Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Investigated Carbons
2.3. Batch Adsorption Tests
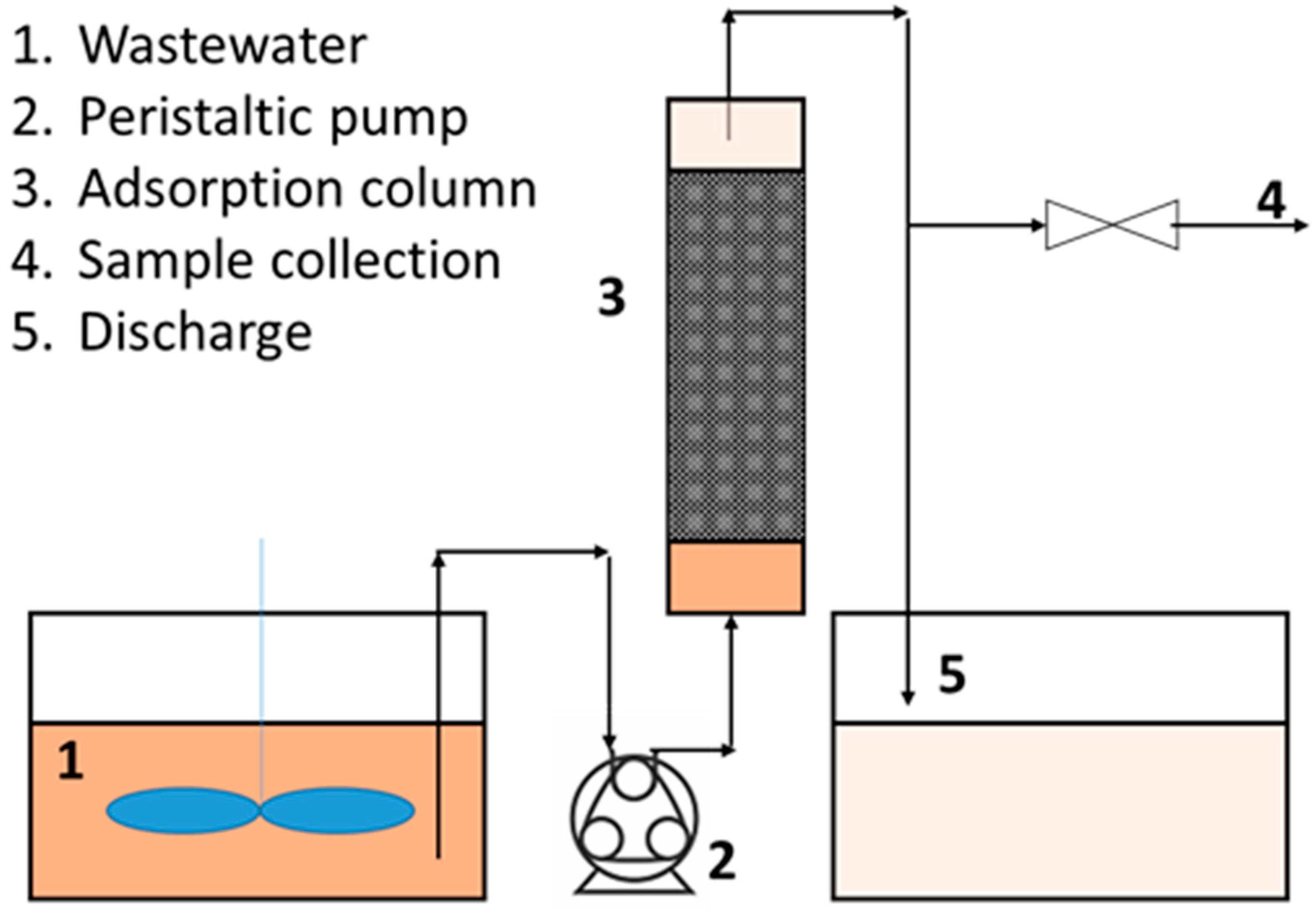
2.4. Continuous Adsorption Tests
3. Results
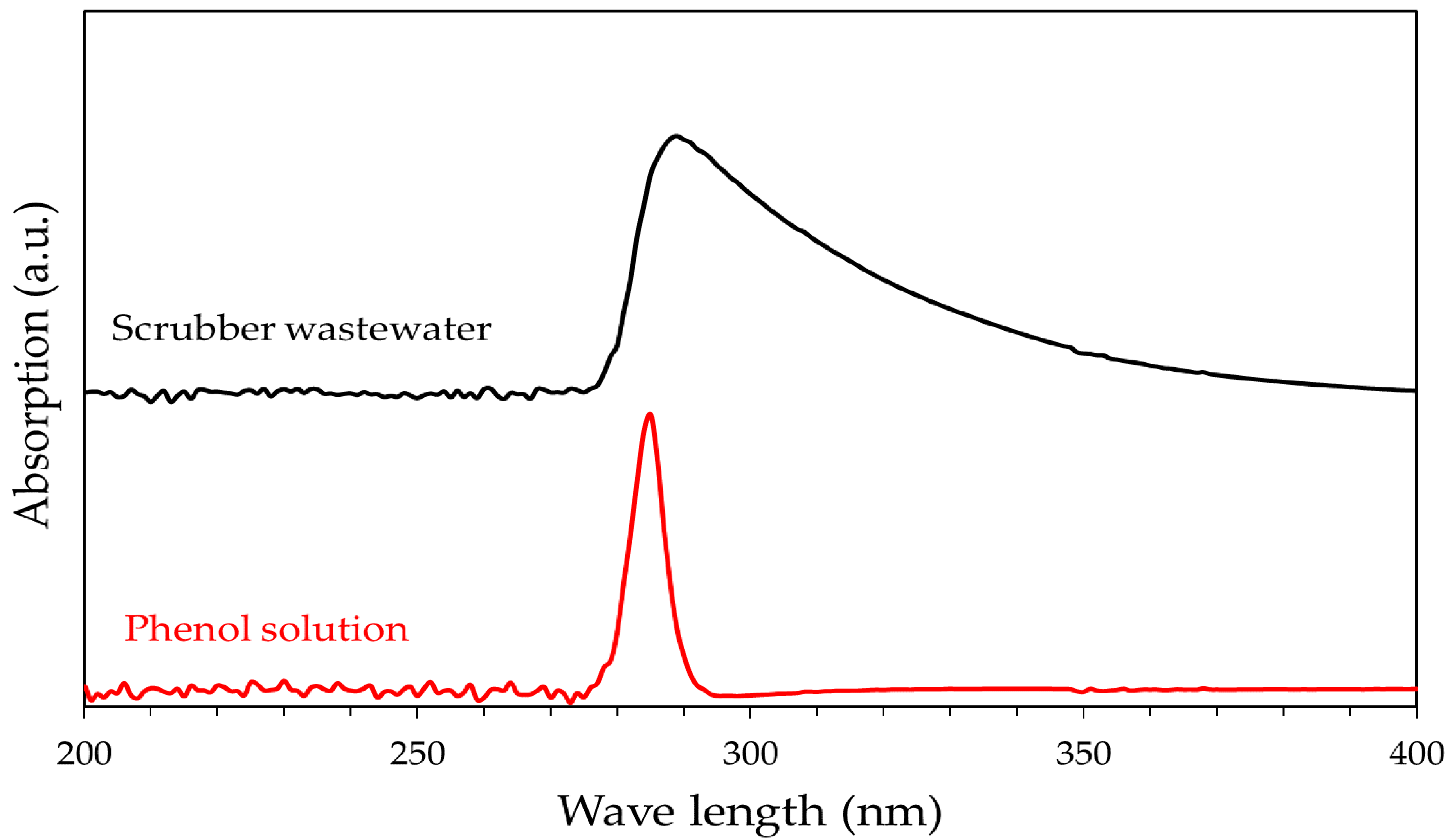
3.1. Characterization of the Samples
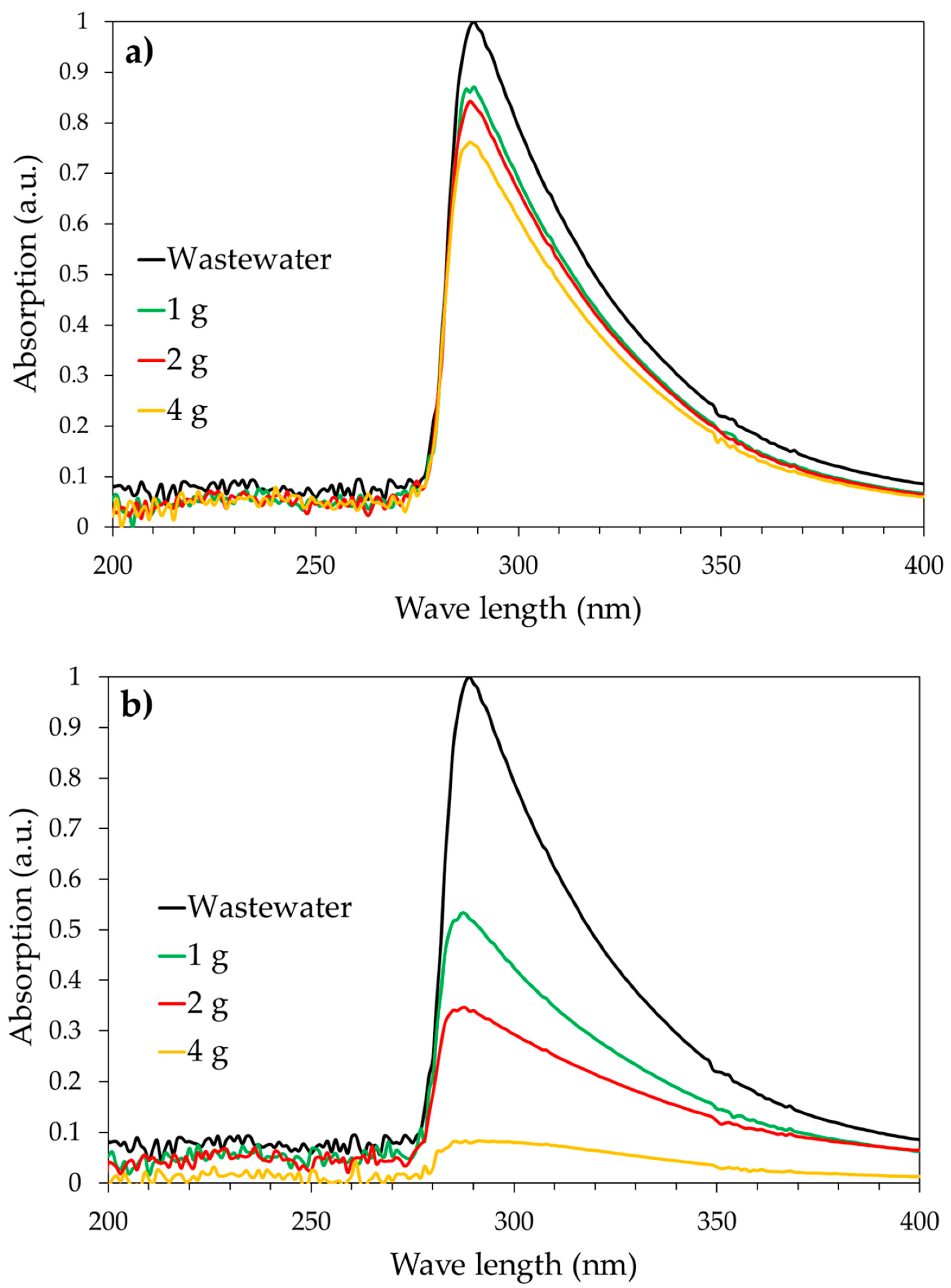
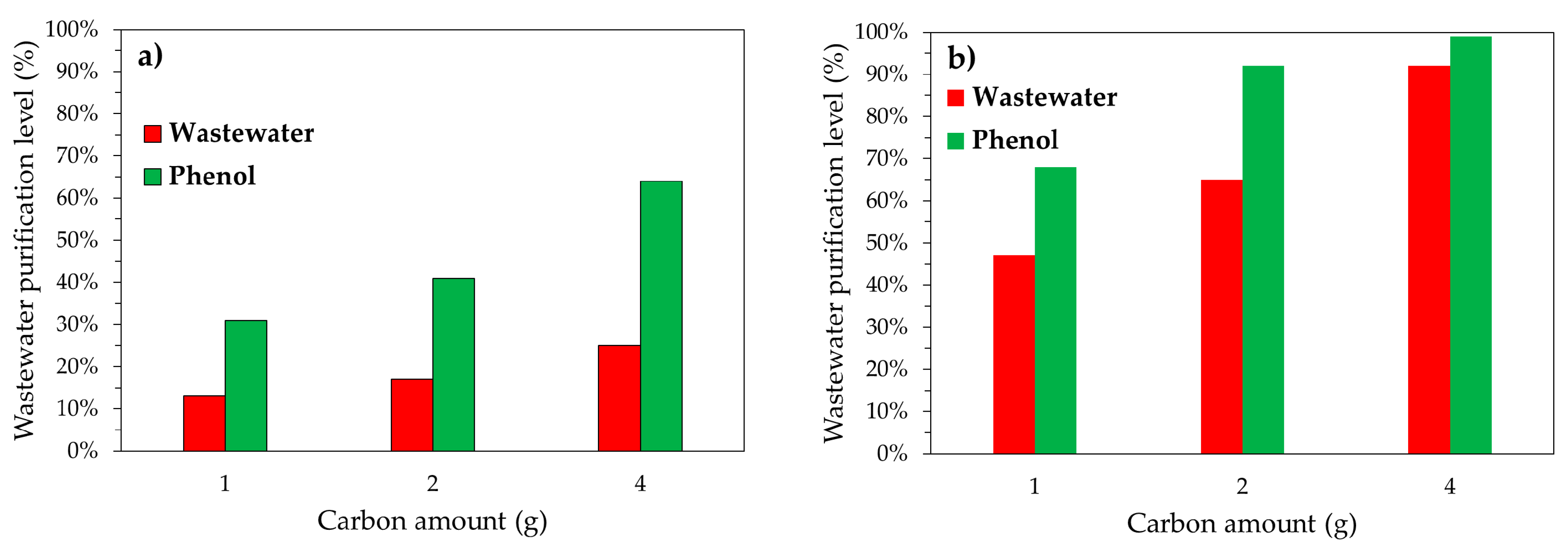
