Stress Salivary Biomarkers Variation during the Work Day in Emergencies in Healthcare Professionals
Abstract
1. Introduction
2. Material and Methods
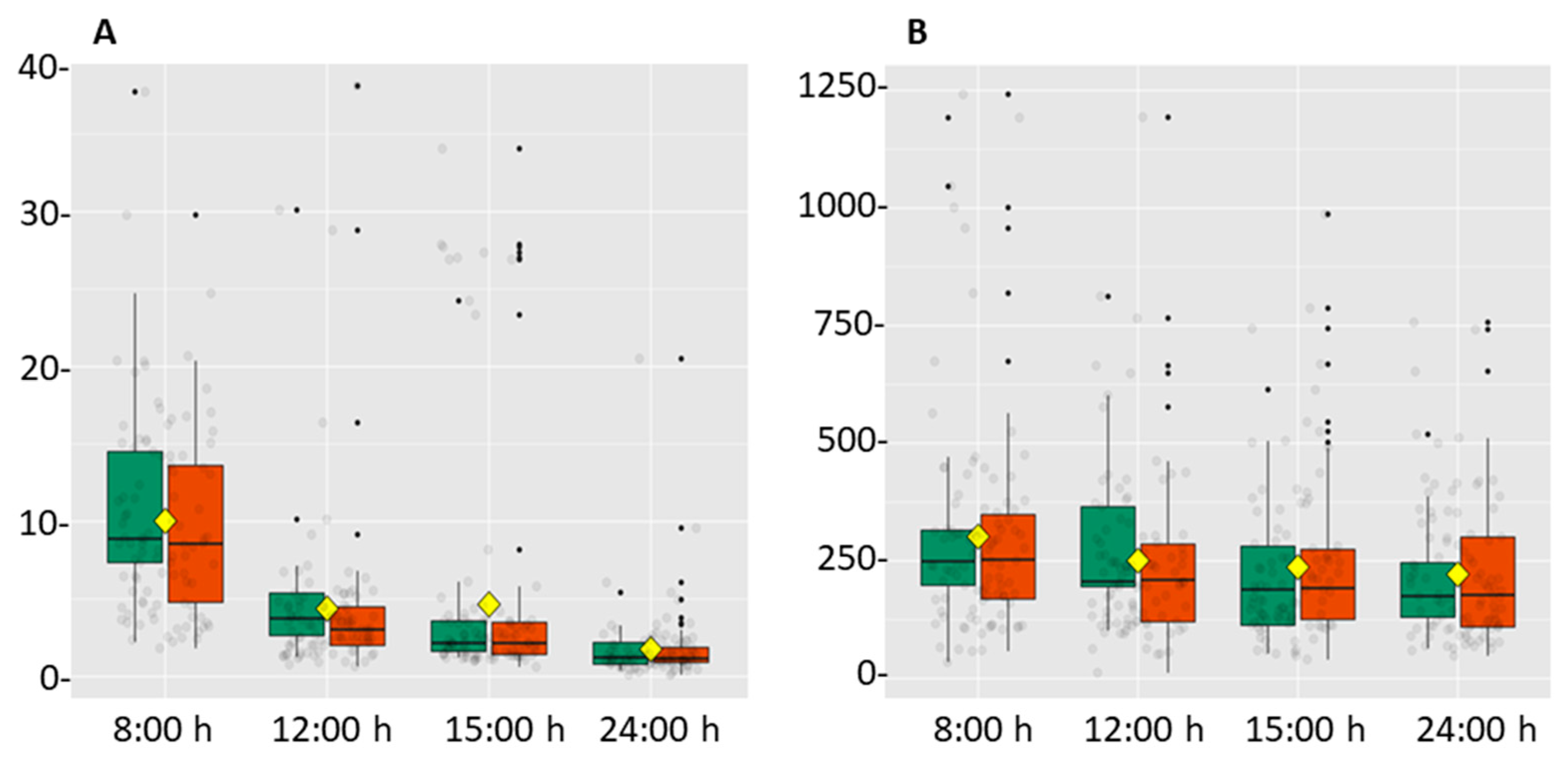
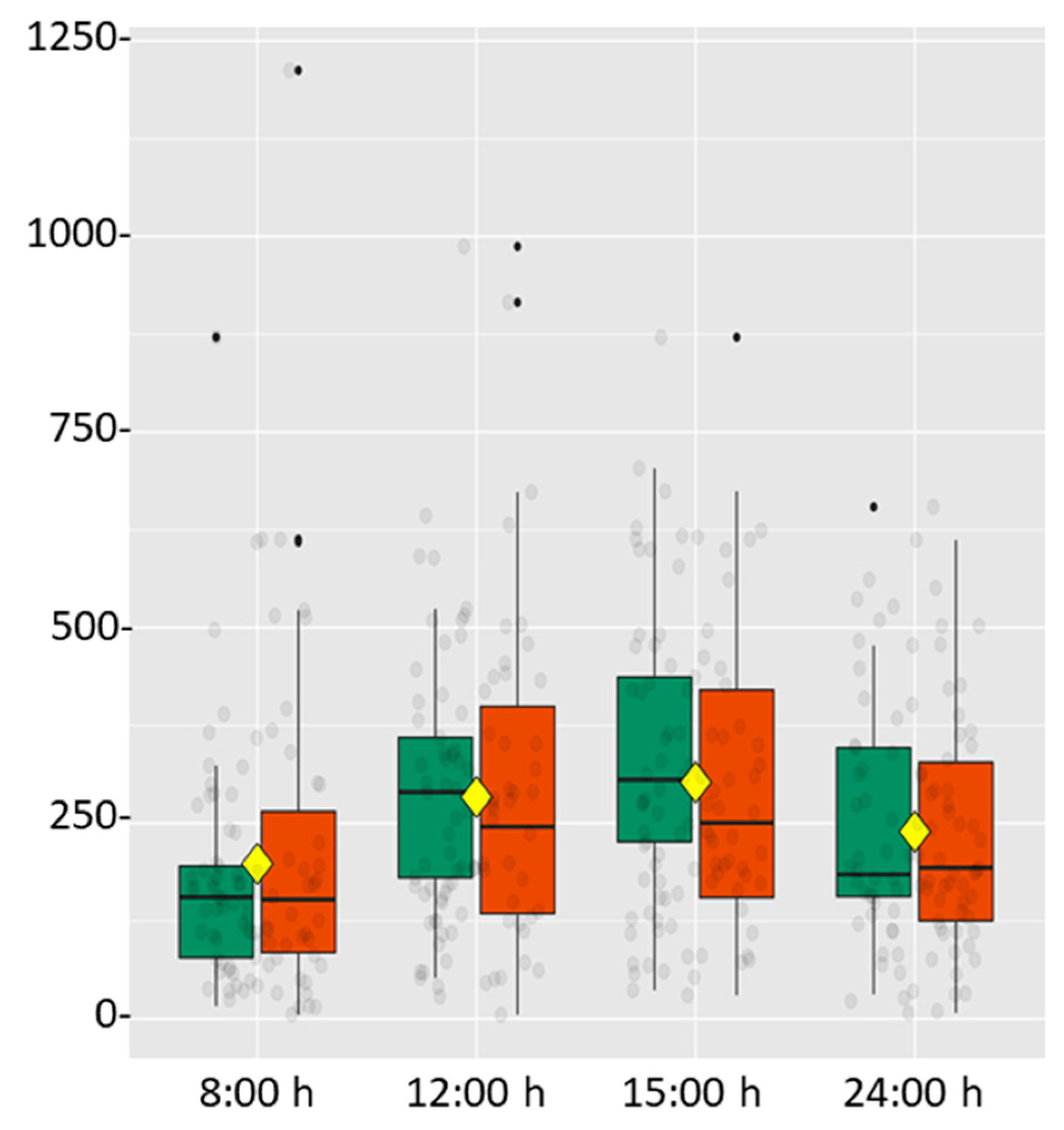
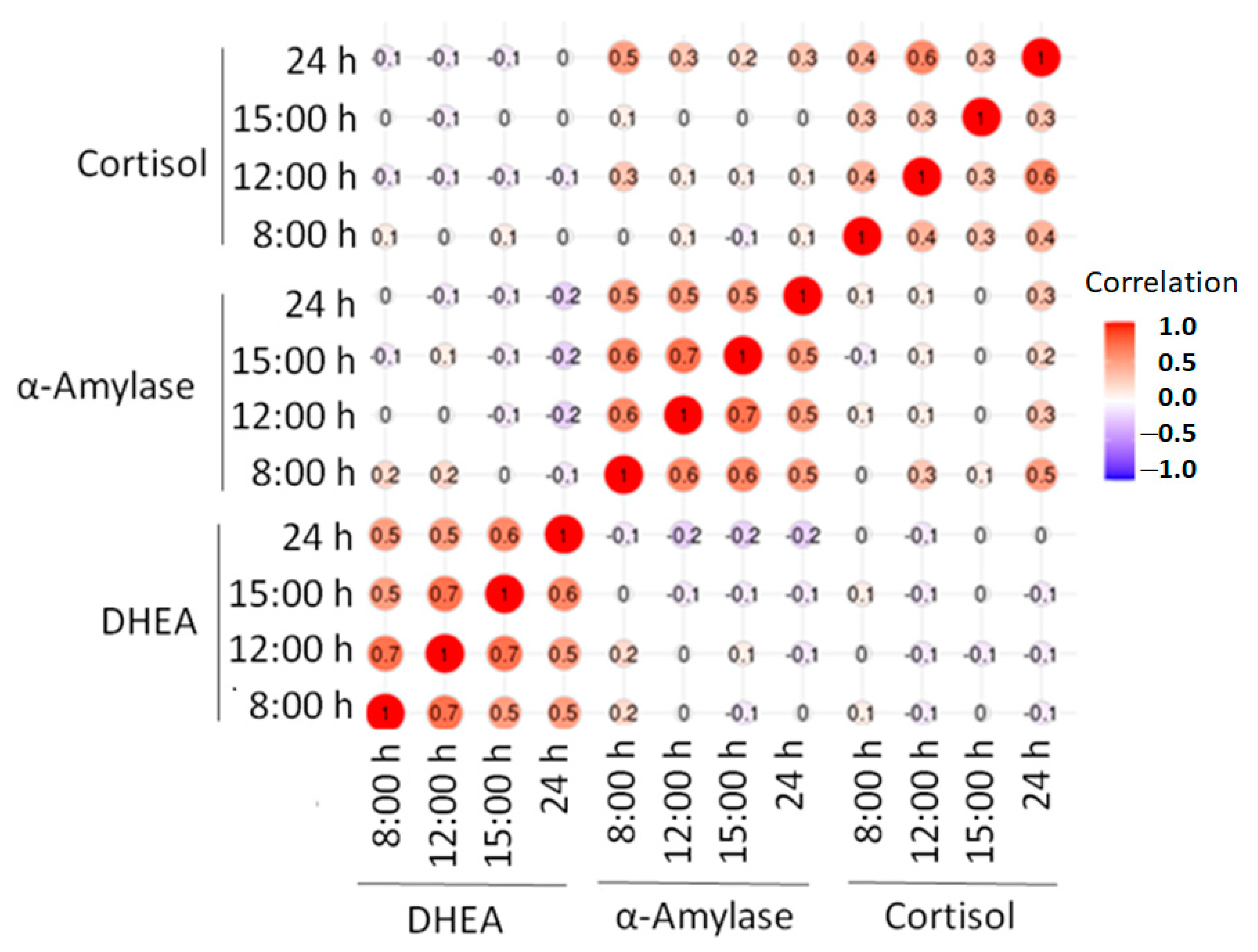
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chojnowska, S.; Ptaszynska-Sarosiek, I.; Kepka, A.; Kna’s, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef]
- Selye, H.; Fortier, C. Adaptive reactions to stress. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1949, 29, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Coben, R.; Evans, J.R. Neurofeedback and Neuromodulation Techniques and Applications; Academic Press-Elsevier: Columbia, SC, USA, 2010. [Google Scholar]
- Córdova, A. Fisiología Dinámica; Masson-Elsevier: Madrid, Spain, 2003. [Google Scholar]
- Laurent, H.K.; Powers, S.I.; Granger, D.A. Refining the multisystem view of the stress response: Coordination among cortisol, alpha-amylase, and subjective stress in response to relationship conflict. Physiol. Behav. 2013, 119, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Valentin, B.; Grottke, O.; Skorning, M.; Bergrath, S.; Fischermann, H.; Rörtgen, D.; Mennig, M.T.; Fitzner, C.; Müller, M.P.; Kirschbaum, C.; et al. Cortisol and alpha-amylase as stress response indicators during pre-hospital emergency medicine training with repetitive high-fidelity simulation and scenarios with standardized patients. Scand. J. Trauma. Resusc. Emerg. Med. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Soo-Quee Koh, D.; Choon-Huat Koh, G. The use of salivary biomarkers in occupational and environmental medicine. Occup. Environ. Med. 2007, 64, 202–210. [Google Scholar] [CrossRef]
