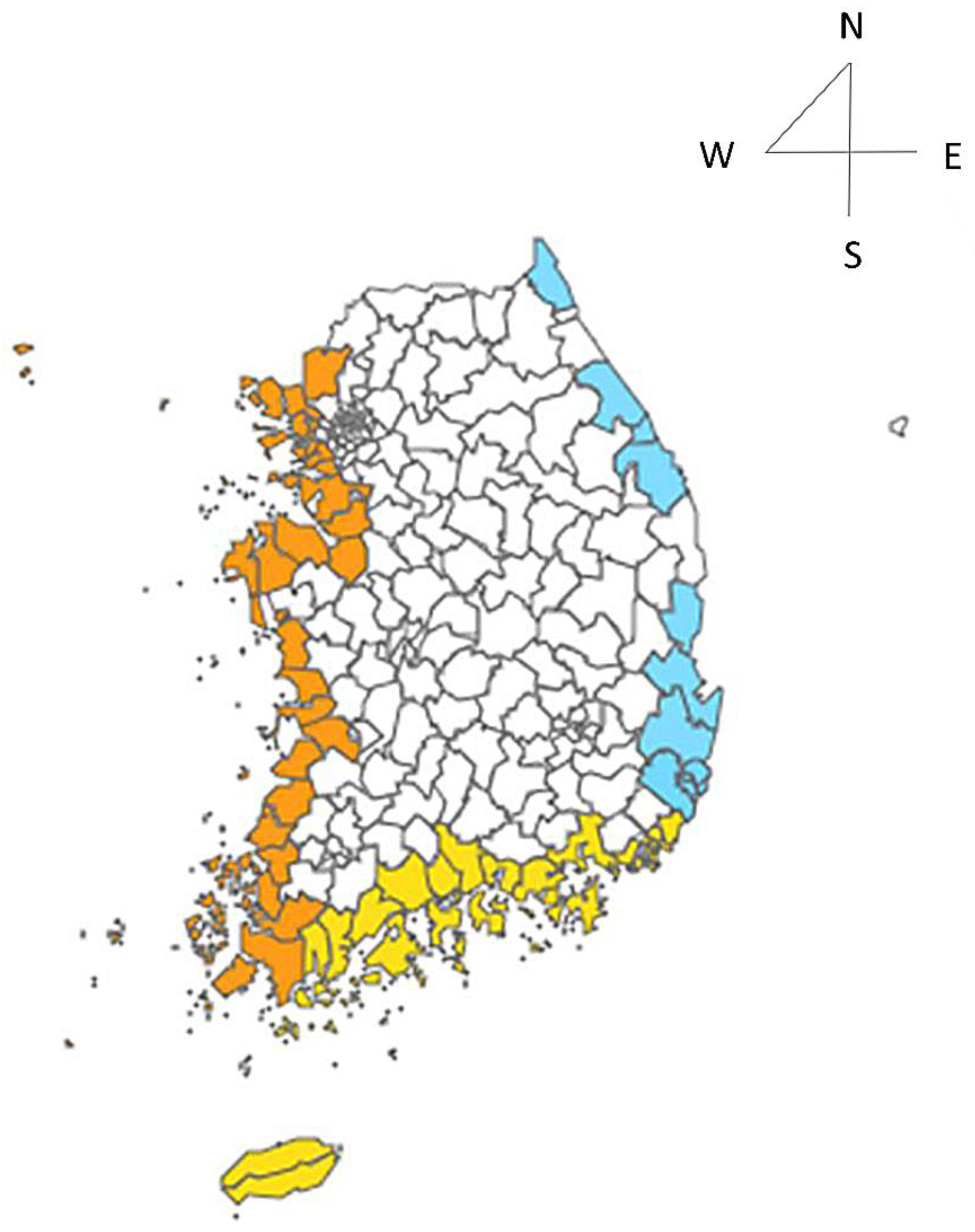
3.2. Epidemiological Characteristics of V. vulnificus Cases in Cities, Counties, and Districts Near the Coast
3.3. Annual Indicators Related to Water Temperature and Seawater Temperature
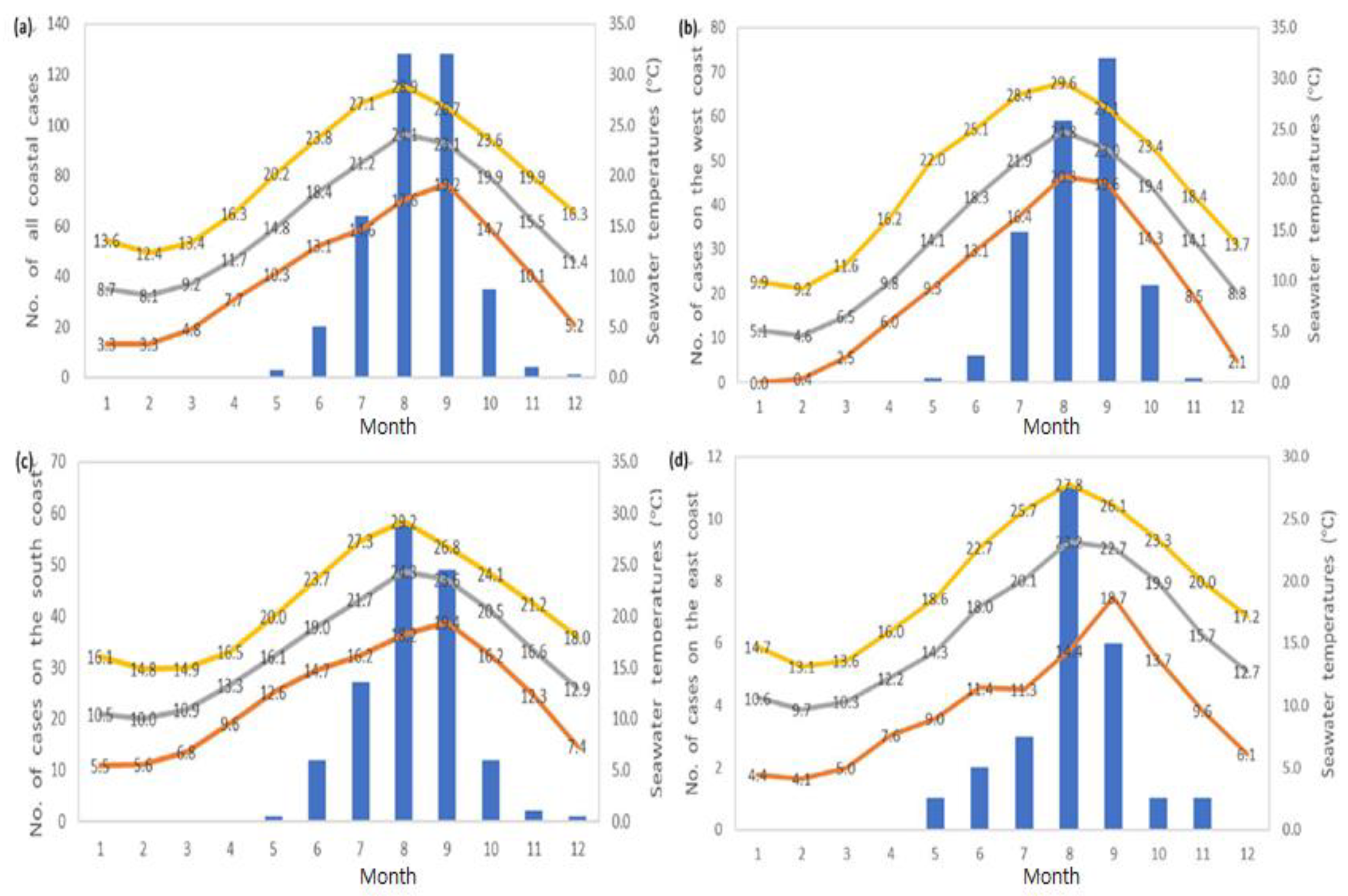
3.4. Monthly Coastal V. vulnificus Cases in Cities, Counties, and Districts and Seawater Temperature
3.5. Assessment of the Correlation between Seawater Temperature and V. vulnificus Cases
3.5.1. Correlation Analysis
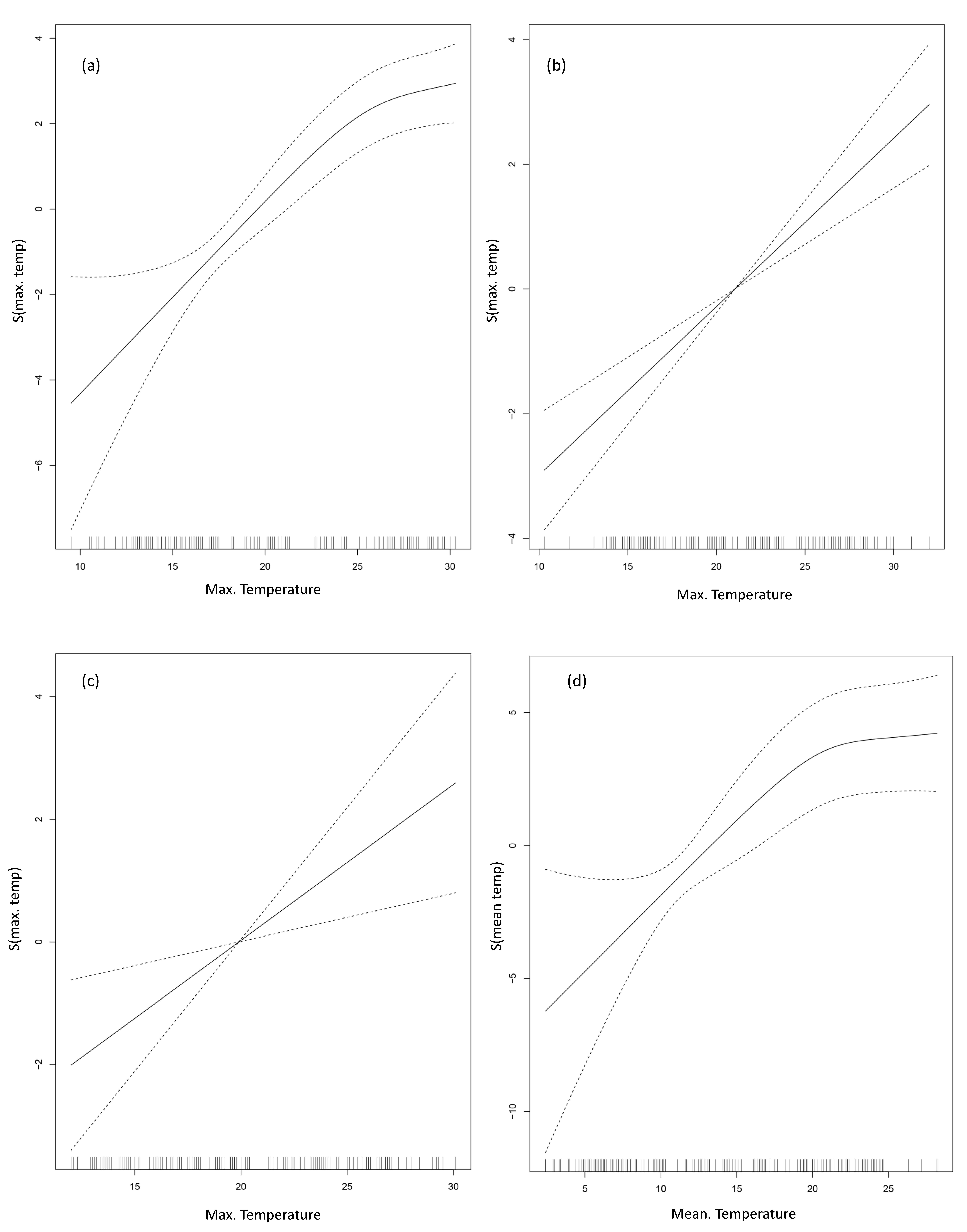
3.5.2. Results of General Additive Model Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horseman, M.A.; Surani, S.A. Comprehensive review of Vibrio vulnificus: An important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 2011, 15, e157–e166. [Google Scholar] [CrossRef]
- Oliver, J.D. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol. Infect. 2005, 133, 383–391. [Google Scholar] [CrossRef]
- Bross, M.H.; Soch, K.; Morales, R.; Mitchell, R.B. Vibrio vulnificus infection: Diagnosis and treatment. Am. Fam. Physician 2007, 76, 539–544. [Google Scholar] [PubMed]
- Oliver, J.D. The biology of Vibrio vulnificus. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Miyasaka, J.; Ono, T.; Ihn, H. The growth of Vibrio vulnificus and the habitat of infected patients in Kumamoto. Biosci. Trends 2007, 1, 134–139. [Google Scholar] [PubMed]
- Inoue, Y.; Ono, T.; Matsui, T.; Miyasaka, J.; Kinoshita, Y.; Ihn, H. Epidemiological survey of Vibrio vulnificus infection in Japan between 1999 and 2003. J. Dermatol. 2008, 35, 129–239. [Google Scholar] [CrossRef]
- Hsueh, P.R.; Lin, C.Y.; Tang, H.J.; Lee, H.C.; Liu, J.W.; Liu, Y.C.; Chuang, Y.C. Vibrio vulnificus in Taiwan. Emerg. Infect. Dis. 2004, 10, 1363–1368. [Google Scholar] [CrossRef]
