Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Analysis
3. Results
3.1. Original Articles That Fulfill Inclusion Criteria for the Systematic Review
3.2. Meta-Analysis Results
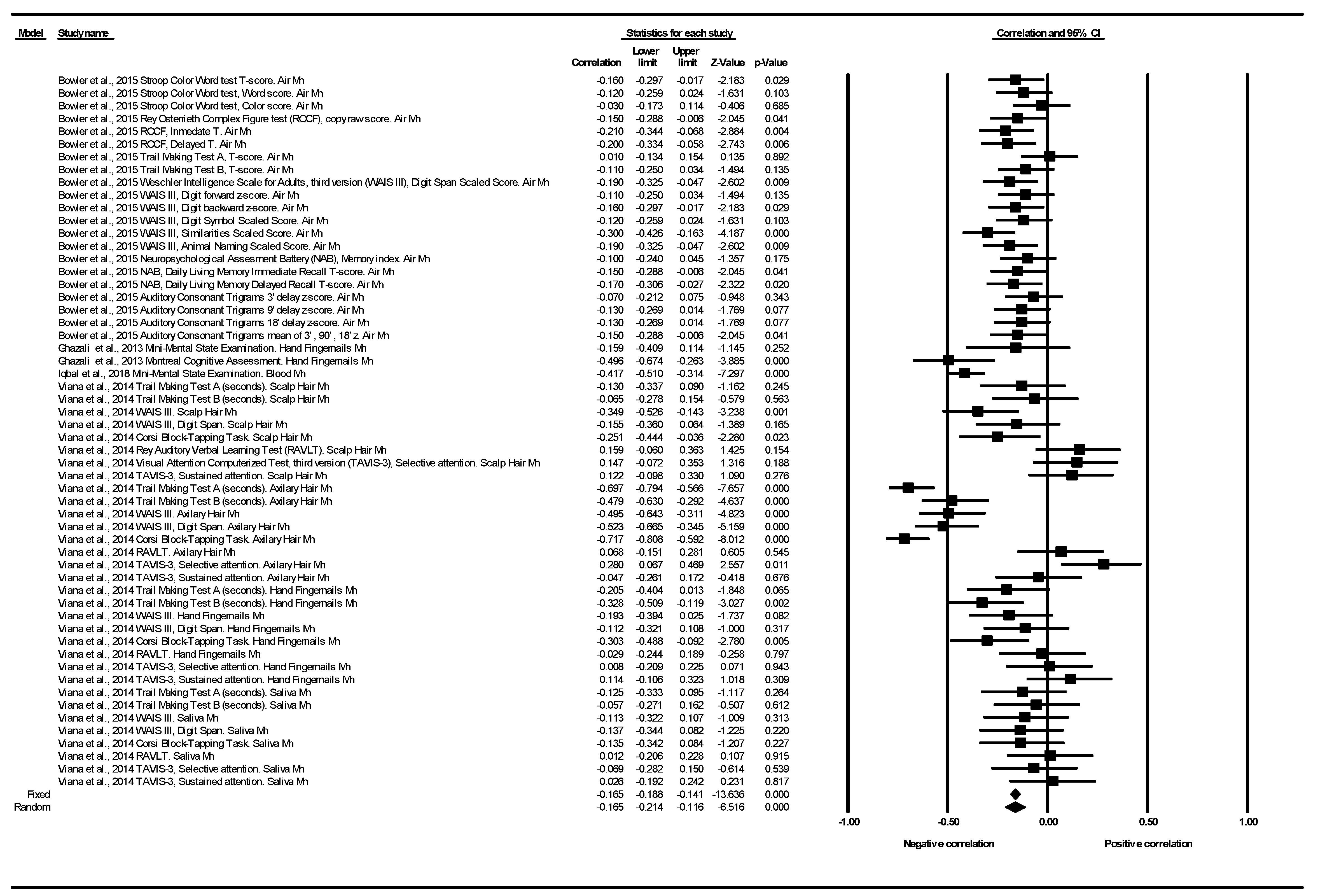
3.2.1. Cognitive Function Correlation
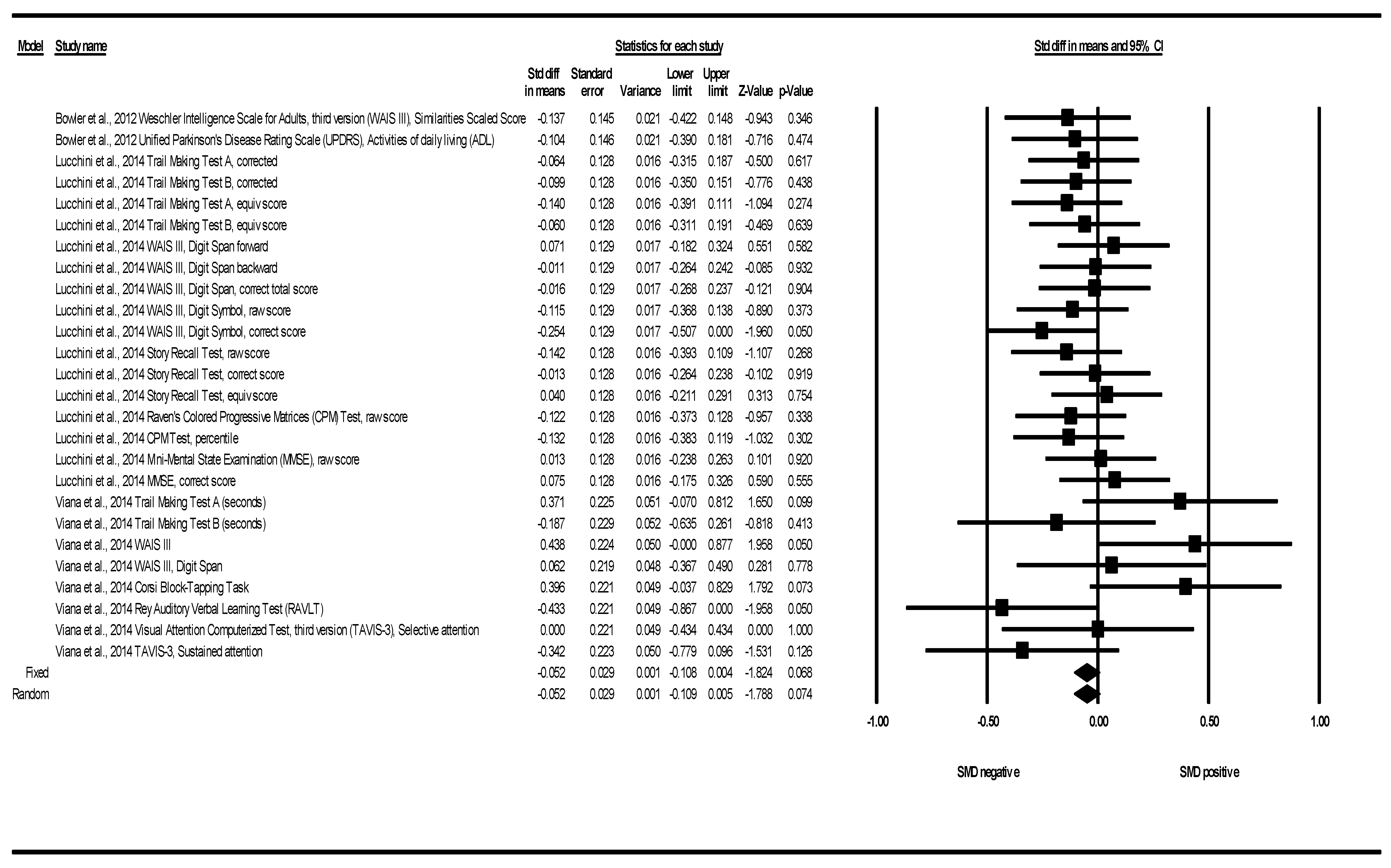
3.2.2. Cognitive Function SMD between Groups
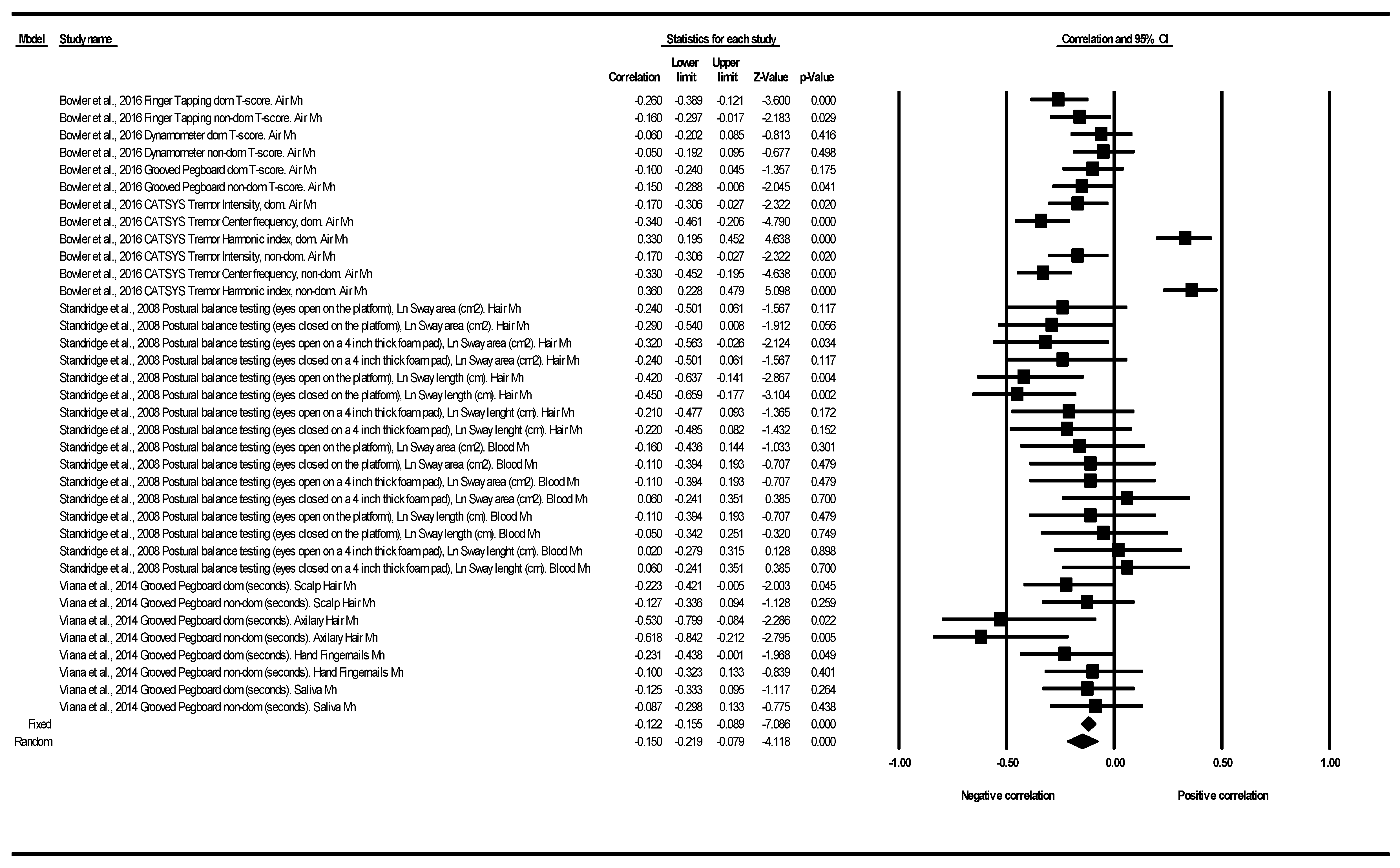
3.2.3. Motor Function Correlation
3.2.4. Motor Function SMD between Groups
3.2.5. Publication Bias
4. Discussion
4.1. Cognitive Function Correlation
4.2. Cognitive Function Standardized Mean Difference (SMD) between Groups
4.3. Motor Function Correlation
4.4. Motor Function Standardized Mean Difference (SMD) between Groups
4.5. Methodological Issues Relating to Meta-Analysis
4.6. Exposure to Airborne Manganese in the Context of Health-Derived Guidelines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of daily living |
| ACT | Auditory Consonant Trigrams |
| CDR | Clinical Dementia Rating Scale |
| CATSYS | Coordination Ability Test System |
| DIADO | Diadocchokinesimetry |
| EKM | Eurythmokinesimetry |
| GDS | Geriatric Depression Scale |
| HRQOL | Health Related Quality of Life |
| HVOT | Hooper visual organization test |
| MSQ | Medical symptoms questionnaires |
| LNNB | Luria Nebraska Neuropsychological Battery |
| MMSE | Mini-Mental State Examination |
| Mn-Air | Mn in ambient air |
| MnH | Mn in hair |
| MnAxH | Mn in axillary hair |
| MnFN | Mn in fingernails |
| MnSal | Mn in saliva |
| MoCA | Montreal Cognitive Assessment |
| NAB | Neuropsychological Assessment Battery |
| CPM | Raven’s Colored Progressive Matrices |
| RAVLT | Rey Auditory Verbal Learning test |
| ROCF | Rey Osterrieth Complex Figure |
| SCL-90R | Symptom Checklist 90 revised |
| TMT | Trail Making Test |
| HI | Tremor Harmonic index |
| DBP | Tremor system of Danish Product Development |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| TAVIS-3 | Visual Attention Computerized Test, third version |
| WAIS III | Wechsler Intelligence Scale for Adults, third version |
References
- Chen, P.; Bornhorst, J.; Aschner, M. Manganese metabolism in humans. Front. Biosci. 2018, 23, 1655–1679. [Google Scholar] [CrossRef]
- Mattison, D.R.; Milton, B.; Krewski, D.; Levy, L.; Dorman, D.C.; Aggett, P.J.; Roels, H.A.; Andersen, M.E.; Karyakina, N.A.; Shilnikova, N.; et al. Severity scoring of manganese health effects for categorical regression. Neurotoxicology 2017, 58, 203–216. [Google Scholar] [CrossRef]