- Farnaud, S.J.C.; Kosti, O.; Getting, S.J.; Renshaw, D. Saliva: Physiology and diagnostic potential in health and disease. Sci. World J. 2010, 10, 434–456. [Google Scholar] [CrossRef]
- Burbeck, R.; Coomber, S.; Robinson, S.; Todd, C. Occupational stress in consultants in accident and emergency medicine: A national survey of levels of stress at work. Emerg. Med. J. 2002, 19, 234–238. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef]
- Morgan, C.A.; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef]
- Cozma, S.; Dima-Cozma, L.C.; Ghiciuc, C.M.; Pasquali, V.; Saponaro, A.; Patacchioli, F.R. Salivary cortisol and α-amylase: Subclinical indicators of stress as cardiometabolic risk. Braz. J. Med. Biol. Res. 2017, 50, e5577. [Google Scholar] [CrossRef]
- Giacomello, G.; Scholten, A.; Parr, M.K. Current methods for stress marker detection in saliva. J. Pharm. Biomed. Anal. 2020, 191, 113604. [Google Scholar] [CrossRef]
- Lala, A.I.; Sturzu, L.M.; Picard, J.P.; Druot, F.; Grama, F.; Bobirnac, G. Coping behavior and risk and resilience stress factors in French regional emergency medicine unit workers: A cross-sectional survey. J. Med. Life 2016, 9, 363–368. [Google Scholar] [PubMed]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry 2014, 26, 139–154. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, J.; Fernández-Prada, M.; Molina-Ruano, R.; Blázquez, A.; Guillén-Solvas, J.; Peinado, J.M. Psychosocial risk at work, self-perceived stress and cortisol in saliva in a sample of emergency physicians in Granada. Emergencias 2012, 24, 101–106. [Google Scholar]
- Kent, J.; Thornton, M.; Allan, F.; Erin, H.; Fitzgibbons, S.; Sava, J. Acute provider stress in high stakes medical care: Implications for trauma surgeons. J. Trauma Acute Care Surg. 2020, 88, 440–445. [Google Scholar] [CrossRef]