- Hlady, W.G.; Klontz, K.C. The epidemiology of Vibrio infections in Florida, 1981–1993. J. Infect. Dis. 1996, 173, 1176–1183. [Google Scholar] [CrossRef]
- Klontz, K.C.; Lieb, S.; Schreiber, M.; Janowski, H.T.; Baldy, L.M.; Gunn, R.A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981–1987. Ann. Intern. Med. 1988, 109, 318–323. [Google Scholar] [CrossRef]
- Dechet, A.M.; Yu, P.A.; Koram, N.; Painter, J. Nonfoodborne Vibrio infections: An important cause of morbidity and mortality in the United States, 1997–2006. Clin. Infect. Dis. 2008, 46, 970–976. [Google Scholar] [CrossRef]
- Leng, F.; Lin, S.L.; Wu, W.; Zhang, J.C.; Song, J.Q.; Zhong, M. Epidemiology, pathogenetic mechanism, clinical characteristics, and treatment of Vibrio vulnificus infection: A case report and literature review. Eur. J. Clin. Microbiol. 2019, 38, 1999–2004. [Google Scholar] [CrossRef]
- Pfeffer, C.S.; Hite, M.F.; Oliver, J.D. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 2003, 69, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl. Environ. Microbiol. 1982, 44, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.S.; Paranjpye, R.N. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes. Infect. 2000, 2, 177–188. [Google Scholar] [CrossRef]
- Froelich, B.A.; Noble, R.T. Vibrio bacteria in raw oysters: Managing risks to human health. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150209. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.P.; Letchumanan, V.; Deng, C.Y.; Ab Mutalib, N.S.; Khan, T.M.; Chuah, L.H.; Chan, K.G.; Goh, B.H.; Pusparajah, P.; Lee, L.H. Vibrio vulnificus: An environmental and clinical burden. Front. Microbiol. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jung, S.I.; Peck, K.R. Historical and clinical perspective of Vibrio vulnificus infections in Korea. Infect. Chemother. 2020, 52, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Bowers, J.C.; Griffitt, K.J.; Molina, V.; Clostio, R.W.; Pei, S.F.; Laws, E.; Paranjpye, R.N.; Strom, M.S.; Chen, A.; et al. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl. Environ. Microbiol. 2012, 78, 7249–7257. [Google Scholar] [CrossRef]