- Dlamini, W.W.; Nelson, G.; Nielsen, S.S.; Racette, B.A. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am. J. Ind. Med. 2020, 63, 36–43. [Google Scholar] [CrossRef]
- Roels, H.; Lauwerys, R.; Buchet, J.; Genet, P.; Sarhan, M.J.; Hanotiau, I.; de Fays, M.; Bernard, A.; Stanescu, D. Epidemiological survey among workers exposed to manganese: Effects on lung, central nervous system, and some biological indices. Am. J. Ind. Med. 1987, 11, 307–327. [Google Scholar] [CrossRef]
- Lucchini, R.; Apostoli, P.; Perrone, C.; Placidi, D.; Albini, E.; Migliorati, P.; Mergler, D.; Sassine, M.P.; Palmi, S.; Alession, L. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology 1999, 20, 287–298. [Google Scholar]
- Mergler, D.; Huel, G.; Bowler, R.; Iregren, A.; Bélanger, S.; Baldwin, M.; Tardif, R.; Smargiassi, A.; Martin, L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994, 64, 151–180. [Google Scholar] [CrossRef]
- Bowler, R.M.; Gysens, E.; Diamond, S.; Nakagawa, M.; Drezgic, M.; Roels, H.A. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 2006, 27, 315–326. [Google Scholar] [CrossRef]
- Bowler, R.M.; Koller, W.; Schulz, P.E. Parkinsonism due to manganism in a welder: Neurological and neuropsychological sequelae. Neurotoxicology 2006, 27, 237–332. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.B. Manganese exposure, essentiality & toxicity. Indian J. Med. Res. 2008, 128, 484–500. [Google Scholar] [PubMed]
- Fernández-Olmo, I.; Mantecón, P.; Markiv, B.; Ruiz-Azcona, L.; Santibáñez, M. A Review on the Environmental Exposure to Airborne Manganese, Biomonitoring, and Neurological/Neuropsychological Outcomes. Rev. Environ. Contam. Toxicol. 2020. [Google Scholar] [CrossRef]
- Leonhard, M.J.; Chang, E.T.; Loccisano, A.E.; Garry, M.R. A systematic literature review of epidemiologic studies of developmental manganese exposure and neurodevelopmental outcomes. Toxicology 2019, 420, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xin, Y.; Li, Q.; Shang, Y.; Ping, Z.; Min, J.; Cahill, C.M.; Rogers, J.T.; Wang, F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ. Health 2020, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barranco, M.; Lacasaña, M.; Aguilar-Garduño, C.; Alguacil, J.; Gil, F.; González-Alzaga, B.; Rojas-García, A. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ. 2013, 454–455, 562–577. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Development and Use of Quality Assessment Tools. Available online: www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascularrisk-reduction/tools (accessed on 12 April 2021).
