Effects of Spatial Characteristics on the Spread of the Highly Pathogenic Avian Influenza (HPAI) in Korea
Abstract
1. Introduction
2. Literature Review
3. Materials and Methods
3.1. Data
3.2. Econometric Procedure
3.2.1. Construction of the Spatial Weight Matrix
3.2.2. Diagnosis of Spatial Autocorrelation
3.2.3. Model Specification
3.3. Testing the Goodness of Fit of the Model
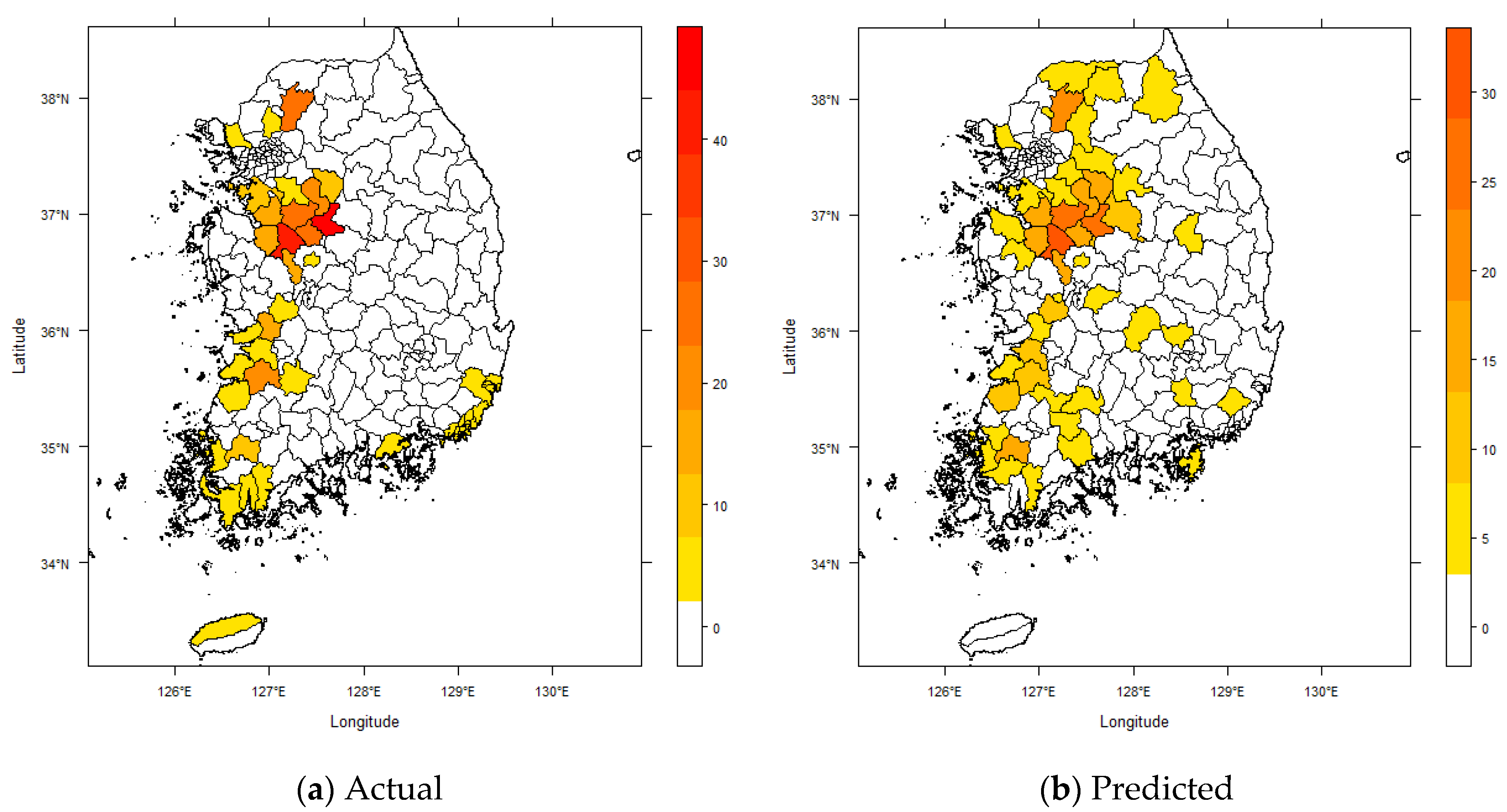
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Food and Rural Affairs & Animal and Plant Quarantine Agency. 17/18 Analysis Report on Epidemiology of Highly Pathogenic Avian Influenza. 2018. Available online: http://ebook.qia.go.kr/src/viewer/main.php?host=main&site=20181219_100247&category=1&page=215&search=AI (accessed on 5 August 2020). (In Korean).