- Di, D.Y.W.; Lee, A.; Jang, J.; Han, D.; Hur, H.G. Season-specific occurrence of potentially pathogenic Vibrio spp. on the southern coast of South Korea. Appl. Environ. Microbiol. 2017, 83, e02680-16. [Google Scholar] [CrossRef]
- Mok, J.S.; Ryu, A.; Kwon, J.Y.; Kim, B.; Park, K. Distribution of Vibrio species isolated from bivalves and bivalve culture environments along the Gyeongnam coast in Korea: Virulence and antimicrobial resistance of Vibrio parahaemolyticus isolates. Food Control. 2019, 106, 106697. [Google Scholar] [CrossRef]
- Chu, C.; Do, Y.; Kim, Y.; Saito, Y.; Lee, S.D.; Park, H.; Lee, J.K. Mathematical modeling of Vibrio vulnificus infection in Korea and the influence of global warming. Osong Public Health Res. Perspect. 2011, 2, 51–58. [Google Scholar] [CrossRef][Green Version]
- Tomenchok, L.E.; Gidley, M.L.; Mena, K.D.; Ferguson, A.C.; Solo-Gabriele, H.M. Children’s abrasions in recreational beach areas and a review of possible wound infections. Int. J. Environ. Res. Public Health 2020, 17, 4060. [Google Scholar] [CrossRef]
- Hernandez-Cabanyero, C.; Sanjuan, E.; Fouz, B.; Pajuelo, D.; Vallejos-Vidal, E.; Reyes-Lopez, F.E.; Amaro, C. The effect of the environmental temperature on the adaptation to host in the zoonotic pathogen Vibrio vulnificus. Front. Microbiol. 2020, 11, 489. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Rhodes, M.; Brown, C.W.; Hood, R.R.; Leight, A.; Long, W.; Wood, R. Modeling and forecasting the distribution of Vibrio vulnificus in Chesapeake Bay. J. Appl. Microbiol. 2014, 117, 1312–1327. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Neogi, S.B.; Kassu, A.; Mai Huong, B.T.; Jahid, I.K.; Islam, M.S.; Ota, F. Occurrence, seasonality and genetic diversity of Vibrio vulnificus in coastal seaweeds and water along the Kii Channel, Japan. FEMS Microbiol. Ecol. 2008, 64, 209–218. [Google Scholar] [CrossRef]