- Nakajima, Y.; Takahashi, T.; Shetty, V.; Yamaguchi, M. Patterns of salivary cortisol levels can manifest work stress in emergency care providers. J. Physiol. Sci. 2012, 62, 191–197. [Google Scholar] [CrossRef]
- Rahmati, F.; Safari, S.; Hashemi, B.; Baratloo, A.; Khosravi, R. Prevalence of depression and personality disorders in the beginning and end of emergency medicine residency program: A prospective cross sectional study. Arch. Acad. Emerg. Med. 2019, 7, e5. [Google Scholar]
- Hammer, J.S.; Jones, J.W.; Lyons, J.S.; Sixsmith, D.; Afficiando, E. Measurement of occupational stress in hospital settings: Two validity studies of a measure of self-reported stress in medical emergency rooms. Gen. Hosp. Psychiatry 1985, 7, 156–162. [Google Scholar] [CrossRef]
- Goldman, N.; Weinstein, M.; Cornman, J.; Singer, B.; Seeman, T.; Chang, M.-C. Sex differentials in biological risk factors for chronic disease: Estimates from population-based surveys. J. Women’s Health 2004, 13, 393–403. [Google Scholar] [CrossRef]
- Boscarino, J.A. Psychobiologic predictors of disease mortality after psychological trauma. J. Nerv. Ment. Dis. 2008, 196, 100–107. [Google Scholar] [CrossRef]
- Noser, E.; Fischer, S.; Ruppen, J.; Ehlert, U. Psychobiological stress in vital exhaustion. Finding for the men stress 40+ study. J. Psychosom. Res. 2018, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gordis, E.; Granger, D.; Susman, E.; Trickett, P. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology 2006, 31, 976–987. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikawa, Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch. Oral. Biol. 2004, 49, 963–968. [Google Scholar] [CrossRef]