- Eom, C.H.; Pak, S.I.; Bae, S.H. Analysis of Potential Infection Site by Highly Pathogenic Avian Influenza Using Model Patterns of Avian Influenza Outbreak Area in Republic of Korea. J. Korean Assoc. Geogr. Inf. Stud. 2017, 20, 60–74. (In Korean) [Google Scholar]
- Hong, S.J.; Park, S.I.; Lee, K.N.; Cho, K.H.; Lee, K.J. Spatial variations of risk factors associated with the diffusion of highly infectious animal diseases. J. Korean Cartogr. Assoc. 2018, 18, 81–91. (In Korean) [Google Scholar] [CrossRef]
- Firestone, S.M.; Christley, R.M.; Ward, M.P.; Dhand, N.K. Adding the spatial dimension to the social network analysis of an epidemic: Investigation of the 2007 outbreak of equine influenza in Australia. Prev. Vet. Med. 2012, 106, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Park, H.S.; Jeong, W.S.; Lee, G.J. Utilizing spatial and temporal information in KAHIS for aiding animal disease control activities. J. Korean Assoc. Geogr. Inf. Stud. 2016, 19, 186–198. (In Korean) [Google Scholar] [CrossRef]
- National Institute of Environmental Research. Reinforce the Surveillance of Influenza in Wild Birds. 2017. Available online: http://www.me.go.kr/home/web/board/read.do?menuId=286&boardMasterId=1&boardCategoryId=39&boardId=816450 (accessed on 11 August 2020). (In Korean).
- Ito, T. Highly pathogenic avian influenza and wild birds. Virus 2009, 59, 53–58. (In Japanese) [Google Scholar] [CrossRef]
- Park, S.L.; Bae, S.H. Link between Service Coverage of Slaughterhouse and the Potential Disease Transmission: Analyzing the Livestock Movements Data for Simulation Exercise (CPX). J. Korean Assoc. Geogr. Inf. Stud. 2016, 16, 67–77. (In Korean) [Google Scholar] [CrossRef]
- Chung, K.; Kim, M.; Song, C. Follow-Up Study Improvement of AI Defense System; Konkuk University: Seoul, Korea, 2016. (In Korean) [Google Scholar]
- Seok, J.H.; Moon, H.; Kim, G.; Reed, M.R. Is Aging the Important Factor for Sustainable Agricultural Development in Korea? Evidence from the Relationship between Aging and Farm Technical Efficiency. Sustainability 2018, 10, 2137. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar] [CrossRef]
- Fang, L.Q.; Cao, C.X.; Chen, G.S.; Lei, F.M.; Liu, Y.L.; Li, C.Y.; Yang, H.; Han, X.N.; Yan, L.; Li, X.W.; et al. Studies on the spatial distribution and environmental factors of highly pathogenic avian influenza in Mainland China, using geographic information system technology. Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi 2005, 26, 839–842. [Google Scholar] [PubMed]
- Martin, V.; Pfeiffer, D.U.; Zhou, X.; Xiao, X.; Prosser, D.J.; Guo, F.; Gilbert, M. Spatial distribution and risk factors of highly pathogenic avian influenza (HPAI) H5N1 in China. PLoS Pathog. 2011, 7, e1001308. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Tavornpanich, S.; Abrial, D.; Gasqui, P.; Charras-Garrido, M.; Thanapongtharm, W.; Xiao, X.; Gilbert, M.; Roger, F.; Ducrot, C. Anthropogenic factors and the risk of highly pathogenic avian influenza H5N1: Prospects from a spatial-based model. Vet. Res. 2010, 41, 28. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Wongnarkpet, S.; Gasqui, P.; Poolkhet, C.; Thongratsakul, S.; Ducrot, C.; Roger, F. Risk factors for highly pathogenic avian influenza (HPAI) H5N1 infection in backyard chicken farms, Thailand. Acta Trop. 2011, 118, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.; Yost, R.; Yanagida, J.; Saksena, S.; Fox, J.; Sultana, N. Spatio-temporal occurrence modeling of highly pathogenic avian influenza subtype H5N1: A case study in the Red River Delta, Vietnam. ISPRS Int. J. Geo-Inf. 2013, 2, 1106–1121. [Google Scholar] [CrossRef]
- Sun, L.; Ward, M.P.; Li, R.; Xia, C.; Lynn, H.; Hu, Y.; Xiong, C.; Zhang, Z. Global spatial risk pattern of highly pathogenic avian influenza H5N1 virus in wild birds: A knowledge-fusion based approach. Prev. Vet. Med. 2018, 152, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Wu, Y.; Sun, X.; Yu, K.; Yu, C.; Qin, A. The risk factors for avian influenza on poultry farms: A meta-analysis. Prev. Vet. Med. 2014, 117, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea, Regional Statistics Division. 2020. Available online: http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01 (accessed on 11 August 2020).