3.2. Adsorption Tests under Batch Conditions
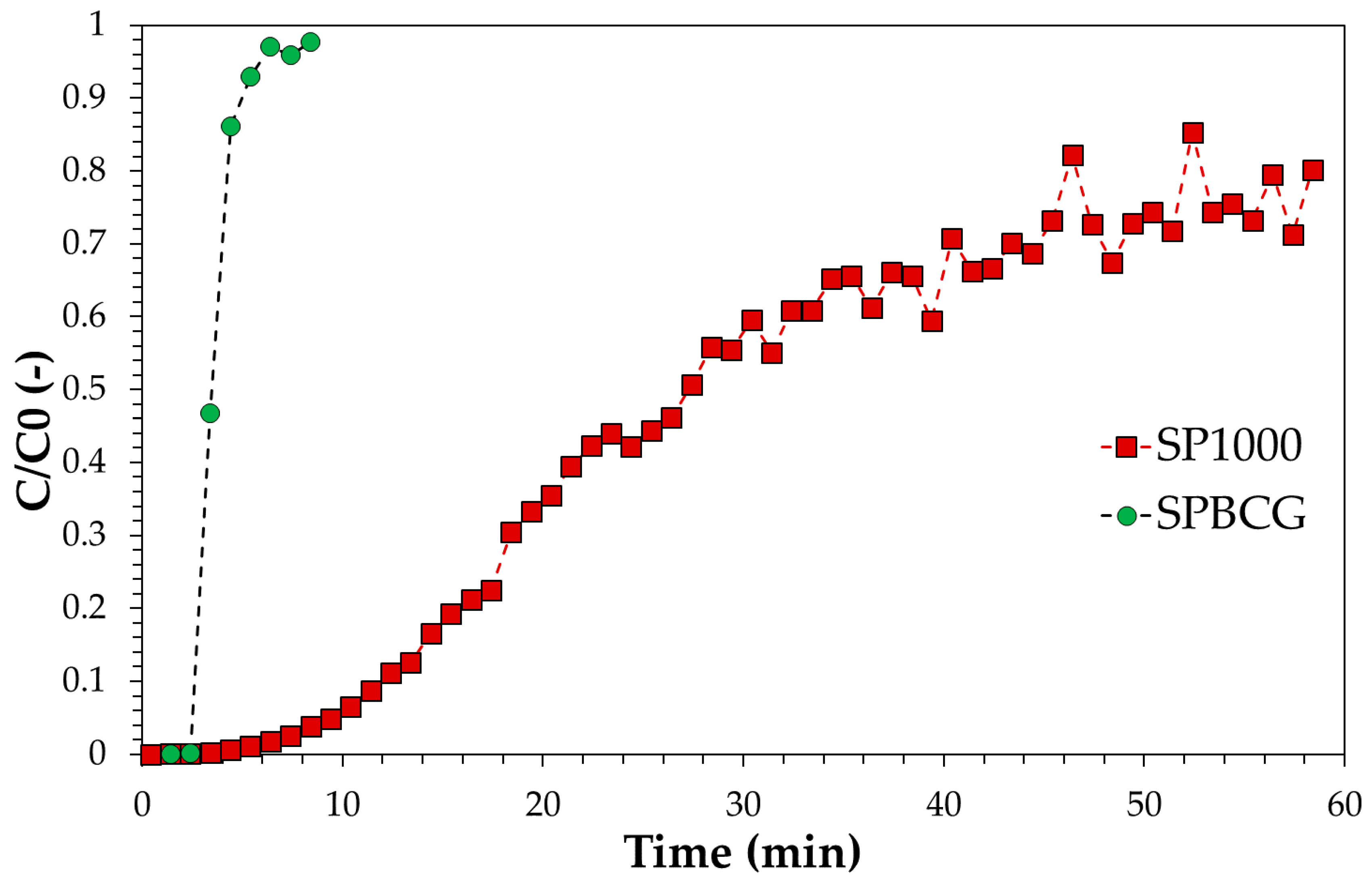
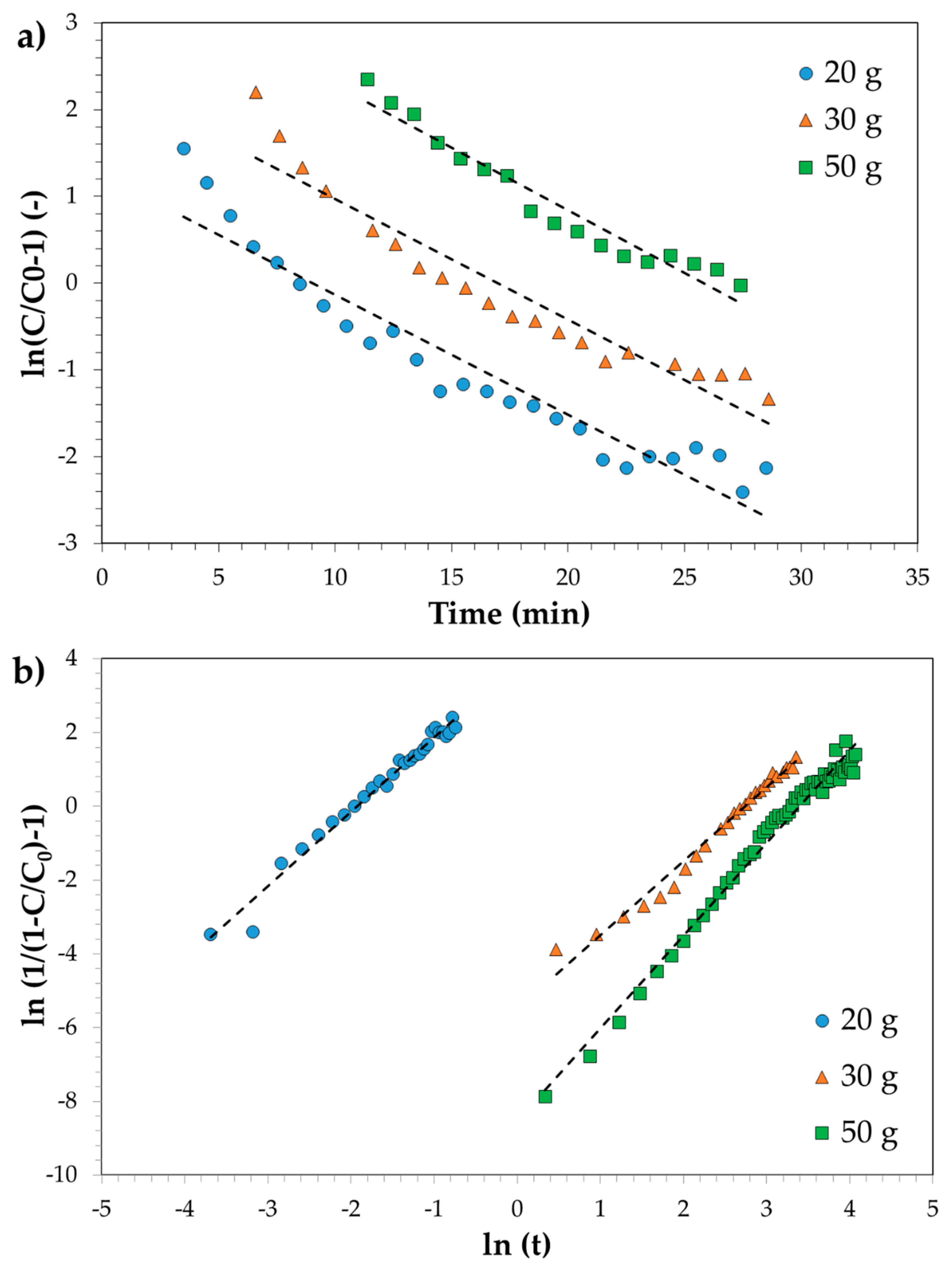
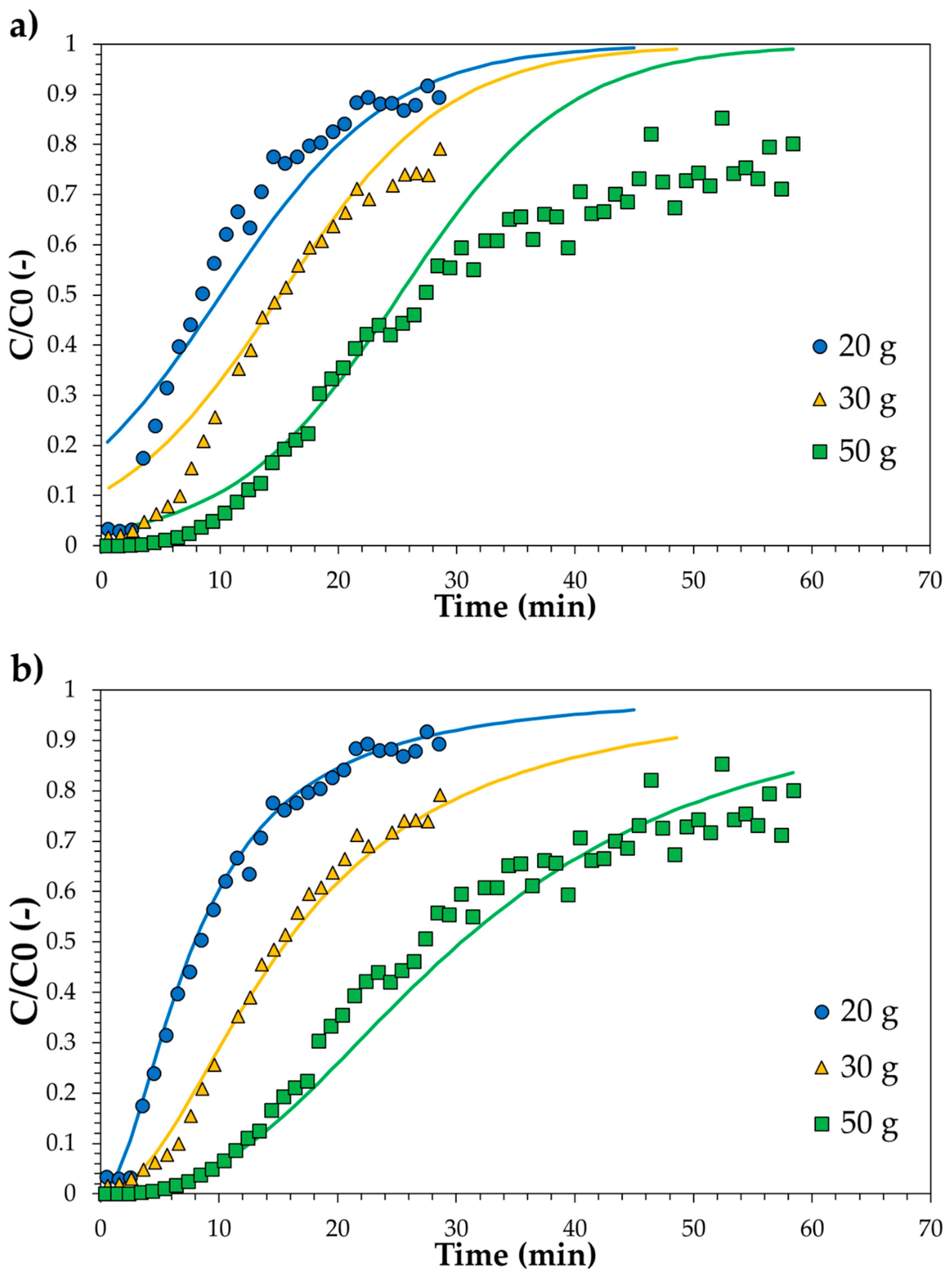
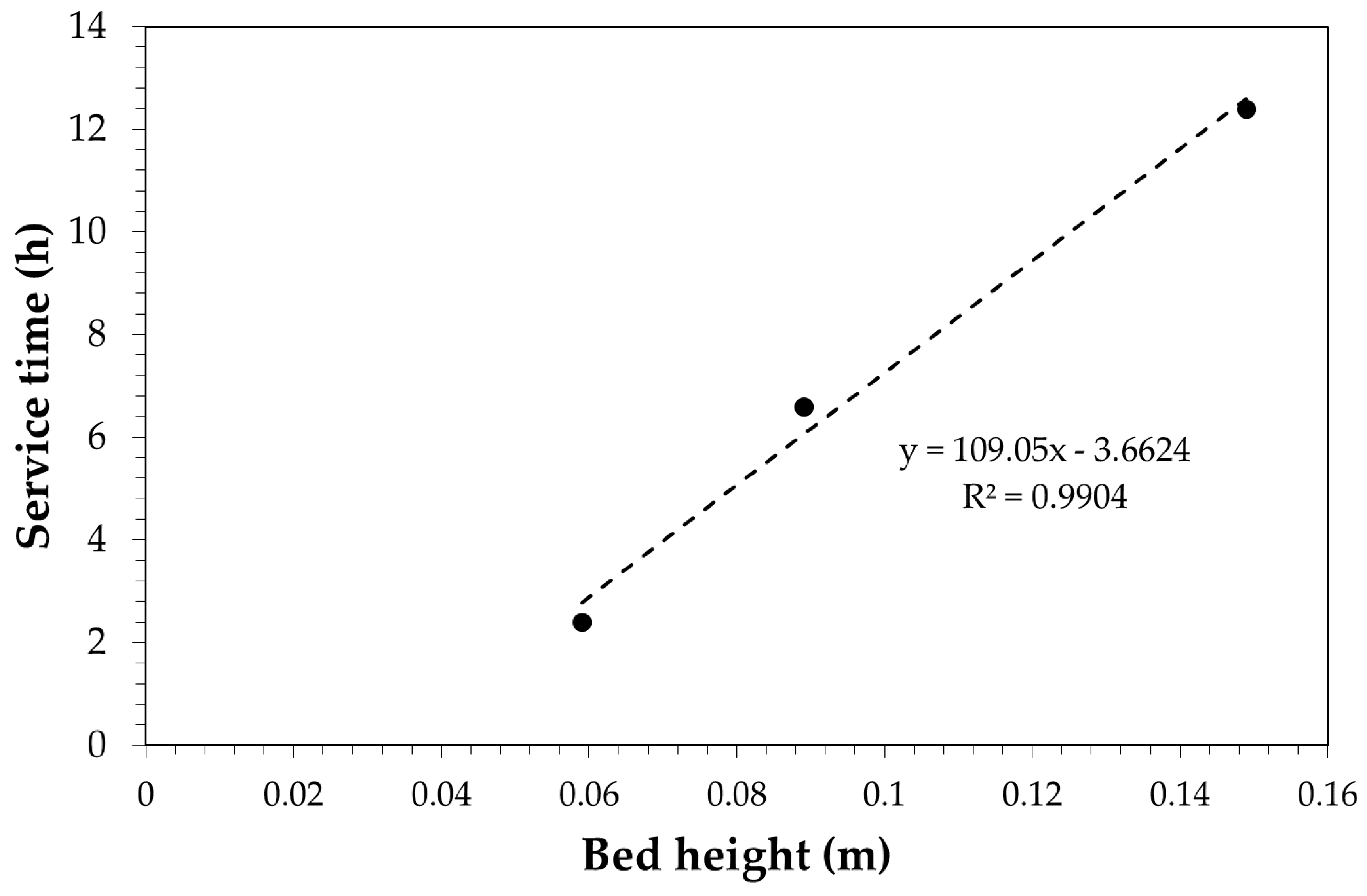
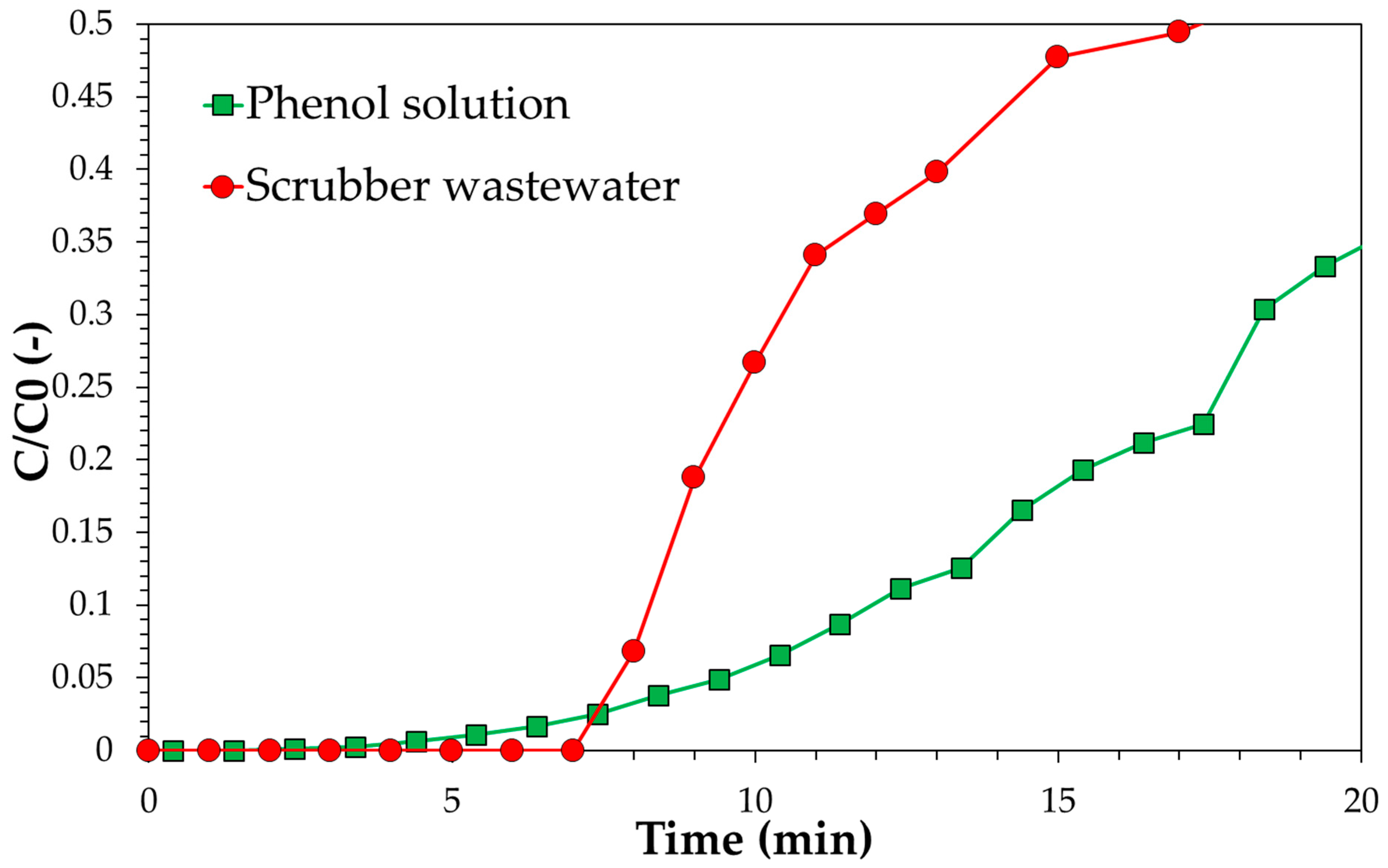
3.3. Adsorption Tests in Continuous Mode: Experimental, Modelling and Scale-Up
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sposato, C.; Catizzone, E.; Romanelli, A.; Marsico, L.; Barisano, D.; Migliori, M. Phenol Removal from Water with Carbons: An Experimental Investigation. Tech. Ital. Ital. J. Eng. Sci. 2020, 64, 143–148. [Google Scholar] [CrossRef]
- Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.303 (accessed on 15 November 2020).
- Galanopoulus, C.; Giuliano, A.; Barletta, D.; Zondervan, E. An Integrated Methodology for the Economic and Environmental Assessment of a Biorafinery Supply Chain. Chem. Eng. Res. Des. 2020, 160, 199–215. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Barisano, D.; Nanna, F.; Villone, A.; De Bari, I.; Cornacchia, G.; Braccio, G. Towards Methanol Economy: A Techno-environmental Assessment for a Bio-methanol OFMSW/Biomass/Carbon Capture-based Integrated Plant. Int. J. Heat Technol. 2019, 37, 665–674. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Milne, T.; Evans, R.J.; Abatzoglou, N. Biomass Gasifier “Tars”: Their Nature, Formation, and Conversion. NREL/TP-570-25357. Available online: https://www.nrel.gov/docs/fy99osti/25357.pdf (accessed on 15 November 2020).