- Müller, M.P.; Hansel, M.; Fichtner, A.; Hardt, F.; Weber, S.; Kirschbaum, C.; Rüder, S.; Walcher, F.; Koch, T.; Eich, C. Excellence in performance and stress reduction during two different full scale simulator training courses: A pilot study. Resuscitation 2009, 80, 919–924. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary alpha-amylase as a biomarker of stress in behavioral medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Bedini, S.; Braun, F.; Weibel, L.; Aussedat, M.; Pereira, B.; Dutheil, F. Stress and salivary cortisol in emergency medical dispatchers: A randomized shifts control trial. PLoS ONE 2017, 12, e0177094. [Google Scholar] [CrossRef] [PubMed]
- Jeckel, C.M.; Lopes, R.P.; Berleze, M.C.; Luz, C.; Feix, L.; Argimon, I.I.L.; Stein, L.M.; Bauer, M.E. Neuroendocrine and immunological correlates of chronic stress in ‘strictly healthy’ populations. Neuroimmunomodulation 2010, 17, 9–18. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, S., Jr.; Bryson, E.O.; Mooney, T.J.; Silverstein, J.H.; Reich, D.L.; Bodian, C.; Levine, A.I. Adding emotional stressors to training in simulated cardiopulmonary arrest enhances participant performance. Med. Educ. 2010, 44, 1006–1015. [Google Scholar] [CrossRef]
- Kudielka, B.M.; Schommer, N.C.; Hellhammer, C.K. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology 2004, 29, 983–992. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Kopin, I.J. Evolution of concepts of stress. Stress 2007, 10, 109–120. [Google Scholar] [CrossRef]
- Hassan, I.; Weyers, P.; Maschuw, K.; Dick, B.; Gerdes, B.; Rothmund, M.; Zielke, A. Negative stress-coping strategies among novices in surgery correlate with poor virtual laparoscopic performance. Br. J. Surg. 2006, 93, 1554–1559. [Google Scholar] [CrossRef]