- Korea Land and Housing Corporation. City Planning Status. Available online: https://www.lh.or.kr/eng/index.do (accessed on 25 September 2020). (In Korean).
- Ministry of Agriculture, Food and Rural Affairs. ASF∙AI∙FMD∙BSE. 2016. Available online: http://www.mafra.go.kr/FMD-AI2/ (accessed on 30 August 2020). (In Korean).
- Anselin, L. Spatial Econometrics: Method and Models; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Lee, S.W.; Yoon, S.D.; Park, J.Y.; Min, S.H. Applied Spatial Econometric Model; Pakyoungsa: Seoul, Korea, 2006. (In Korean) [Google Scholar]
- Anselin, L.; Rey, S. Properties of tests for spatial dependence in linear regression models. Geogr. Anal. 1991, 23, 112–131. [Google Scholar] [CrossRef]
- Ullah, A. Spatial dependence in linear regression models with an introduction to spatial econometrics: Regression models with an Anselin Bera. In Handbook of Applied Economic Statistics; CRC Press: Boca Raton, FL, USA, 1998; pp. 257–259. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 October 2020).
- Bivand, R.S.; Pebesma, E.J.; Gomez-Rubio, V.; Pebesma, E.J. Applied Spatial Data Analysis with R; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Bivand, R.S.; Piras, G. Comparing implementations of estimation methods for spatial econometrics. J. Stat. Softw. 2015, 63, 1–36. [Google Scholar] [CrossRef]
- Gilbert, M.; Chaitaweesub, P.; Parakamawongsa, T.; Premashthira, S.; Tiensin, T.; Kalpravidh, W.; Wagner, H.; Slingenbergh, J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg. Infect. Dis. 2006, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Ji, I.; Han, K. Analysis of HPAI Incidence Factors in Duck Farms using Negative Binomial Regression Model. J. Rural Dev. 2020, 43, 25–43. (In Korean) [Google Scholar]
- Kim, H.R.; Park, C.K.; Oem, J.K.; Bae, Y.C.; Choi, J.G.; Lee, O.S.; Lee, Y.J. Characterization of H5N2 influenza viruses isolated in South Korea and their influence on the emergence of a novel H9N2 influenza virus. J. Gen. Virol. 2010, 91 Pt 8, 1978–1983. [Google Scholar] [CrossRef]
- Lee, C.W.; Suarez, D.L.; Tumpey, T.M.; Sung, H.W.; Kwon, Y.K.; Lee, Y.J.; Choi, J.G.; Joh, S.J.; Kim, M.C.; Lee, E.K.; et al. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J. Virol. 2005, 79, 3692–3702. [Google Scholar] [CrossRef] [PubMed]