- Boer, S.I.; Heinemeyer, E.A.; Luden, K.; Erler, R.; Gerdts, G.; Janssen, F.; Brennholt, N. Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German North Sea. Microb. Ecol. 2013, 65, 1052–1067. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.; Oliver, J.D. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol. Ecol. 2013, 84, 213–222. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, J.Y.; Jeon, S.M.; Park, M.S.; Kim, S.H. Genotypic characterization of Vibrio vulnificus clinical isolates in Korea. Osong Public Health Res. Perspect. 2011, 2, 8–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semenza, J.C.; Trinanes, J.; Lohr, W.; Sudre, B.; Lofdahl, M.; Martinez-Urtaza, J.; Nichols, G.L.; Rocklov, J. Environmental suitability of Vibrio infections in a warming climate: An early warning system. Environ. Health Persp. 2017, 125, 107004. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D. Vibrio vulnificus: New insights into a deadly opportunistic pathogen. Environ. Microbiol. 2018, 20, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.K.; Oliver, J.D. Effects of temperature abuse on survival of Vibrio vulnificus in oysters. Appl. Environ. Microbiol. 1992, 58, 2771–2775. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Classification | N | (%) |
|---|---|---|---|
| Sex | |||
| Male | 329 | (85.9) | |
| Female | 54 | (14.1) | |
| Age (years) | |||
| ≤39 | 13 | (3.4) | |
| 40–64 | 269 | (70.2) | |
| ≥65 | 101 | (26.4) | |
| Occupation (N = 351) | |||
| Fishery and fishery product related workers | 35 | (10.0) | |
| Agriculture | 65 | (18.5) | |