- Tsiligianni, I.; Kocks, J.; Tzanakis, N.; Siafakas, N.; van der Molen, T. Factors that influence disease-specific quality of life or health status in patients with COPD: A review and meta-analysis of Pearson Correlations. Prim. Care Respir. J. 2011, 20, 257–268. [Google Scholar]
- Shadish, W.R.; Haddock, C.K. Combining estimates of effect size. In The Handbook of Research Synthesis; Cooper, H., Hedges, L.V., Eds.; Russell Sage Foundation: New York, NY, USA, 1994; pp. 265–266. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Metaanalysis; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Almeida, K.M.; Fonseca, S.T.; Figueiredo, P.R.P.; Aquino, A.A.; Mancini, M.C. Effects of interventions with therapeutic suits (clothing) on impairments and functional limitations of children with cerebral palsy: A systematic review. Braz. J. Phys. Ther. 2017, 21, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Jiménez, C.; Sarabia, R.; Paz-Zulueta, M.; Paras-Bravo, P.; Pellico, A.; Ruiz Azcona, L.; Blanco, C.; Madrazo, M.; Agudo, M.J.; Sarabia, C.; et al. Impact of Active Video Games on Body Mass Index in Children and Adolescents: Systematic Review and Meta-Analysis Evaluating the Quality of Primary Studies. Int. J. Environ. Res. Public Health 2019, 16, 2424. [Google Scholar] [CrossRef]
- Collado-Garrido, L.; Parás-Bravo, P.; Calvo-Martín, P.; Santibáñez-Margüello, M. Impact of Resistance Therapy on Motor Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4513. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences, 2nd ed.; Lawrence Earlbaum: Mahwah, NJ, USA, 1988. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available online: www.cochrane-handbook.org (accessed on 12 April 2021).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Comprehensive Meta-Analysis Version 2; Biostat: Englewood, NJ, USA, 2005. [Google Scholar]
- Bowler, R.M.; Beseler, C.L.; Gocheva, V.V.; Colledge, M.; Kornblith, E.S.; Julian, J.R.; Kim, Y.; Bollweg, G.; Lobdell, D.T. Environmental exposure to manganese in air: Associations with tremor and motor function. Sci. Total Environ. 2016, 541, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Danish Product Development (DPD). TREMOR 7.0 User’s Manual; Danish Product Development Ltd.: Snekkersten, Denmark, 2000. [Google Scholar]
- Mergler, D.; Baldwin, M.; Bélanger, S.; Larribe, F.; Beuter, A.; Bowler, R.; Panisset, M.; Edwards, R.; de Geoffroy, A.; Sassine, M.P.; et al. Manganese neurotoxicity, a continuum of dysfunction: Results from a community-based study. Neurotoxicology 1999, 20, 327–342. [Google Scholar] [PubMed]
- Beuter, A.; Edwards, R.; de Geoffroy, A.; Mergler, D.; Hundnell, K. Quantification of neuromotor function for detection of the effects of manganese. Neurotoxicology 1999, 20, 355–366. [Google Scholar] [PubMed]
- Santos-Burgoa, C.; Rios, C.; Mercado, L.A.; Arechiga-Serrano, R.; Cano-Valle, F.; Eden-Wynter, R.A.; Texcalac-Sangrador, J.L.; Villa-Barragan, J.P.; Rodriguez-Agudelo, Y.; Montes, S. Exposure to manganese: Health effects on the general population, a pilot study in Central Mexico. Environ. Res. 2001, 85, 90–104. [Google Scholar] [CrossRef]
- Rodríguez-Agudelo, Y.; Riojas-Rodríguez, H.; Ríos, C.; Rosas, I.; Sabido Pedraza, E.; Miranda, J.; Siebe, C.; Texcalac, J.L.; Santos-Burgoa, C. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci. Total Environ. 2006, 368, 542–556. [Google Scholar] [CrossRef]
- Standridge, J.S.; Bhattacharya, A.; Succop, P.; Cox, C.; Haynes, E.N. Effect of chronic low-level manganese exposure on postural balance: A pilot study of residents in Southwest Ohio. J. Occup. Environ. Med. 2008, 50, 1421–1429. [Google Scholar] [CrossRef]
- Solís-Vivanco, R.; Rodríguez-Agudelo, Y.; Riojas-Rodríguez, H.; Ríos, C.; Rosas, I.; Montes, S. Cognitive impairment in an adult Mexican population non-occupationally exposed to manganese. Environ. Toxicol. Pharmacol. 2009, 28, 172–178. [Google Scholar] [CrossRef]
- Kim, Y.; Bowler, R.M.; Abdelouahab, N.; Harris, M.; Gocheva, V.; Roels, H.A. Motor function in adults of an Ohio community with environmental manganese exposure. Neurotoxicology 2011, 32, 606–614. [Google Scholar] [CrossRef]
- Bowler, R.M.; Harris, M.; Gocheva, V.; Wilson, K.; Kim, Y.; Davis, S.I.; Bollweg, G.; Lobdell, D.T.; Ngo, L.; Roels, H.A. Anxiety affecting parkinsonian outcome and motor efficiency in adults of an Ohio community with environmental airborne manganese exposure. Int. J. Hyg. Environ. Health 2012, 215, 393–405. [Google Scholar] [CrossRef]
- Ghazali, A.R.; Kamarulzaman, F.; Normah, C.D.; Ahmad, M.; Ghazali, S.E.; Ibrahim, N.; Said, Z.; Shahar, S.; Angkat, N.; Razali, R. Levels of metallic elements and their potential relationships to cognitive function among elderly from Federal Land Development Authority (FELDA) settlement in Selangor Malaysia. Biol. Trace Elem. Res. 2013, 153, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Guarneros, M.; Ortiz-Romo, N.; Alcaraz-Zubeldia, M.; Drucker-Colín, R.; Hudson, R. Nonoccupational environmental exposure to manganese is linked to deficits in peripheral and central olfactory function. Chem. Senses 2013, 38, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Guazzetti, S.; Zoni, S.; Benedetti, C.; Fedrighi, C.; Peli, M.; Donna, F.; Bontempi, E.; Borgese, L.; Micheletti, S.; et al. Neurofunctional dopaminergic impairment in elderly after lifetime exposure to manganese. Neurotoxicology 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Viana, G.F.S.; Carvalho, C.F.; Nunes, L.S.; Rodrigues, J.L.G.; Ribeiro, N.S.; Almeida, D.A.; Ferreira, J.R.D.; Abreu, N.; Menezes-Filho, J.A. Noninvasive biomarkers of manganese exposure and neuropsychological effects in environmentally exposed adults in Brazil. Toxicol. Lett. 2014, 231, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bowler, R.M.; Kornblith, E.S.; Gocheva, V.V.; Colledge, M.A.; Bollweg, G.; Kim, Y.; Beseler, C.L.; Wright, C.W.; Adams, S.W.; Lobdell, D.T. Environmental exposure to manganese in air: Associations with cognitive functions. Neurotoxicology 2015, 49, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, G.; Zada, W.; Mannan, A.; Ahmed, T. Elevated heavy metals levels in cognitively impaired patients from Pakistan. Environ. Toxicol. Pharmacol. 2018, 60, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.S.C.; Marinho-Reis, A.P.; Almeida, A.; Ordens, C.M.; Silva, M.M.V.G.; Freitas, S.; Simões, M.R.; Moreira, P.I.; Dinis, P.A.; Diniz, M.L.; et al. Human predisposition to cognitive impairment and its relation with environmental exposure to potentially toxic elements. Environ. Geochem. Health 2018, 40, 1767–1784. [Google Scholar] [CrossRef]
- Kornblith, E.S.; Casey, S.L.; Lobdell, D.T.; Colledge, M.A.; Bowler, R.M. Environmental exposure to manganese in air: Tremor, motor and cognitive symptom profiles. Neurotoxicology 2018, 64, 152–158. [Google Scholar] [CrossRef]
- Rafiee, A.; Delgado-Saborit, J.M.; Sly, P.D.; Quémerais, B.; Hashemi, F.; Akbari, S.; Hoseini, M. Environmental chronic exposure to metals and effects on in the general population. Sci. Total Environ. 2020, 705, 135911. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Tian, Q.; Resnick, S.M.; Studenski, S.A. Olfaction Is Related to Motor Function in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1067–1071. [Google Scholar] [CrossRef]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019, Volume One. US Department of Health and Human Services. 2019; ISBN 92 4 153063 4. Available online: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf (accessed on 25 March 2021).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Williams, M.; Todd, G.D.; Roney, N.; Crawford, J.; Coles, C.; McClure, P.R.; Garey, J.D.; Zaccaria, K.; Citra, M. Toxicological Profile for Manganese; Agency for Toxic Substances and Disease Registry (US ATSDR): Atlanta, GA, USA, 2012.