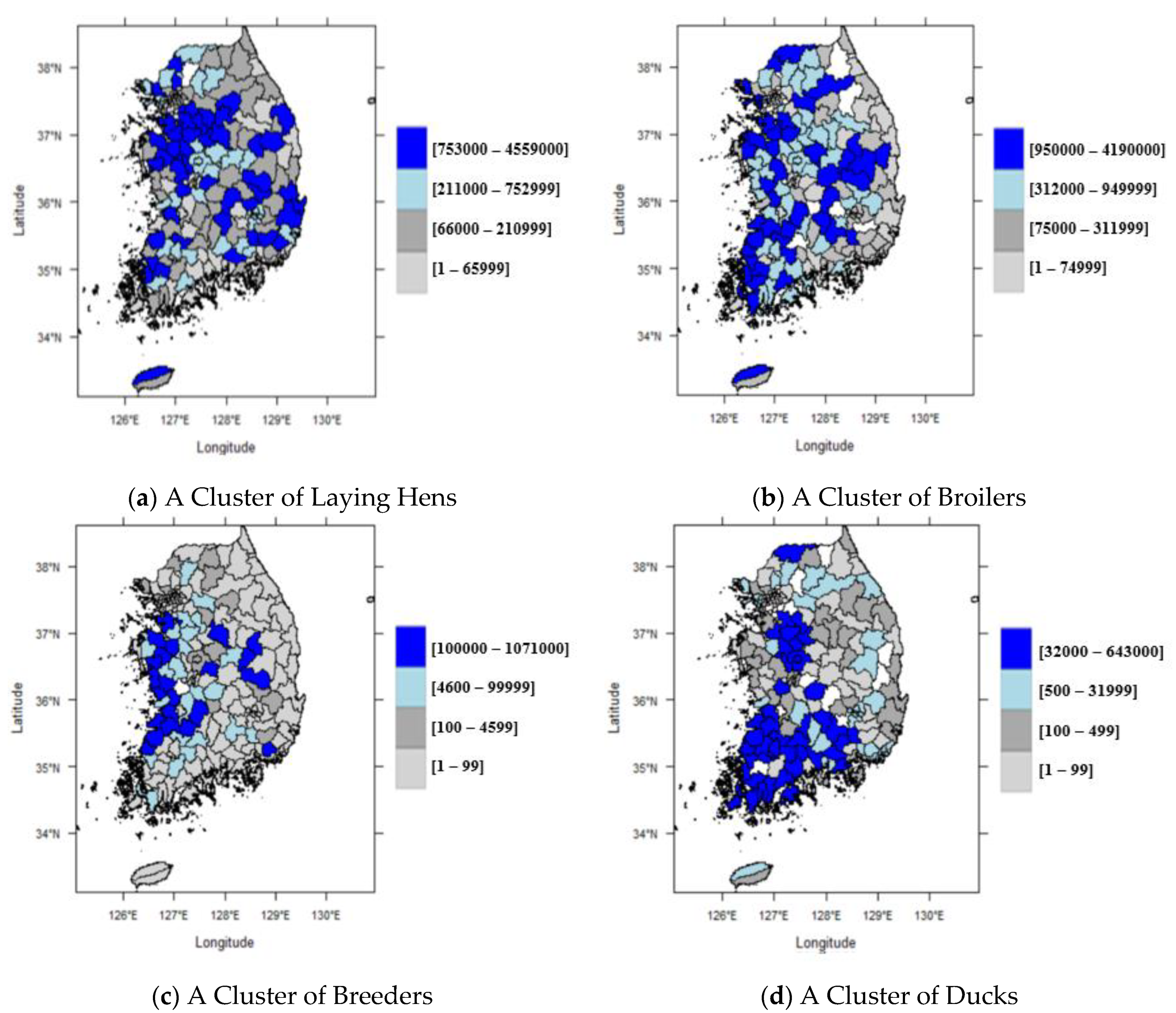
| Variables | Mean | Std. Dev. | Max. | Min. | |
|---|---|---|---|---|---|
| Dependent variable | Farms affected by HPAI (N/county) | 2.59 | 6.82 | 46.00 | 0.00 |
| Poultry number (1000 head) | Laying hens | 444.26 | 743.71 | 4558.81 | 0.00 |
| Broilers | 505.68 | 737.57 | 4190.00 | 0.00 | |
| Breeders | 70.46 | 156.12 | 1070.05 | 0.00 | |
| Ducks | 36.92 | 94.30 | 642.59 | 0.00 | |
| Poultry Farms | Farm Number (N) | 43.54 | 32.55 | 197.00 | 1.00 |
| Climate | Temperature (°C) | 13.52 | 1.28 | 17.00 | 8.10 |
| Precipitation (mm) | 112.16 | 88.66 | 1056.00 | 60.49 | |
| Humidity (%) | 69.69 | 5.98 | 91.90 | 56.70 | |
| Geographic and environmental | Migratory birds (0 or 1) | 0.20 | 0.40 | 1.00 | 0.00 |
| Livestock vehicles (N) | 301.70 | 212.56 | 845.94 | 6.75 | |
| Demographic and sociological | Population growth (%) | 0.38 | 2.98 | 26.40 | −0.52 |
| Migration (1000) | 0.00 | 12.81 | 43.53 | −14.03 | |
| Population density (N/km2) | 1058.00 | 2474.00 | 16,408.00 | 19.00 | |
| Aging level (%) | 20.55 | 8.24 | 37.49 | 7.51 | |
| Urbanization (%) | 65.06 | 25.75 | 100.00 | 19.06 | |
| Moran’s I | Z-Score | Expectation | Variance | p-Value |
|---|---|---|---|---|
| 0.2283 | 5.1670 | −0.0062 | 0.0021 | 0.0001 |
| Independent Variable | OLS Model | Spatial Lag Model | Spatial Error Model | General Spatial Model |
|---|---|---|---|---|
| Constant | 15.700 ** | 12.499 ** | 14.815 ** | 11.376 ** |
| (6.342) | (5.327) | (7.217) | (4.646) | |
| Laying hens | 0.006 *** | 0.005 *** | 0.005 *** | 0.005 *** |
| (0.001) | (0.001) | (0.001) | (0.001) | |
| Broilers | 0.002 *** | 0.001 | 0.001 | 0.001 |
| (0.001) | (0.001) | (0.001) | (0.001) | |
| Breeders | 0.004 | 0.004 * | 0.005 * | 0.004 * |
| (0.003) | (0.002) | (0.003) | (0.002) | |
| Ducks | 0.023 *** | 0.020 *** | 0.021 *** | 0.018 *** |
| (0.004) | (0.004) | (0.004) | (0.003) | |
| Poultry farms | 0.052 ** | 0.040 ** | 0.042 * | 0.033 * |
| (0.023) | (0.019) | (0.022) | (0.018) | |
| Temperature | 0.239 | 0.081 | −0.028 | 0.130 |
| (0.291) | (0.244) | (0.312) | (0.212) | |
| Precipitation | −0.005 | −0.004 | −0.006 * | −0.002 |
| (0.004) | (0.004) | (0.004) | (0.003) | |
| Relative humidity | −0.107 * | −0.099 * | −0.082 | −0.109 ** |