- Luo, X.; Wu, T.; Shi, K.; Song, M.; Rao, Y. Chapter 1–Bionmass Gasification: An Overview of Technological Barriers and Socio-Environmental Impact. In Gasification of Low Grade Feedstock; Yun, Y., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Zwart, R.W.R. Gas Cleaning Downstream Biomass Gasification–Status Report 2009. ECN-E--08-078. Available online: www.ieatask33.org (accessed on 15 November 2020).
- Singh, R.N.; Singh, S.P.; Balwanshi, J.B. Tar Removal from Producer Gas: A Review. Res. J. Eng. Sci. 2014, 3, 16–22. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A Review of the Primary Measures for Tar Elimination in Biomass Gasification Processes. Biomass Bioenerg. 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Heidenreich, S.; Müller, M.; Foscolo, P.U. Advanced Biomass Gasification New Concepts for Efficiency Increase and Product Flexibility; Academic Press: Cambridge, MA, USA, 2016; pp. 1–134. [Google Scholar]
- Madav, V.; Das, D.; Kumar, M.; Surwade, M.; Parikh, P.P.; Sethi, V. Studies for Removal of Tar from Producer Gas in Small Scale Biomass Gasifiers Using Biodiesel. Biomass Bioenerg. 2019, 123, 123–133. [Google Scholar] [CrossRef]
- Hrbek, J. Status Report on Thermal Biomass Gasification in Countries Participating in IEA Bioenergy Task 33; University of Technology: Viena, Austria, April 2016; Available online: http://www.ieatask33.org/app/webroot/files/file/2016/Status_report.pdf (accessed on 15 November 2020).
- De Bari, I.; Barisano, D.; Cardinale, M.; Matera, D.; Nanna, F.; Viggiano, D. Air Gasification of Biomass in a Downdraft Fixed Bed: A Comparative Study of the Inorganic and Organic Products Distribution. Energy Fuels 2000, 14, 889–898. [Google Scholar] [CrossRef]
- Akhlas, J.; Bertucco, A.; Ruggeri, F.; Collodi, G. Treatment of Wastewater from Syngas Wet Scrubbing: Model-based Comparison of Phenol Biodegradation Basin Configurations. Canad. J. Chem. Eng. 2017, 95, 1652–1660. [Google Scholar] [CrossRef]
- Panchratna, D.; Sethi, V.; Mukherji, S. Sorption of Simulated Biomass Gasification Wastewater on Rice Husk Char and Activated Carbon. Int. J. Environ. Eng. Manag. 2013, 4, 489–496. [Google Scholar]
- Li, H.-Q.; Han, H.-j.; Du, M.-A.; Wang, W. Removal of Phenols, Thiocyanate and Ammonium from Coal Wastewater Using Moving Bed Biofilm Reactor. Biores. Technol. 2011, 102, 4667–4673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, X.; Gong, Z.; Yang, F. Removal of COD, Phenols and Ammonium from Lurgi Coal Gasification Wastewater Using A2O-MBR System. J. Hazard. Mater. 2012, 235-236, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bianco, L.; D’Amico, E.; Villone, A.; Nanna, F.; Barisano, D. Bioremediation of Wastewater Stream from Syngas Cleaning via Wet Scrubbing. Chem. Eng. Trans. 2020, 80, 31–36. [Google Scholar]
- Wong, S.; Ngadi, N.; Inuwa, I.M.; Hassan, O. Recent Advances in Applications of Activated Carbon from Biowaste for Wastewater Treatment: A Short Review. J. Clean. Prod. 2018, 175, 361–375. [Google Scholar] [CrossRef]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, M.S.; Hossain, M.A.; Ahmed, M.B. Efficacies of Carbon-Based Adsorbents for Carbon Dioxide Capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Marsh, H. Chapter 2–Activated Carbon: Structure Characterization Preparation and Applications. In Introduction to Carbon Technologies; Rodriguez-Reinoso, F., Ed.; University of Alicante: Alicante, Spain, 1997; p. 35. [Google Scholar]
- Kilic, M.; Apaydin-Varol, E.; Putun, A.E. Adsorptive Removal of Phenol from Aqueous Solutions on Activated Carbon Prepared from Tobacco Residues: Equilibrium, Kinetics and Thermodynamics. J. Hazard. Mater. 2011, 189, 397–403. [Google Scholar] [CrossRef]
- El-Naas, M.; Al-Zuhair, S.; Alhaija, M. Removal of Phenol from Petroleum Refinery Wastewater through Adsorption on Date-pit Activated Carbon. Chem. Eng. J. 2010, 162, 997–1005. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Pinto da Silva, M.L.C.; Alvarez-Mendes, M.O.; dos reis Coutinho, A.; Thim, G.P. Phenol Removal from Aqueous Solution by Activated Carbon Produced from Avocado Kernel Seeds. Chem. Eng. J. 2011, 174, 49–57. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Zeng, G.; Cai, Y.; Tang, J.; Pang, Y.; Zhou, Y.; Liu, Y.; Wang, J.; Zhang, S.; et al. Simultaneous Removal of Lead and Phenol Contamination from Water by Nitrogen-functionalized Magnetic Ordered Mesoporous Carbon. Chem. Eng. J. 2015, 259, 854–864. [Google Scholar] [CrossRef]
- Beker, U.; Ganbold, B.; Dertlu, H.; Gulbayir, D.D. Adsorption of Phenol by Activated Carbon: Influence of Activation Methods and Solution pH. Energ. Conv. Manag. 2010, 51, 235–240. [Google Scholar] [CrossRef]