- Chen, J.; Davis, L.S.; Davis, K.G.; Pan, W.; Daraiseh, N.M. Physiological and behavioural response patterns at work among hospital nurses. J. Nurs. Manag. 2011, 19, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.H.; Sprung, J.; Clark, M.M.; Shanafelt, T.D.; Johnson, B.D.; Kruseet, T.N.; Chantigian, D.P.; Carter, J.R.; Long, T.R. The psychological and physiological effects of acute occupational stress in new anesthesiology residents: A pilot trial. Anesthesiology 2014, 121, 878–893. [Google Scholar] [CrossRef]
- Takai, N.; Yamaguchi, M.; Aragaki, T.; Eto, K.; Uchihashi, K.; Nishikaw, Y. Gender-specific differences in salivary biomarker responses to acute psychological stress. Ann. N. Y. Acad. Sci. 2007, 1098, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Klauer, T.; Filipp, S.H.; Hellhammer, D.H. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995, 57, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, J.H.; Verbeek, J.H.; Mariné, A.; Consol, S. Preventing occupational stress in healthcare workers. Cochrane Database Syst. Rev. 2014, 12, 1–155. [Google Scholar]
| Professional | n | % |
|---|---|---|
| Total | 97 | 20.6/79.4 (Men/Women) |
| Nurses | 59 | 16.9/83.1 (Men/Women) |
| Medical Doctors | 38 | 26.3/73.7 (Men/Women) |
| HCUV | 45 | 46.4 |
| HSBS | 52 | 53.6 |
| Biomarker (Units) | 8:00 h | 12:00 h | 15:00 h | 24 h |
|---|---|---|---|---|
| HCUV + HSBS | ||||
| Cortisol (ng/mL) | 10.0 ± 1.2 | 4.4 ± 1.1 a | 4.7 ± 1.5 a | 1.8 ± 0.5 a |
| α-Amylase (U/mL) | 197.6 ± 37.1 | 283.8 ± 35.3 a | 302.4 ± 35.6 a | 239.7 ± 29.2 a |
| DHEA (pg/mL) | 301.9 ± 44.6 | 250.9 ± 35.3 a | 235.7 ± 33.6 a | 221.4 ± 30.3 a |
| DHEA/Cortisol Ratio | 41.2 ± 9.1 | 89.7 ± 17.4 | 107.8 ± 18.5 | 224.3 ± 67.2 |
| HCUV | ||||
| Cortisol (ng/mL) | 10.6 ± 2.3 | 4.9 ± 2.1 | 5.6 ± 2.3 | 2.23 ± 1.04 |
| α-Amylase (U/mL) | 231.8 ± 68.3 c | 317.9 ± 59.9 a,c | 326.2 ± 58.3 a | 241.0 ± 4.4 a |