- WHO. Manganese and Its Compounds: Environmental Aspects (Concise International Chemical Assessment Document; 63). ISBN 92 4 153063 4. Available online: https://www.who.int/ipcs/publications/cicad/cicad63_rev_1.pdf (accessed on 25 March 2021).
- US Environmental Protection Agency (EPA). Printout of Reference Concentration (RfC) for Chronic Inhalation Exposure for Manganese as Verified 9/23/93, Dated 12/93; US EPA: Washington, DC, USA, 1993.
- Menezes-Filho, J.A.; Paes, C.R.; Pontes, A.M.; Moreira, J.C.; Sarcinelli, P.N.; Mergler, D. High levels of hair manganese in children living in the vicinity of a ferro-manganese alloy production plant. Neurotoxicology 2009, 30, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.A.; Goodman, J.E.; Beck, B.D. Proposal for a revised Reference Concentration (RfC) for manganese based on recent epidemiological studies. Regul. Toxicol. Pharmacol. 2009, 55, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Gentry, P.R.; Van Landingham, C.; Fuller, W.G.; Sulsky, S.I.; Greene, T.B.; Clewell, H.J., 3rd; Andersen, M.E.; Roels, H.A.; Taylor, M.D.; Keene, A.M. A tissue dose-based comparative exposure assessment of manganese using physiologically based pharmacokinetic modeling-The importance of homeostatic control for an essential metal. Toxicol. Appl. Pharmacol. 2017, 322, 27–40. [Google Scholar] [CrossRef]
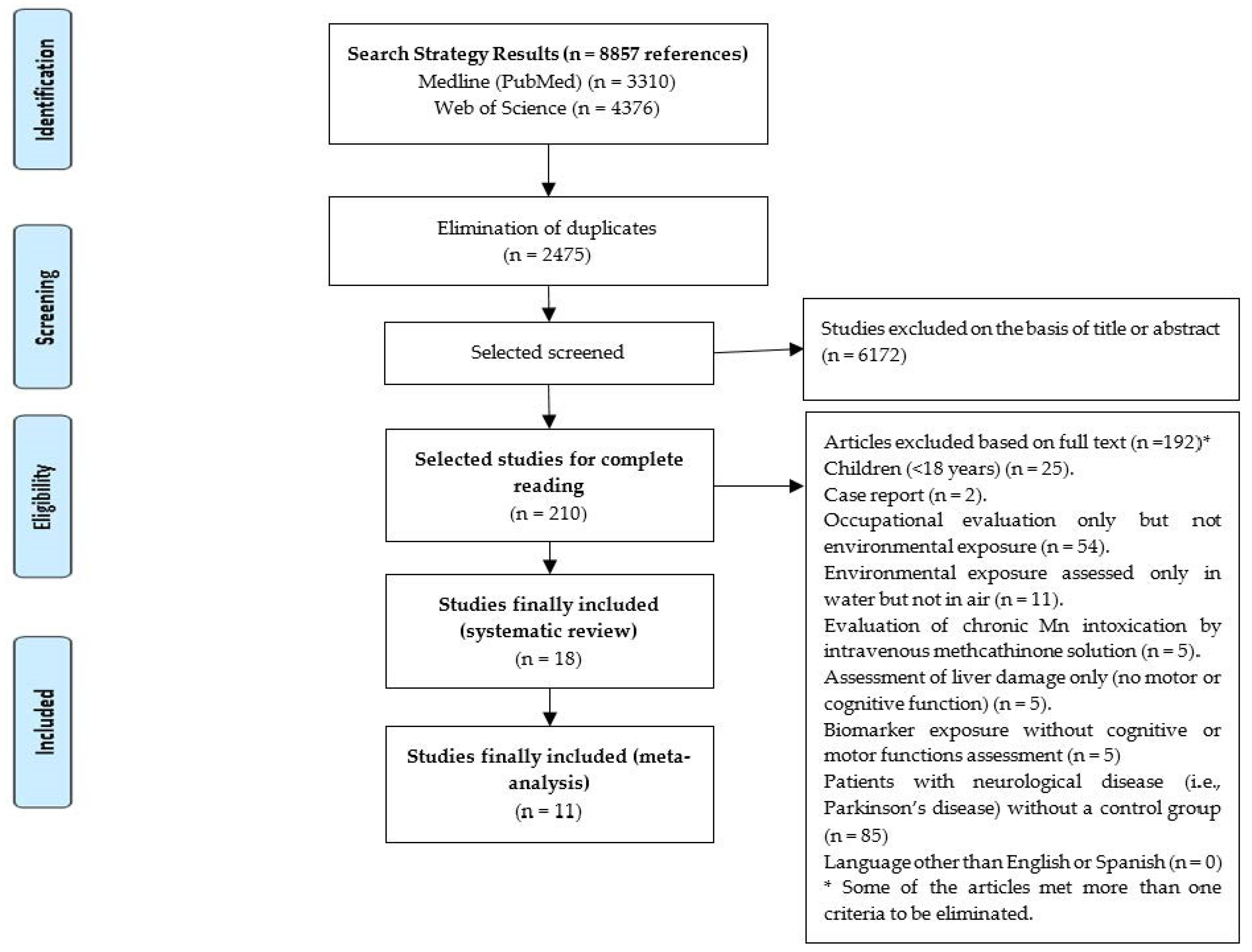


| Inclusion Criteria |
| • Primary epidemiological articles (original papers) in adults (≥18 years). • Mn as a heavy metal. • Environmental exposure determined in air and/or biomarkers. • Assessment of cognitive or motor functions. • Language: written in English or Spanish. |
| Exclusion Criteria |
| • Case reports or reviews. • Developed in rats, other animals or in human cell models. • Children (<18 years). • Mn referred to the enzyme “Manganese superoxide dismutase” (not as a heavy metal). • Mn as “trace element” or “micronutrient”, evaluating for example its role in a “micronutrient supplementation”. • Occupational evaluation only but not environmental exposure. • Environmental exposure only assessed by ingestion (e.g., water). • Evaluation of chronic Mn intoxication by intravenous methcathinone solution. • Determination of exposure using biomarkers but without assessing cognitive or motor functions. • Assessment of liver damage only (no motor or cognitive function). • Only in patients with neurological disease (i.e., Parkinson’s disease) without a control group. |
| Author/(Year)/Country | Design. Study Population | Exposure | Neurological and Cognitive Tests | Neuromotor Evaluation Test (Motor/Tremor) | Control of Biases. Results |
|---|---|---|---|---|---|
| Mergler et al. (1999)/Canada d [30] | Cross-sectional. 273 healthy population (without cognitive impairment) from Southwest Quebec, (after removing persons with sequelae of neurological illness and persons with heavy alcohol consumption), where a former Mn alloy production plant existed. | Biomarkers of Mn exposure (Blood). Arithmetic mean was used for categorical analyses between exposed and non-exposed (<7.5 µg/L, >= 7.5 µg/L). Same study as Beuter et al. (1999). | Memory Assessment Scale, Rey-15-item visual memory and reproduction, Wechsler Intelligence Scale for Adults, third version (WAIS III) Digit Span test, Cancellation H, Trail Making Test * (TMT), Stroop color/word test, Near visuality acuity chart, AMTI acusway system. | Eurythmokinesimetry (EKM) *, Purdue Pegboard Test, Diadocchokinesimetry (DIADO), TREMOR system of Danish Product Development (DBP). | Results stratified by age and gender. Confounding controlling by multivariate analysis (educational level, tobacco and alcohol consumption and others). Analyses of the individual measures revealed that people in the higher Mn category performed less well on the pointing task, EKM, manifesting more irregularity and higher Fitt’s constant and a tendency to make more multiple contacts on the target. On DIADO, those in the higher Mn category displayed slower velocity. For the other measures of motor performance, handarm tremor and tapping movements, showed no relation with Mn. |
| Beuter et al. (1999)/Canada e [31] | Cross-sectional. Same study population as Mergler et al. (1999). | Same exposure as Mergler et al. (1999). | Same tests as Mergler et al. (1999), but presenting results with different analysis strategy. | Same tests as Mergler et al. (1999), but presenting results with different analysis strategy. | Confounding controlling by multivariate analysis (age, gender, educational level, tobacco and alcohol consumption and others). Mn exposure was found to be associated with a decrease in ability to perform regular, rapid and precise pointing movements and a decrease in ability to attain high maximum rotation speeds in rapid alternated movement, and an increase in regularity of tremor oscillations. |
| Santos Burgoa et al. (2001)/Mexico e [32] | Cross-sectional. Two communities living within a Mn mining district in central Mexico: Community A (n = 44) was 2 km from the primary ore refining plant, residing in the uphill area surrounding the plant. Community B (n = 27) was 25 km downhill and downstream from the point source. The name of the communities is not detailed, but probably is the same state as Guarneros et al. (2013); Rodríguez-Agudelo et al. (2006); and Solís Vivanco et al. (2009) studies. | Biomarkers of Mn exposure (Blood). Median Blood Mn 15 μg/L; range (7.5–88). The upper quartile started at 20 μg/L; the upper 10% was above 25 μg/L. | The Hooper visual organization test (HVOT), TMT *, WAIS III Digit span, Animal naming, Mini-Mental State Examination (MMSE). | The neuropsychological scheme (motor behaviour). | Multivariate analysis including most frequently as covariates: age, schooling, community, alcohol; and occasionally age and sex. Mn increased the risk of deficient cognitive performance 11.7 times (Mini-Mental score of less than 17). The models with the highest explanation of the effects are those related to motor strength, coordination, and cognitive performance. The motor test employed was fingertip touching. The most relevant of these are the results of the Mini-Mental Examination. A lack of trend for the Mini-Mental test with increasing Mn concentrations, while the estimated risk ratios for each tertile of Mn for reduced Mini-Mental score, and other tests displaying a U-shaped dose–response curve. |
