Surveillance for Severe Acute Respiratory Infections among Hospitalized Subjects from 2015/2016 to 2019/2020 Seasons in Tuscany, Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Analysis
2.3. Sequencing Methods
2.4. Influenza Hemagglutinin Multiple-Sequence Alignment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 22 June 2020).
- Trombetta, C.M.; Marchi, S.; Manini, I.; Kistner, O.; Li, F.; Piu, P.; Manenti, A.; Biuso, F.; Sreenivasan, C.; Druce, J.; et al. Influenza D Virus: Serological Evidence in the Italian Population from 2005 to 2017. Viruses 2019, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. U.S. Influenza Surveillance System: Purpose and Methods. Available online: https://www.cdc.gov/flu/weekly/overview.htm (accessed on 17 March 2021).
- Centers for Disease Control and Prevention. Selecting Viruses for the Seasonal Influenza Vaccine. Available online: https://www.cdc.gov/flu/prevent/vaccine-selection.htm (accessed on 18 November 2020).
- ECDC. Sentinel Surveillance. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/surveillance-and-disease-data/facts-sentinel-surveillance (accessed on 18 November 2020).
- Ministero della Salute. Monitoraggio delle Forme Gravi e Complicate. Available online: http://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=4246&area=influenza&menu=vuoto (accessed on 18 November 2020).
- Fitzner, J.; Qasmieh, S.; Mounts, A.W.; Alexander, B.; Besselaar, T.; Briand, S.; Brown, C.; Clark, S.; Dueger, E.; Gross, D.; et al. Revision of clinical case definitions: Influenza-like illness and severe acute respiratory infection. Bull. World Heal. Organ. 2017, 96, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute. Sistema di Sorveglianza InfluNet. Available online: http://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=704&area=influenza&menu=vuoto (accessed on 18 November 2020).
- Gasparini, R.; Bonanni, P.; Amicizia, D.; Bella, A.; Donatelli, I.; Cristina, M.L.; Panatto, D.; Lai, P.L. Influenza epidemiology in Italy two years after the 2009-2010 pandemic: Need to improve vaccination coverage. Hum. Vaccin. Immunother. 2013, 9, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Ministero della Salute, Monitoraggio Dell’andamento Delle Forme Gravi e Complicate di Influenza Confermata, Stagione 2019–2020. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=71972&parte=1%20&serie=null (accessed on 30 November 2020).
- Istituto Superiore di Sanità. FluNews—Italia Rapporto Della Sorveglianza Integrata Dell’influenza. Available online: https://www.epicentro.iss.it/influenza/FluNews18-19 (accessed on 18 November 2020).
- Macias, A.E.; Mc Elhaney, J.E.; Chaves, S.S.; Nealon, J.; Nunes, M.C.; Samson, S.I.; Seet, B.T.; Weinke, T.; Yu, H. The disease burden of influenza beyond respiratory illness. Vaccine 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Kroneman, M.; Wiegers, T.; El Guerche-Séblain, C.; Paget, J. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: A systematic literature review. Influ. Other Respir. Viruses 2018, 12, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Gesualdo, F.; Loconsole, D.; Pandolfi, E.; Bella, A.; Orsi, A.; Guarona, G.; Panatto, D.; Icardi, G.; Napoli, C.; et al. Moderate Vaccine Effectiveness against Severe Acute Respiratory Infection Caused by A(H1N1)pdm09 Influenza Virus and No Effectiveness against A(H3N2) Influenza Virus in the 2018/2019 Season in Italy. Vaccines (Basel) 2020, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Rondy, M.; Gherasim, A.; Casado, I.; Launay, O.; Rizzo, C.; Pitigoi, D.; Mickiene, A.; Marbus, S.D.; Machado, A.; Syrjanen, R.K.; et al. Low 2016/17 season vaccine effectiveness against hospitalised influenza A(H3N2) among elderly: Awareness warranted for 2017/18 season. Euro. Surveill 2017, 22, 17–00645. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Snacken, R.; Melidou, A.; Ionescu, S.; Penttinen, P.; Network, T.E.I.S. Dominant influenza A(H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively. Eurosurveillance 2018, 23, 18–00146. [Google Scholar] [CrossRef] [PubMed]
- Rondy, M.; Kissling, E.; Emborg, H.D.; Gherasim, A.; Pebody, R.; Trebbien, R.; Pozo, F.; Larrauri, A.; McMenamin, J.; Valenciano, M. group. Interim 2017/18 influenza seasonal vaccine effectiveness: Combined results from five European studies. Euro. Surveill 2018, 23, 18–00086. [Google Scholar] [CrossRef] [PubMed]
- Bellino, S.; Bella, A.; Puzelli, S.; Di Martino, A.; Facchini, M.; Punzo, O.; Pezzotti, P.; Castrucci, M.R.; the InfluNet Study Group. Moderate influenza vaccine effectiveness against A(H1N1)pdm09 virus, and low effectiveness against A(H3N2) subtype, 2018/19 season in Italy. Expert Rev. Vaccines 2019, 18, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.; Restivo, V.; Amodio, E.; Colomba, G.M.E.; Vitale, F.; Tramuto, F. A mid-term estimate of 2018/2019 vaccine effectiveness to prevent laboratory confirmed A(H1N1)pdm09 and A(H3N2) influenza cases in Sicily (Italy). Vaccine 2019, 37, 5812–5816. [Google Scholar] [CrossRef]
- ECDC. Regional Situation Assessment Seasonal Influenza. Influenza Season 2019–2020: Early Situation Assessment. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/influenza-situation-assessment-18-December-2019.pdf (accessed on 2 March 2021).