| DHEA (pg/mL) | 325.1 ± 65.8 | 298.5 ± 50.1 | 264.7 ± 51.6 | 258.0 ± 45.5 |
| DHEA/Cortisol Ratio | 45.3 ± 16.5 | 102.4 ± 29.6 | 106.9 ± 20.4 | 192.7 ± 40.4 |
| HSBS | ||||
| Cortisol (ng/mL) | 10.4 ± 1.7 | 1.4 ± 0.3 | 4.5 ± 2.0 | 1.4 ± 0.3 |
| α-Amylase (U/mL) | 151.4 ± 50.4 | 233.7 ± 51.1 a | 260.9 ± 51.0 a | 220.2 ± 47.3 a |
| DHEA (pg/mL) | 311.2 ± 76.4 | 229.2 ± 61.3 | 233.0 ± 54.5 | 204.4 ± 43.4 |
| DHEA/Cortisol Ratio | 40.2 ± 13.1 | 85.3 ± 5.2 | 119.6 ± 36.1 | 273.8 ± 138.6 |
| Medical doctors | ||||
| Cortisol (ng/mL) | 10.0 ± 1.9 | 4.0 ± 1.9 | 3.0 ± 1.3 | 2.2 ± 1.1 |
| α-Amylase (U/mL) | 243.2 ± 68.9 | 318.8 ± 53.1 a | 367.7 ± 49.5 a,b | 283.2 ± 53.0 a |
| DHEA (pg/mL) | 299.4 ± 64.4 | 262.3 ± 65.4 | 245.4 ± 55.9 | 205.3 ± 38.3 |
| DHEA/Cortisol Ratio | 42.2 ± 13.7 | 102.2 ± 35.0 | 116.3 ± 32.2 | 242.5 ± 152.8 |
| Nurses | ||||
| Cortisol (ng/mL) | 10.1 ± 1.7 | 4.7 ± 1.3 | 5.8 ± 2.2 | 1.5 ± 0.3 |
| α-Amylase (U/mL) | 168.3 ± 38.1 | 259.4 ± 46.0 a | 210.2 ± 34.5 a | 211.9 ± 34.6 a |
| DHEA (pg/mL) | 301.6 ± 60.6 | 244.6 ± 40.8 | 233.6 ± 41.0 | 235.0 ± 40.0 |
| DHEA/Cortisol Ratio | 40.3 ± 11.3 | 80.5 ± 16.9 | 102.3 ± 24.1 | 208.1 ± 41.8 |
| Day shift | ||||
| Cortisol (ng/mL) | 10.5 ± 1.8 | 4.4 ± 1.4 | 5.72 ± 2.2 | 1.5 ± 0.3 |
| α-Amylase (U/mL) | 185.0 ± 41.8 | 275.7 ± 50.2 | 278.2 ± 52.2 d | 215.1 ± 36.3 d |
| DHEA (pg/mL) | 333.6 ± 67.1 | 273.9 ± 53.5 | 258.9 ± 45.9 d | 248.8 ± 44.2 d |
| DHEA/Cortisol Ratio | 46.4 ± 13.7 | 101.8 ± 27.2 | 122.7 ± 29.7 | 275.4 ± 109.1 |
| Afternoon shift | ||||
| Cortisol (ng/mL) | 9.6 ± 1.9 | 4.4 ± 1.83 | 3.1 ± 1.3 | 2.1 ± 1.0 |
| α-Amylase (U/mL) | 220.0 ± 68.1 | 296.9 ± 49.3 | 342.2 ± 44.9 | 280.0 ± 51.1 |
| DHEA (pg/mL) | 259.0 ± 52.8 | 219.6 ± 40.2 | 208.5 ± 50.0 | 185.4 ± 35.9 |