- Gundogdo, A.; Duran, C.; Senturk, H.B.; Soylak, M.; Ozdes, D.; Serencam, H.; Imamoglu, M. Adsorption of Phenol from Aqueous Solution on a Low-Cost Activated Carbon Produced from Tea Industry Waste: Equilibrium, Kinetic, and Thermodynamic Study. J. Chem. Eng. Data 2012, 57, 2733–2743. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, S.; Misra, A.K.; Fan, M. Removal of Phenols from Water Environment by Activated Carbon, Bagasse Ash and Wood Charcoal. Chem. Eng. J. 2007, 129, 133–142. [Google Scholar] [CrossRef]
- Akl, M.A.; Dawy, M.B.; Serage, A.A. Efficient Removal of Phenol from Water Samples Using Sugarcane Bagasse Based Activated Carbon. J. Anal. Bioanal. Techn. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Alhamed Yahia, A. Adsorption Kinetics and Performance of Packed Bed Adsorber for Phenol Removal Using Activated Carbon from Dates’ Stones. J. Hazard. Mater. 2009, 170, 763–770. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Girgis, B.S. Removal of Some Substituted Phenols by Activated Carbon Obtained from Agricultural Waste. Water Res. 1998, 32, 1169–1177. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Vuono, D.; Catizzone, E.; Aloise, A.; Policicchio, A.; Agostino, R.G.; Migliori, M.; Giordano, G. Modelling of Adsorption of Textile Dyes over Multi-walled Carbon Nanotubes: Equilibrium and Kinetic. Chin. J. Chem. Eng. 2017, 25, 523–532. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Daana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Benmahdi, F.; Semra, S.; Haddad, D.; Mandin, P.; Kolli, M.; Bouhelassa, M. Breakthrough Curves Analysis and Statistical Design of Phenol Adsorption on Activated Carbon. Chem. Eng. Technol. 2019, 42, 355–369. [Google Scholar] [CrossRef]
- Hasan, S.H.; Ranjan, D.; Talat, M. Agro-industrial Waste “Wheat Bran” for the Biosorptive Remediation of Selenium through Continuous Up-flow Fixed-bed Column. J. Hazard. Mater. 2010, 181, 1134–1142. [Google Scholar] [CrossRef]
- Girish, C.R.; Ramachandra, M.V. Removal of Phenol from Wastewater in Packed Bed and Fluidized Bed Columns: A Review. Int. Res. J. Environ. Sci. 2013, 2, 96–100. [Google Scholar]
- Srivastava, V.C.; Prasad, B.; Mishra, I.M.; Mall, I.D.; Swamy, M.M. Prediction of Breakthrough Curves for Sorptive Removal of Phenol by Bagasse Fly Ash Packed Bed. Ind. Eng. Chem. Res. 2008, 47, 1603–1613. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of an Activated Carbon Packed Bed for the Adsorption of Phenols from Petroleum Refinery Wastewater. Environ. Sci. Pollut. Res. 2017, 24, 7511–7520. [Google Scholar] [CrossRef] [PubMed]
- Negrea, A.; Mihailescu, M.; Mosoarca, G.; Ciopec, M.; Duteanu, N.; Negrea, P.; Minzatu, V. Estimation of Fixed-bed Column Paramters Pf Breakthrough Behaviours for Gold Recovery by Adsorption onto Modifies/Functionalized Amberlite XAD7. Int. J. Environ. Res. Public Health 2020, 17, 6868. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Xiao, W.; Han, R. Adsorption Behavior of Light Green Anionic Dye Using Cationic Surfactant-modified Wheat Straw in Batch and Column Mode. Environ. Sci. Pollut. Res. 2013, 20, 5558–5568. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Adsorption of Acid Dyes on to Granular Activated Carbon in Fixed Beds. Water Res. 1997, 31, 2093–2101. [Google Scholar] [CrossRef]
- Fryda, L.; Visserr, R. Biochar for Soil Improvement: Evaluation of Biochar from Gasification and Slow Pyrolysis. Agriculture 2015, 5, 1076–1115. [Google Scholar] [CrossRef]
- Dias, D.; Lapa, N.; Bernardo, M.; Godinho, D.; Fonseca, I.; Miranda, M.; Pinto, F.; Lemos, F. Properties of Chars from the Gasification and Pyrolysis of Rice Waste Streams Towards Their Valorisation as Adsorbent Materials. Waste Manag. 2017, 65, 186–194. [Google Scholar] [CrossRef]
- Bordbar, M. Biosynthesis of Ag/Almond Shell Nanocomposite as a Cost-effective and Efficient Catalyst for Degradation of 4-nitrophenol and Organic Dyes. RSC Adv. 2017, 7, 180. [Google Scholar] [CrossRef]
- Xue, J.; Wu, Y.; Li, M.; Sun, X.; Wang, H.; Liu, B. Characteristic Assessment of Diesel-degrading Bacteria Immobilized on Natural Organic Carriers in Marine Environment: The Degradation Activity and Nutrient. Sci. Rep. 2017, 7, 8635–8644. [Google Scholar] [CrossRef]
- Singhal, A.; Jaiswal, P.K.; Jha, P.K.; Thapliyal, A.; Thakur, I.S. Assessment of Cryptococcus albidus for Biopulping of Eucalyptus. Biochem. Biotechnol. 2013, 43, 735–749. [Google Scholar]
- Lorenc-Grabowska, E.; Diez, M.A.; Gryglewicz, G. Influence of Pore Size Distribution on the Adsorption of Phenol on PET-baserd Activated Carbons. J. Coll. Interf. Sci. 2016, 469, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Teng, H. Liquid-phase Adsorption of Phenol onto Activated Carbons Prepared with Different Activation Levels. J. Coll. Interf. Sci. 2000, 230, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Din, A.T.M.; Hameed, B.H.; Ahmad, A.L. Batch Adsorption of Phenol onto Physciochemical-activated Coconut Schell. J. Hazard. Mat. 2009, 161, 1522–1529. [Google Scholar]