| White-collar workers, professional | 18 | (5.1) | |
| Service, distribution industry, self-employed | 32 | (9.1) | |
| Blue-collar workers * | 44 | (12.6) | |
| Housewife, unemployed | 157 | (44.7) | |
| Drinking (N = 304) | |||
| Yes | 257 | (84.5) | |
| No | 7 | (2.3) | |
| Unknown | 40 | (13.2) | |
| Smoking (N = 223) | |||
| Yes | 128 | (57.4) | |
| No | 20 | (9.0) | |
| Unknown | 75 | (33.6) | |
| Underlying diseases (N = 352) | |||
| Yes | 340 | (96.6) | |
| No | 8 | (2.3) | |
| Unknown | 4 | (1.1) | |
| V. vulnificus cases by year | |||
| 2003 | 42 | (11.0) | |
| 2004 | 29 | (7.6) | |
| 2005 | 30 | (7.8) | |
| 2006 | 44 | (11.5) | |
| 2007 | 26 | (6.8) | |
| 2008 | 16 | (4.2) | |
| 2009 | 8 | (2.1) | |
| 2010 | 37 | (9.7) | |
| 2011 | 25 | (6.5) | |
| 2012 | 31 | (8.1) | |
| 2013 | 25 | (6.5) | |
| 2014 | 33 | (8.6) | |
| 2015 | 15 | (3.9) | |
| 2016 | 22 | (5.7) | |
| Coastal area | |||
| West coast | 196 | (51.2) | |
| South coast | 162 | (42.3) | |
| East coast | 25 | (6.5) | |
| Route of infection | |||
| Seafood consumption | 336 | (87.7) | |
| Seawater exposure | 22 | (5.7) | |
| Seafood consumption and seawater exposure | 24 | (6.3) | |
| Unknown | 1 | (0.3) |
| Year | Min. Seawater Temperature (°C) | Mean Seawater Temperature (°C) | Max. Seawater Temperature (°C) |
|---|---|---|---|
| 2003 | 9.9 | 15.1 | 20.1 |
| 2004 | 10.3 | 15.5 | 20.6 |
| 2005 | 9.7 | 15.3 | 21.0 |
| 2006 | 9.6 | 15.1 | 20.2 |
| 2007 | 10.8 | 15.8 | 20.5 |
| 2008 | 7.6 | 15.7 | 20.9 |
| 2009 | 11.1 | 16.0 | 20.3 |
| 2010 | 9.0 | 15.0 | 20.1 |
| 2011 | 9.1 | 14.9 | 20.0 |
| 2012 | 10.8 | 15.4 | 20.1 |
| 2013 | 11.1 | 15.7 | 19.6 |
| 2014 | 12.0 | 16.0 | 19.8 |
| 2015 | 11.4 | 15.7 | 19.4 |
| 2016 | 12.1 | 16.2 | 19.5 |
| Mean | 10.3 | 15.5 | 20.2 |
| Cases | Mean Seawater Temperature (°C) | Maximum Seawater Temperature (°C) | Minimum Seawater Temperature (°C) | |