| Rodríguez-Agudelo et al. (2006)/Mexico e [33] | Cross-sectional. 288 healthy participants (168 women and 120 men) from eight communities at various distances from Mn extraction or processing facilities in the district of Molango (Hidalgo state) were studied. Same district as Santos-Burgoa et al.. (2001) and Guarneros et al. (2013) study, and same study population as Solís Vivanco et al. (2009). | Air Mn evaluation. Range: 0.003 to 5.86 µg/ m3. Geometric mean = 0.10 µg/m3 (median 0.13). A cut-off point of 0.05 and 0.1 µg/m3 was used to dichotomously categorize Mn exposure. Biomarkers of Mn exposure (Blood). Blood Mn range: 5.0 µg/L to 31.0 µg/L (geometric mean: 9.44 µg/L). | _ | Ostrosky-Solís’s neuropsychological battery. | Multivariate analysis including as possible covariates alcoholism, gender, age, socioeconomic status, blood lead, and scholarship if p < 0.1. Considering cumulative exposure index in quartiles (to have variability below and above the cutting point), there was an association between air Mn concentrations and motor tests that assessed the coordination of two movements: OR = 3.69; 95%CI (0.9 to 15.13) and position changes in hand movements, reaching statistical significance: OR = 3.09; (95%CI 1.07 to 8.92). An association with tests evaluating conflictive reactions (task that explores verbal regulations of movements) was also found: OR = 2.30; 95%CI (1.00 to 5.28). No associations were found between blood Mn and poorer motor tests results. |
| Standridge et al. (2008)/United States (US) c,d [34] | Cross-sectional. Healthy population (n = 29 without cognitive impairment) from 19 to 68 years (mean = 50) from Marietta, Ohio (same area as Bowler et al. studies), a town near a ferroMn refinery. | Biomarkers of Mn exposure (Hair and Blood). Mean hair Mn 4.4 μg/g; range (1.2–12.4). Mean blood Mn of 9.4 μg/L; range (4.2–21.7). | _ | Postural Balance Testing * | Multivariate analysis including gender, age and height/weight ratio (HT/WT). Caffeine, tobacco and alcohol included if p ≤ 0.10. Pearson correlation coefficients between measures of postural balance and natural logarithm (Ln) transformed hair Mn were all positive (the higher levels, the worse motor function) and reached statistical significance for sway length (SL) under eyes open (EO) and eyes closed (EC) on the platform. Following covariate adjustment within the linear regression analysis, Ln hair Mn reached statistical significance with sway area (SA) and SL under EO and EC test conditions. |
| Solís Vivanco et al. (2009)/Mexico e [35] | Cross-sectional. Same study population as Rodríguez-Agudelo et al. 2006 and same district as Guarneros et al. 2013 study, where there are important Mn extraction and processing facilities. | Air Mn evaluation. Biomarkers of Mn exposure (Blood). See Rodríguez-Agudelo et al. 2006 data for details. | MMSE, Digit Span, Word Association Test, Clock Test, Word List test, Semicomplex Desing test. | _ | Multivariate analysis including age, education, gender, tobacco and alcohol consumption, and blood Pb concentration. When using the 0.1 μg/m3 cut-off point of air Mn, there was a risk of poor performance on the digit span test (attention impairment): OR = 1.75; 95%CI (1.01 to 3.06). When using the 0.05 μg/m3 cut-off point there was no risk of poor performance on any test (e.g., OR digit span test = 1.24; 95%CI (0.67 to 2.29). There was no association between blood Mn concentration and cognitive function (e.g., OR MMSE = 1.17, 95%CI (0.99 to 1.38). |
| Kim et al. (2011)/US d [36] | Cross-sectional. Healthy participants (without cognitive impairment) from “Marietta”, Washington County, Ohio (n = 100, exposed to high levels of Mn) are compared with “Mount Vernon”, Knox County, Ohio (n = 90, non-exposed to high levels of Mn). | Air Mn evaluation. Modeled air Mn (Mn-Air) reported only for Marietta. Mean ±SD, 0.18 µg/m3 ± 0.13 µg/m3. Median = 0.16 µg/m3. Range 0.04–0.96 µg/m3. Biomarkers of Mn exposure (Blood). Mean in Blood ±SD = 9.65 µg/L ± 3.21 µg/L. Range: 4.91–24.60 µg/L (Marietta, exposed). Mean ±SD = 9.48 µg/L ± 3.16 µg/L. Range: 3.75–18.90 µg/L (Mount Vernon, non-exposed). | Unified Parkinson’s Disease Rating Scale (UPDRS)-Activities of daily living (ADL) *. | Coordination Ability Test System (CATSYS)*, UPDRS (motor and bradykinesia)*. | Multivariate analysis using different models. Model 1: adjustment for age, sex, ethnicity, smoking, alcohol, educational level, household income, and insurance status. Model 2 and 3 incorporates more covariates in addition. The Mn-exposed group (Marietta) showed significantly higher postural sway scores under eyes-open conditions according to CATSYS assessment than the comparison group (Mount Vernon), but the effect sizes were small to medium (0.23–0.42). The overall means of the UPDRS Motor and Bradykinesia scores were significantly higher in the exposed group than in the comparison group. However, the effect sizes were small (Motor: 0.22; Bradykinesia: 0.20). UPDRS Motor or Bradykinesia scores did not correlate with exposure indices such as Mn-B, or modeled air-Mn (data not shown). The risks of abnormal UPDRS Motor and Bradykinesia scores (scores >0) were in the exposed group respectively 2.43- and 2.90-fold higher than in the comparison group after adjustment for confounding variables. |
| Bowler et al. (2012)/US b,d [37] | Cross-sectional. Same study population as Kim et al. (2011). | Same exposure as Kim et al. (2011). | Medical symptoms questionnaire (MSQ), UPDRS-ADL *, the Symptom Checklist-90-Revised (SCL-90-R), the Environmental Worry Scale (EWS), The Health-Related Quality of Life (HRQOL) Scale, Similarities subtest from WAIS III, The Rey 15-Item Test, Victoria Symptom Validity Test. | Grooved Pegboard *, Grip Strength (Dynamometer), Finger Tapping Test, UPDRS (tremor and motor)*. | Multivariate analysis incorporating age, sex, diabetes, education, health insurance status, and psychiatric medication as a function of the test used as dependent variable. The Mn-exposed participants showed a slightly higher average T score (mean±SD, 54.1±9.0) than comparison participants (51.6±7.0) (p = 0.035) with an effect size of 0.308. Scores on two of the UPDRS scales differed; the exposed group had higher levels of bradykinesia (p = 0.04) and motor disturbance (p = 0.034). However, these effect sizes were small (0.196 and 0.222). WAIS III no significant difference was found (p = 0.915) between mean scores of those in the exposed group (10.8±3.1) and those in the comparison group (11.2±2.7). The Finger Tapping scores were dichotomized and 40% had worse function for the dominant hand, and 46% had worse function for the nondominant hand. For the three UPDRS variables, the crude prevalence of impairment ranged from 19% to 53%. There was no statistically significant association detected between generalized anxiety and the two Finger Tapping Tests. |