| (0.063) | (0.053) | (0.066) | (0.047) | |
| Migratory birds | 1.042 | 1.300 * | 1.310 | 1.221 * |
| (0.916) | (0.768) | (0.859) | (0.702) | |
| Livestock vehicles | −0.016 *** | −0.011 *** | −0.011 *** | −0.010 *** |
| (0.004) | (0.003) | (0.004) | (0.003) | |
| Population growth | 0.078 | 0.032 | 0.041 | 0.004 |
| (0.142) | (0.120) | (0.129) | (0.111) | |
| Migration | 0.000 | 0.000 | 0.000 | 0.000 |
| (0.000) | (0.000) | (0.000) | (0.000) | |
| Population density | 0.000 | 0.000 | 0.000 | 0.000 |
| (0.000) | (0.000) | (0.000) | (0.000) | |
| Aging level | −0.313 *** | −0.201 *** | −0.229 *** | −0.172 *** |
| (0.077) | (0.066) | (0.073) | (0.063) | |
| Urbanization | −0.066 *** | −0.047 ** | −0.050 ** | −0.043 ** |
| (0.025) | (0.021) | (0.022) | (0.020) | |
| — | 0.463 *** | — | 0.562 *** | |
| (0.070) | (0.079) | |||
| Λ | — | — | 0.570 *** | −0.343 * |
| (0.084) | (0.183) | |||
| Adj. R2 | 0.612 | — | — | |
| Log likelihood | −455.910 | −439.187 | −448.071 | −437.764 |
| AIC | 943.819 | 912.371 | 930.141 | 911.528 |
| BIC | 947.171 | 915.936 | 933.703 | 915.299 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, M.; Vitale, J.; Han, K.; Ng’ombe, J.N.; Ji, I. Effects of Spatial Characteristics on the Spread of the Highly Pathogenic Avian Influenza (HPAI) in Korea. Int. J. Environ. Res. Public Health 2021, 18, 4081. https://doi.org/10.3390/ijerph18084081
An M, Vitale J, Han K, Ng’ombe JN, Ji I. Effects of Spatial Characteristics on the Spread of the Highly Pathogenic Avian Influenza (HPAI) in Korea. International Journal of Environmental Research and Public Health. 2021; 18(8):4081. https://doi.org/10.3390/ijerph18084081
Chicago/Turabian StyleAn, Meilan, Jeffrey Vitale, Kwideok Han, John N. Ng’ombe, and Inbae Ji. 2021. "Effects of Spatial Characteristics on the Spread of the Highly Pathogenic Avian Influenza (HPAI) in Korea" International Journal of Environmental Research and Public Health 18, no. 8: 4081. https://doi.org/10.3390/ijerph18084081
APA StyleAn, M., Vitale, J., Han, K., Ng’ombe, J. N., & Ji, I. (2021). Effects of Spatial Characteristics on the Spread of the Highly Pathogenic Avian Influenza (HPAI) in Korea. International Journal of Environmental Research and Public Health, 18(8), 4081. https://doi.org/10.3390/ijerph18084081