- Lorenc-Grabowska, E.; Gryglewicz, G.; Diez, M.A. Kinetics and Equilibrium Study of Phenol Adsorption on Nitrogen-enriched Activated Carbons. Fuel 2013, 114, 235–243. [Google Scholar] [CrossRef]
- Teng, H.; Hsieh, C.-T. Influence of Surface Characteristics on Liquid-phase Adsorption of Phenol by Activated Carbons Prepared from Bituminous Coal. Ind. Eng. Chem. Res. 1998, 37, 3618–3624. [Google Scholar] [CrossRef]
- Lütke, S.F.; Igansi, A.V.; Pegoraro, L.; Dotto, G.L.; Pinto, L.A.A.; Cadaval Jr, T.R.S. Preparation of Activated Carbon from Black Wattle Bark Waste and Its Application for Phenol Adsorption. J. Environ. Chem. Eng. 2019, 7, 103396–103406. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Liquid-phase Adsorption of Dyes and Phenols Using Pinewood-based Activated Carbons. Carbon 2003, 41, 487–495. [Google Scholar] [CrossRef]
- Lukchis, G.M. Adsorption Systems, Part I-Design by Mass-Transfer-Zone Concept. Chem. Eng. 1973, 30, 111–116. [Google Scholar]
- Kuo, J. Practical Design Calculations for Groundwater and Soil Remediation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.-H. A Critical Review on Sustainable Biochar System through Gasification: Energy and Environmental Applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Asudullah, M. Biomass Gasification Gas Cleaning for Downstream Applications: A Comparative Critical Review. Renew. Sustain. Energy Rev. 2014, 40, 118–132. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Li, Z.; Chen, D. Characteristics of Tar Formation during Cellulose, Hemicellulose and Lignin Gasification. Fuel 2014, 118, 250–256. [Google Scholar] [CrossRef]
- Coll, R.; Salvadò, J.; Farriol, X.; Montané, D. Steam Reforming Model Compounds of Biomass Gasification Tars: Conversion at Different Operating Conditions and Tendency Towards Coke Formation. Fuel Process. Technol. 2001, 74, 19–31. [Google Scholar] [CrossRef]
- Wolfesberger, U.; Aigner, I.; Hofbauer, H. Tar Content and Composition in Produced Gas of Fluidized Bed Gasification of Wood–influence of Temperature and Pressure. Environ. Prog. Sustain. Energy 2009, 28, 372–379. [Google Scholar] [CrossRef]
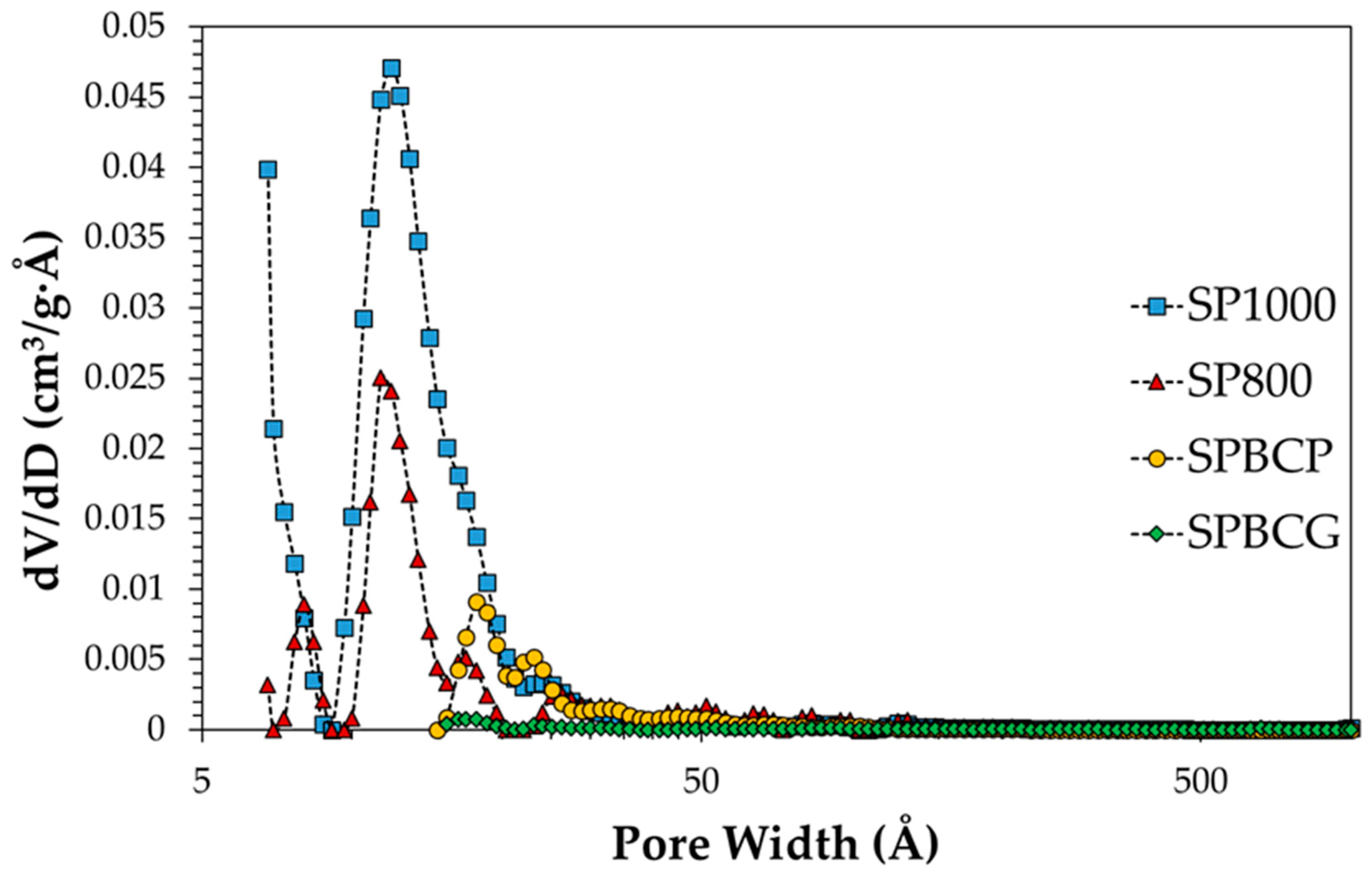




| Sample | B.E.T. SSA (m2/g) | B.E.T C-Value (−) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| SP1000 | 1281 | 6289 | 0.643 |
| SP800 | 698 | 6155 | 0.413 |
| SPBCP | 211 | 81 | 0.126 |
| SPBCG | 63 | 844 | 0.069 |
| Carbon Amount (g) | Bed Height (cm) | Total Adsorption Capacity (mg/g) | Breakthrough Time a (min) | Breakthrough Adsorption Capacity b (mg/g) | Stoichiometric Time c (min) |
|---|---|---|---|---|---|
| 20 | 5.9 | 133 | 3 | 40 | 11 |
| 30 | 8.9 | 152 | 6 | 49 | 21 |
| 50 | 14.9 | 177 | 12 | 71 | 37 |
| Sample | Carbon Amount (g) | Thomas | MDR | ||||
|---|---|---|---|---|---|---|---|
| Kth × 102 (min/mL/mg) | qth (mg/g) | R2 | a (−) | qMDR (mg/g) | R2 | ||
| SP1000 | 20 | 2.76 | 118 | 0.959 | 1.8 | 109 | 0.998 |
| 30 | 2.78 | 155 | 0.987 | 2.0 | 144 | 0.997 | |
| 50 | 2.88 | 142 | 0.985 | 2.5 | 168 | 0.984 | |
| SPBCG | 50 | 14.01 | 13.1 | 0.949 | 5.6 | 19.5 | 0.995 |
| CUR (kg/h) | SP1000 | SP800 | SPBCG | SPBCP |
|---|---|---|---|---|
| C0 = 5000 ppm | 41 | 46 | 187 | 132 |
| C0 = 2500 ppm | 21 | 24 | 110 | 84 |
| C0 = 1000 ppm | 8 | 11 | 64 | 55 |
| C0 = 500 ppm | 4 | 7 | 48 | 45 |
| C0 = 100 ppm | 1 | 4 | 36 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catizzone, E.; Sposato, C.; Romanelli, A.; Barisano, D.; Cornacchia, G.; Marsico, L.; Cozza, D.; Migliori, M. Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons. Int. J. Environ. Res. Public Health 2021, 18, 4247. https://doi.org/10.3390/ijerph18084247
Catizzone E, Sposato C, Romanelli A, Barisano D, Cornacchia G, Marsico L, Cozza D, Migliori M. Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons. International Journal of Environmental Research and Public Health. 2021; 18(8):4247. https://doi.org/10.3390/ijerph18084247
Chicago/Turabian StyleCatizzone, Enrico, Corradino Sposato, Assunta Romanelli, Donatella Barisano, Giacinto Cornacchia, Luigi Marsico, Daniela Cozza, and Massimo Migliori. 2021. "Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons" International Journal of Environmental Research and Public Health 18, no. 8: 4247. https://doi.org/10.3390/ijerph18084247
APA StyleCatizzone, E., Sposato, C., Romanelli, A., Barisano, D., Cornacchia, G., Marsico, L., Cozza, D., & Migliori, M. (2021). Purification of Wastewater from Biomass-Derived Syngas Scrubber Using Biochar and Activated Carbons. International Journal of Environmental Research and Public Health, 18(8), 4247. https://doi.org/10.3390/ijerph18084247