| Ghazali et al. (2013)/Malaysia a [38] | Cross-sectional. 54 elderlies from Selangor, aged 60 and above. Based on cut-off score of 24 for MMSE and 26 for MoCA, the subjects were considered as having normal cognitive function from MMSE score (64.8% ≥ 24, 35.2% < 24), but found to be cognitively impaired based on MoCA score (7.4% ≥ 26, 92.6% < 26). | Biomarkers of Mn exposure (fingernails). Levels of heavy metals and trace elements (μg/g) in fingernails and reference range were showed. Mn Mean ±SD = 1.00 µg/g ±0.23 µg/g. Reference Range:0.10–1.48 µg/g. | MMSE and Montreal Cognitive Assessment (MoCA). | _ | Bivariate analysis only. Concentrations of Mn in fingernail were found to be inversely correlated with MoCA score r = −0.496, p < 0.001) and MMSE score (r = −0.159, p = 0.250). |
| Guarneros et al. (2013)/Mexico d [39] | Cross-sectional. Subjects from a Mn mining district living <1 km from a Mn processing plant (Tolago/Chiconcoac), in the central Mexican Molango state (n = 30), were compared to non-exposed subjects living 50 km from the closest source of exposure (n = 30) (same state as the rest of Mexican studies). | Biomarkers of Mn exposure (hair). The exposed subjects had significantly higher concentrations of Mn in hair (MnH) than the control subjects: median scores = 9.73 μg/g versus 1.01 μg/g, p < 0.001. | _ | Sniffin’ Sticks Test battery (olfactory function as surrogate of early motor function decline). | Exposed and non-exposed groups were matched for gender. Bivariate analysis only. As overall performance for each subject in the Sniffin’ Sticks Test is a 3 subtest battery, the results of the 3 subtests were summed to give a composite threshold–discrimination–identification (TDI) score (maximum of 16 + 16 + 13 = 45). A tendential negative correlation was found between MnH and the performance of subjects within each group on each of the olfactory tests of threshold, discrimination, identification, and TDI scores, but specific correlation values are not reported. Median scores in the overall results of the 3 subtests, were higher in exposed (p < 0.001). |
| Lucchini et al. (2014)/Italy b,d [40] | 255 elderly healthy subjects (≥60 years, without cognitive impairment) out of a total of 365 originally enrolled, from two regions, one Industrial, next to closed Mn alloy plants (Valcamonica, n = 153) exposed to significantly higher environmental levels than the reference region (Garda Lake reference area, n = 102). | Air Mn evaluation. Mean airborne Mn = 26.41 ng/m3 (median 18.42) in Valcamonica and 20.96 ng/m3 (median 17.62) in the reference area. Biomarkers of Mn exposure (Blood, Urine). Blood Mean Valcamonica = 8.4 Range: 3.6–19.5 µg/L. Blood Mean Garda Lake = 10.2 Range: 3.6–21.6 µg/L. Urine Mean Valcamonica = 0.3 Range: 0.1–6 µg/L. Urine Mean Garda Lake = 0.4. Range: 0.1–9.4 µg/L. | MMSE, Story Recall Test, The Raven’s Colored Progressive Matrices (CPM) test, TMT *, WAIS III Digit Span, WAIS III Digit Symbol. | Luria Nebraska Neuropsychological Battery (LNNB), Finger Tapping Test, Simple Visual Reaction Time *, CATSYS *, Sniffin’ Sticks Test battery. | Multivariate analysis including age, gender, tobacco, alcohol, distance from the source and Pb Blood levels. Results also stratified by geographic area. A negative significant association between the motor coordination test of the LNNB, and airborne Mn (p = 0.0237) and the distance from the nearest ferroMn plant point source (p = 0.0035) was found. For the odor identification score of the Sniffin’ Sticks Test, an association was observed with soil Mn (p = 0.0006). Significant dose–responses resulted also for the Raven’s Colored Progressive Matrices with the distance from exposure point source (p = 0.0025) and Mn in soil (p = 0.09), and for the TMT, with urinary Mn (p = 0.0074). |
| Viana et al. (2014)/Brazil a,b,c,d [41] | Cross-sectional. Healthy population (without cognitive impairment), from two communities of the town of Simões Filho, Bahia: Cotegipe and Santa Luzia villages. These communities are situated at an approximate distance of 1.5 and 2.5 km, respectively, from the ferroMn alloy plant. | Biomarkers of Mn exposure (scalp hair, axillary hair, fingernails and saliva) (µg/g). Cotegipe Mn exposure: Mn scalp hair (MnH) Median = 2.7; range (0.6–44,6). Mn axillary hair (MnAxH) Median = 5.8 µg/g; range (3.8 µg/g–17.2 µg/g). Mn fingernails (MnFN) Median = 4.0; range (0.7–16.1). Mn saliva (MnSal) median = 3.0; range (0.4–43.3) Santa Luzia Mn exposure: MnH Median = 10.5; range (0.9–42,0). MnAxH Median = 21.8; range (4.4–85.6). MnFN Median = 6.5; range (1.1–22.2). MnSal median = 3.7; range (0.6–81.6). | WAIS III, Rey Auditory Verbal Learning Test (RAVLT), Visual Attention Computerized Test, third version (TAVIS-3) *, TMT *, WAIS III Digit Span, Corsi Block-Tapping Task. | Grooved Pegboard * | Multivariate analysis studying as possible covariates: gender, local of residence, time in years of residence in the communities, drinking habits, age, and family income. Significant correlations were observed between MnH levels and WAIS III IQ scores (r = −0.349, p = 0.002), Corsi Block-Tapping Task visual working memory (r = −0.251, p = 0.024) and with motor function for the dominant hand according to the GPT (r = 0.223, p = 0.045). MnAxH was negatively associated with IQ scores (r = −0.495, p = 0.043), visual working memory (r = −0.717, p = 0.001), Digit Span from the WAIS III verbal working memory (r = −0.303, p = 0.009); and with motor function for the dominant (r = 0.530, p = 0.024) and nondominant hand (r = 0.618, p = 0.005). MnFN were negatively associated with visual working memory (r = −0.717, p = 0.009) and with motor function for the dominant hand (r = 0.231, p = 0.05). MnSal was not significantly correlated with any of the neuropsychological functions evaluated. Statistically nonsignificant differences in medians, were reported between Cotegipe and Santa Luzia communities. |