|---|---|---|---|---|
| Cases | 1 | |||
| Mean seawater temperature (°C) | 0.693 * | 1 | ||
| Maximum seawater temperature (°C) | 0.686 * | 0.981 * | 1 | |
| Minimum seawater temperature (°C) | 0.667 * | 0.959 * | 0.914 * | 1 |
| Coast | Seawater Temperature Index | Relative Risk * | 95% Confidence Interval | p |
|---|---|---|---|---|
| All coasts | Maximum seawater temperature | 1.37 | 1.24–1.52 | 0.001 |
| South coast | Maximum seawater temperature | 1.35 | 1.19–1.53 | 0.001 |
| East coast | Maximum seawater temperature | 1.30 | 1.06–1.59 | 0.011 |
| West coast | Mean seawater temperature | 1.34 | 1.20–1.51 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Chun, B.C. Effect of Seawater Temperature Increase on the Occurrence of Coastal Vibrio vulnificus Cases: Korean National Surveillance Data from 2003 to 2016. Int. J. Environ. Res. Public Health 2021, 18, 4439. https://doi.org/10.3390/ijerph18094439
Kim J, Chun BC. Effect of Seawater Temperature Increase on the Occurrence of Coastal Vibrio vulnificus Cases: Korean National Surveillance Data from 2003 to 2016. International Journal of Environmental Research and Public Health. 2021; 18(9):4439. https://doi.org/10.3390/ijerph18094439
Chicago/Turabian StyleKim, Jungsook, and Byung Chul Chun. 2021. "Effect of Seawater Temperature Increase on the Occurrence of Coastal Vibrio vulnificus Cases: Korean National Surveillance Data from 2003 to 2016" International Journal of Environmental Research and Public Health 18, no. 9: 4439. https://doi.org/10.3390/ijerph18094439
APA StyleKim, J., & Chun, B. C. (2021). Effect of Seawater Temperature Increase on the Occurrence of Coastal Vibrio vulnificus Cases: Korean National Surveillance Data from 2003 to 2016. International Journal of Environmental Research and Public Health, 18(9), 4439. https://doi.org/10.3390/ijerph18094439