| DHEA/Cortisol Ratio | 34.1 ± 7.3 | 73.9 ± 14.7 | 88.5 ± 16.5 | 150.7 ± 36.0 |
| SCALE MPSS-R | HCUV | HSBS |
|---|---|---|
| Organizational stress | 21.2 ± 3.9 | 16.3 ± 3.4 |
| Negative patient attitudes | 14.2 ± 3.0 | 11.6 ± 3.3 |
| Job dissatisfaction | 15.9 ± 2.9 | 15.0 ± 2.6 |
| Somatic distress | 20.4 ± 3.2 | 16.6 ± 3.1 |
| Total stress | 71.7 ± 5.6 | 59.5 ± 5.8 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Valdecantos, D.; Caballero-García, A.; Del Castillo-Sanz, T.; Bello, H.J.; Roche, E.; Córdova, A. Stress Salivary Biomarkers Variation during the Work Day in Emergencies in Healthcare Professionals. Int. J. Environ. Res. Public Health 2021, 18, 3937. https://doi.org/10.3390/ijerph18083937
Pérez-Valdecantos D, Caballero-García A, Del Castillo-Sanz T, Bello HJ, Roche E, Córdova A. Stress Salivary Biomarkers Variation during the Work Day in Emergencies in Healthcare Professionals. International Journal of Environmental Research and Public Health. 2021; 18(8):3937. https://doi.org/10.3390/ijerph18083937
Chicago/Turabian StylePérez-Valdecantos, Daniel, Alberto Caballero-García, Teodosia Del Castillo-Sanz, Hugo J. Bello, Enrique Roche, and Alfredo Córdova. 2021. "Stress Salivary Biomarkers Variation during the Work Day in Emergencies in Healthcare Professionals" International Journal of Environmental Research and Public Health 18, no. 8: 3937. https://doi.org/10.3390/ijerph18083937
APA StylePérez-Valdecantos, D., Caballero-García, A., Del Castillo-Sanz, T., Bello, H. J., Roche, E., & Córdova, A. (2021). Stress Salivary Biomarkers Variation during the Work Day in Emergencies in Healthcare Professionals. International Journal of Environmental Research and Public Health, 18(8), 3937. https://doi.org/10.3390/ijerph18083937