| Bowler et al. (2015)/US a [42] | Cross-sectional. Healthy population (without cognitive impairment), belonging to two towns (Marietta, n = 100 and East Liverpool, n = 86, from Ohio) both highly exposed to environmental Mn from industrial sources. The Marietta exposure group is the same in Bowler et al. (2012, 2015 and 2016) studies. | Air Mn evaluation. Mean in Air ±SD = 0.2 µg/m3 ± 0.2 µg/m3. Median = 0.2 (Marietta). Mean in Air ±SD = 0.9 µg/m3 ± 1.2 µg/m3. Median = 0.3 (East Liverpool). Biomarkers of Mn exposure (Blood) not presented. | Stroop Color Word test, Rey Osterrieth complex figure, TMT *, Neuropsychological Assesment Battery (NAB), WAIS III Digit Span, WAIS III Digit Symbol, WAIS III similarities, animal naming, Victoria Sympton Validity Test, Auditory Consonant Trigrams (ACT). | _ | Multivariate analysis using town of residence and education (for tests not already adjusted for education) and age when appropriate. Controlling for ‘‘town’’ (as reported by authors) effectively and parsimoniously controls for any differences between them (e.g., age, income, ethnicity, years of residence). No significant differences appeared for any of the neuropsychological test variables using independent sample t-tests. Significant inverse relationships occurred between modeled air-Mn concentrations and test performance for cognitive measures of visuospatial memory (Rey-O Immediate and Delayed) and verbal skills (WAIS Similarities and Animal Naming). Significant relationships (p < 0.05) were found between modeled air-Mn exposure and performance on working and visuospatial memory (e.g., Rey-O Immediate β = −0.19, Rey-O Delayed β = −0.16) and verbal skills (e.g., WAIS Similarities β = −0.19), after controlling for education and town of residence. |
| Bowler et al. (2016)/US c [28] | Cross-sectional. Bowler et al. (2015 and 2016) studies are the same studies, but one shows cognitive function and the other motor function respectively. | Same exposure as Bowler et al. (2015). | _ | Finger Tapping Test, Hand Dynamometer, Grooved Pegboard *, CATSYS*. | Unadjusted Bayesian path analysis models used. Significant town differences were seen for all means comparisons in tremor test z-scores, and motor function test T-scores with the exception of Grooved Pegboard, nondominant. Air-Mn exposure was significantly correlated for the combined towns, with the tremor test (CATSYS) for intensity, center frequency and HI. Finger Tapping T–scores were also significantly negatively correlated with air-Mn, as were the Grooved Pegboard nondominant hand T–scores. |
| Iqbal et al. (2018)/Pakistan a [43] | Cross-sectional. 183 patients diagnosed with cognitive impairment (MMSE score ≤24); mild (n = 72) (MMSE scores range 21–24), moderate (n = 86) (MMSE scores range 10–20) and severe (n = 25) (MMSE score < 10), were compared to age-matched healthy controls (n = 90) (MMSE scores ranged 25–30). | Biomarkers of Mn exposure (Blood) Mean ±SD is reported for each group (μg/L). Mn levels were significantly higher in severe (92.08 ± 6.8 μg/L), moderate (77.8 ± 2.4 μg/L) and mild (64.97 ± 3.76 μg/L) cognitively impaired group as compared to the age-matched healthy control group (52.8 ± 2.8 μg/L), p < 0.001, p < 0.001 and p < 0.05 respectively. | MMSE. | _ | Cognitive impairment patients matched by ages with healthy controls. Bivariate analysis only. Results showed that Mn and the rest of elements studied were significantly higher in the cognitively impaired patients and increasing concentration was strongly correlated with the increase in severity of the disease. Person’s correlation test revealed negative correlations between the metal concentration and MMSE scores. The maximum correlation was observed with Al (r = −0.638; p < 0.001) followed by Cu (r = −0.610; p < 0.001), Pb (r = −0.554; p < 0.001), Cd (r = −0.418; p < 0.001), Mn (r = −0.417; p < 0.001) and Zn (r = −0.329; p < 0.001) respectively. |
| Cabral Pinto et al. (2018)/Portugal e [44] | Cross-sectional. 103 permanent residents from the industrial city of Estarrejal (>55 years old). 40.2% of the subjects had a normal performance on neurological tests assessing cognitive status. 18.3% showed a mild cognitive impairment compatible with Mild Cognitive Impairment (MCI) condition (considering the cut-off for MCI established in Portuguese validation studies and Clinical Dementia Rating Scale (CDR) = 0.5) and 36.6% had a cognitive performance suggestive of dementia condition (CDR and MMSE and MoCA scores below the respective thresholds). | Biomarkers of potentially toxic elements exposure (urine) including aluminium, cadmium, zinc and Mn exposure (among others). Mean of Mn concentration = 46.4 ± 217 μg/g, Mode = 0.83. Max 1694. P5-P95 = 0.39–57. Reference for healthy people (0.11–1.32). Also groundwater levels assessed. | MMSE, MoCA, CDR, Geriatric Depression Scale (GDS). | _ | Multivariate linear regression models showed that aluminium (R2 = 38%), cadmium (R2 = 11%) and zinc (R2 = 6%) were good predictors of the scores of the MMSE cognitive test. Mn was not shown as a good predictor (the specific R2 result for Mn is not reported). Specific covariates included in the multivariate models to control confounding not reported. |
| Kornblith et al. (2018)/US e [45] | Cross-sectional. Same study population as Bowler et al. (2015 and 2016) studies: residents of Marietta (n = 99) and East Liverpool (n = 83). | See Bowler et al. (2015) for means and medians details. | UPDRS-ADL *, Animal naming, Stroop color word, TMT *, Rey Osterrieth Complex Figure, ACT. | CATSYS*, UPDRS (tremor and motor)*. | Two-step cluster analyses were used. Four distinct symptom clusters were identified in this sample: The largest identified group (Cluster 1: Non-Impaired) contained 60% of the sample and was characterized by average scores (within one standard deviation of the overall sample mean) on measures of gait disturbance, bradykinesia/rigidity, and tremor, and the absence of EF impairment. The second-largest group (Cluster 3: Executive Dysfunction) contained 20% of the sample and consisted of average scores on measures of tremor, gait disturbance and bradykinesia/rigidity, but all members met criteria for EF impairment. The third-largest group (Cluster 2: Tremor) contained 11% of the sample and was characterized by high tremor and average bradykinesia and rigidity. The smallest group (Cluster 4: No Tremor) contained 7% of the sample and had high levels of gait disturbance and bradykinesia/rigidity with relatively lower levels of tremor. |
| Rafiee et al. (2019)/Iran e [46] | Cross-sectional. 200 healthy volunteered participants (110 men and 90 women), aged 14–70 years, without cognitive impairment from Tehran. | Chronic exposure to metals (Cd, Be, Co, Hg, Sn, V, Al, Ba, Cr, Cu, Fe, Li, Mn, Ni, Pb, and Zn) and metalloids (As, B, Sb) through hair samples. Biomarkers of Mn exposure (Hair). Median MnH 3.05 μg/g; range (1.2–12.4). Mean MnH 3.86 μg/L± 3.37, range (0.8–18.4). | TMT * | Multivariate analysis using the following selected variables after studying their effect as confounders: age, gender, self-reported residential traffic exposure, existence of dental amalgam implants, cigarette smoking, water-pipe smoking and insecticide use. Mn levels in hair were significantly associated with poorer participants’ performance scores in the TMT test (more time in seconds), (p < 0.05). 0.201 and 0.204 more seconds per one 1 µg/g of Mn in TMT-A and B score respectively |
| Cognitive Function | N of Studies | N of Determinations | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Correlation | Q | df | p (Chi2) | I2 (%) | Tau2 | Tau | ||
| ACT | 1 | 4 | 0.67 | 3.00 | 0.879 | 0.00 | 0.00 | 0.00 |
| Corsi Block-Tapping Task | 1 | 4 | 27.60 | 3.00 | 0.000 | 89.13 | 0.10 | 0.32 |
| Mini-Mental State Examination | 2 | 2 | 3.45 | 1.00 | 0.06 | 71.04 | 0.03 | 0.17 |
| Montreal Cognitive Assessment | 1 | 1 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 |
| NAB | 1 | 3 | 0.49 | 2.00 | 0.781 | 0.00 | 0.00 | 0.00 |
| RAVLT | 1 | 4 | 1.59 | 3.00 | 0.661 | 0.00 | 0.00 | 0.00 |
| ROCF | 1 | 3 | 0.40 | 2.00 | 0.817 | 0.00 | 0.00 | 0.00 |
| Stroop Color Word Test | 1 | 3 | 1.65 | 2.00 | 0.437 | 0.00 | 0.00 | 0.00 |
| TAVIS-3 | 1 | 8 | 7.66 | 7.00 | 0.364 | 8.59 | 0.00 | 0.03 |
| Trail Making Test | 2 | 10 | 57.71 | 9.00 | 0.000 | 84.40 | 0.06 | 0.24 |
| WAIS III | 2 | 14 | 29.19 | 13.00 | 0.006 | 55.47 | 0.01 | 0.10 |
| All studies and determinations | 4 | 56 | 233.92 | 55.00 | 0.000 | 76.49 | 0.03 | 0.16 |
| Cognitive Function | N of Studies | N of Determinations | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| SMD | Q | df | p (Chi2) | I2 (%) | Tau2 | Tau | ||
| Corsi Block-Tapping Test | 1 | 1 | 0.0 | 0.00 | 1.000 | 0.00 | 0.00 | 0.00 |
| CPM | 1 | 2 | 0.00 | 1.00 | 0.958 | 0.00 | 0.02 | 0.00 |
| MMSE | 1 | 2 | 0.12 | 1.00 | 0.729 | 0.00 | 0.02 | 0.00 |
| RAVLT | 1 | 1 | 0.00 | 0.00 | 1.000 | 0.00 | 0.00 | 0.00 |
| StoryRecall Test | 1 | 3 | 1.07 | 2.00 | 0.586 | 0.00 | 0.02 | 0.00 |
| TAVIS-3 | 1 | 2 | 1.18 | 1.00 | 0.277 | 15.29 | 0.08 | 0.09 |
| Trail Making Test | 2 | 6 | 4.46 | 5.00 | 0.485 | 0.00 | 0.01 | 0.00 |
| UPDRS | 1 | 1 | 0.00 | 0.00 | 1.000 | 0.00 | 0.00 | 0.00 |
| WAIS III | 3 | 8 | 9.11 | 7.00 | 0.245 | 23.20 | 0.01 | 0.08 |
| All tests | 3 | 26 | 25.67 | 25.00 | 0.425 | 2.62 | 0.01 | 0.02 |
| Motor Function | N of Studies | N of Determinations | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Correlation | Q | df | p (Chi2) | I2 (%) | Tau2 | Tau | ||
| CATSYS | 1 | 6 | 99.607 | 5.00 | 0.000 | 94.98 | 0.10 | 0.32 |
| Dynamometer | 1 | 2 | 0.01 | 1.00 | 0.924 | 0.00 | 0.00 | 0.00 |
| Finger Tapping | 1 | 2 | 1.00 | 1.00 | 0.316 | 0.34 | 0.00 | 0.00 |
| Grooved Pegboard | 2 | 10 | 9.75 | 9.00 | 0.371 | 7.74 | 0.00 | 0.03 |
| Postural Balance | 1 | 16 | 16.40 | 15.00 | 0.356 | 8.53 | 0.00 | 0.05 |
| All studies and determinations | 3 | 36 | 139.97 | 35.00 | 0.000 | 74.99 | 0.03 | 0.18 |
| Motor Function | N of Studies | N of Determinations | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| SMD | Q | df | p (Chi2) | I2 (%) | Tau2 | Tau | ||
| CATSYS | 2 | 32 | 31.05 | 31.00 | 0.464 | 0.15 | 0.00 | 0.00 |
| Dynamometer | 1 | 2 | 0.34 | 1.00 | 0.557 | 0.00 | 0.00 | 0.00 |
| EKM | 1 | 4 | 0.82 | 3.00 | 0.846 | 0.00 | 0.00 | 0.00 |
| Finger Tapping | 2 | 4 | 1.34 | 3.00 | 0.719 | 0.00 | 0.00 | 0.00 |
| Grooved Pegboard | 2 | 4 | 2.2 | 3.00 | 0.530 | 0.00 | 0.00 | 0.00 |
| Luria Nebraska | 1 | 6 | 3.55 | 5.00 | 0.616 | 0.00 | 0.00 | 0.00 |
| Postural Balance | 1 | 8 | 7.11 | 7.00 | 0.417 | 1.59 | 0.02 | 0.04 |
| Purdue Pegboard | 1 | 2 | 13.10 | 1.00 | 0.000 | 92.36 | 0.41 | 0.64 |
| Simple Visual Reaction Time | 1 | 2 | 0.18 | 1.00 | 0.668 | 0.00 | 0.00 | 0.00 |
| Sniffin’ sticks | 2 | 5 | 5.78 | 4.00 | 0.216 | 30.83 | 0.02 | 0.15 |
| UPDRS | 1 | 2 | 0.01 | 1.00 | 0.903 | 0.00 | 0.00 | 0.00 |
| All test | 7 | 71 | 146.69 | 70.00 | 0.000 | 52.28 | 0.02 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Azcona, L.; Fernández-Olmo, I.; Expósito, A.; Markiv, B.; Paz-Zulueta, M.; Parás-Bravo, P.; Sarabia-Cobo, C.; Santibáñez, M. Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4075. https://doi.org/10.3390/ijerph18084075
Ruiz-Azcona L, Fernández-Olmo I, Expósito A, Markiv B, Paz-Zulueta M, Parás-Bravo P, Sarabia-Cobo C, Santibáñez M. Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(8):4075. https://doi.org/10.3390/ijerph18084075
Chicago/Turabian StyleRuiz-Azcona, Laura, Ignacio Fernández-Olmo, Andrea Expósito, Bohdana Markiv, María Paz-Zulueta, Paula Parás-Bravo, Carmen Sarabia-Cobo, and Miguel Santibáñez. 2021. "Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 8: 4075. https://doi.org/10.3390/ijerph18084075
APA StyleRuiz-Azcona, L., Fernández-Olmo, I., Expósito, A., Markiv, B., Paz-Zulueta, M., Parás-Bravo, P., Sarabia-Cobo, C., & Santibáñez, M. (2021). Impact of Environmental Airborne Manganese Exposure on Cognitive and Motor Functions in Adults: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(8), 4075. https://doi.org/10.3390/ijerph18084075