- Ministero Della Salute. Dati Coperture Vaccinali. Available online: http://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=679&area=influenza&menu=vuoto (accessed on 13 July 2020).
- Rondy, M.; Larrauri, A.; Casado, I.; Alfonsi, V.; Pitigoi, D.; Launay, O.; Syrjanen, R.K.; Gefenaite, G.; Machado, A.; Vucina, V.V.; et al. Grp 2015/16 seasonal vaccine effectiveness against hospitalisation with influenza A(H1N1) pdm09 and B among elderly people in Europe: Results from the I-MOVE plus project. Eurosurveillance 2017, 22, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Broberg, E.; Melidou, A.; Prosenc, K.; Bragstad, K.; Hungnes, O.; Region, E.I. Surveillance Network members of the reporting countries. Predominance of influenza A(H1N1)pdm09 virus genetic subclade 6B.1 and influenza B/Victoria lineage viruses at the start of the 2015/16 influenza season in Europe. Eurosurveillance 2016, 21, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Melidou, A.; Broberg, E.N. European region influenza surveillance. Erratum to "Predominance of influenza A(H3N2) virus genetic subclade 3C.2a1 during an early 2016/17 influenza season in Europe–Contribution of surveillance data from World Health Organization (WHO) European region to the WHO vaccine composition consultation for northern hemisphere 2017/18" [Vaccine 35 (2017) 4828-4835]. Vaccine 2018, 36, 2740–2741. [Google Scholar] [PubMed]
- Bella, A.; Gesualdo, F.; Orsi, A.; Arcuri, C.; Chironna, M.; Loconsole, D.; Napoli, C.; Orsi, G.B.; Manini, I.; Montomoli, E.; et al. Effectiveness of the trivalent MF59 adjuvated influenza vaccine in preventing hospitalization due to influenza B and A(H1N1)pdm09 viruses in the elderly in Italy, 2017—2018 season. Expert Rev. Vaccines 2019, 18, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Stuurman, A.L.; Bollaerts, K.; Alexandridou, M.; Biccler, J.; Diez Domingo, J.; Nohynek, H.; Rizzo, C.; Turunen, T.; Riera-Montes, M.; Partners, D.P. Vaccine effectiveness against laboratory-confirmed influenza in Europe–Results from the DRIVE network during season 2018/19. Vaccine 2020, 38, 6455–6463. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Kissling, E.; Emborg, H.D.; Larrauri, A.; McMenamin, J.; Pozo, F.; Trebbien, R.; Mazagatos, C.; Whitaker, H.; Valenciano, M. Interim 2019/20 influenza vaccine effectiveness: Six European studies, September 2019 to January 2020. Euro. Surveil 2020, 25, 153. [Google Scholar] [CrossRef] [PubMed]
| Underlying Conditions | N | % |
|---|---|---|
| Lung disease | 118 | 64.8 |
| Heart disease | 112 | 61.5 |
| Diabetes | 65 | 35.7 |
| Renal disease | 53 | 29.1 |
| Diseases of the hematopoietic organs and hemoglobinopathies | 18 | 9.9 |
| Cancer | 35 | 19.2 |
| Liver disease | 8 | 4.4 |
| Obesity | 17 | 9.3 |
| Anemia and enlarged spleen | 24 | 13.2 |
| Leukemia, lymphoma | 6 | 3.3 |
| Nutritional deficiency | 5 | 2.7 |
| Dementia or stroke | 25 | 13.7 |
| Rheumatologic disease | 17 | 9.3 |
| Congenital and acquired diseases involving deficient antibody production | 8 | 4.4 |
| Immunosuppression due to drugs or HIV | 8 | 4.4 |
| Chronic inflammatory diseases and intestinal malabsorption syndromes | 10 | 5.5 |
| Diseases associated with an increased risk of aspiration of respiratory secretions | 12 | 6.6 |
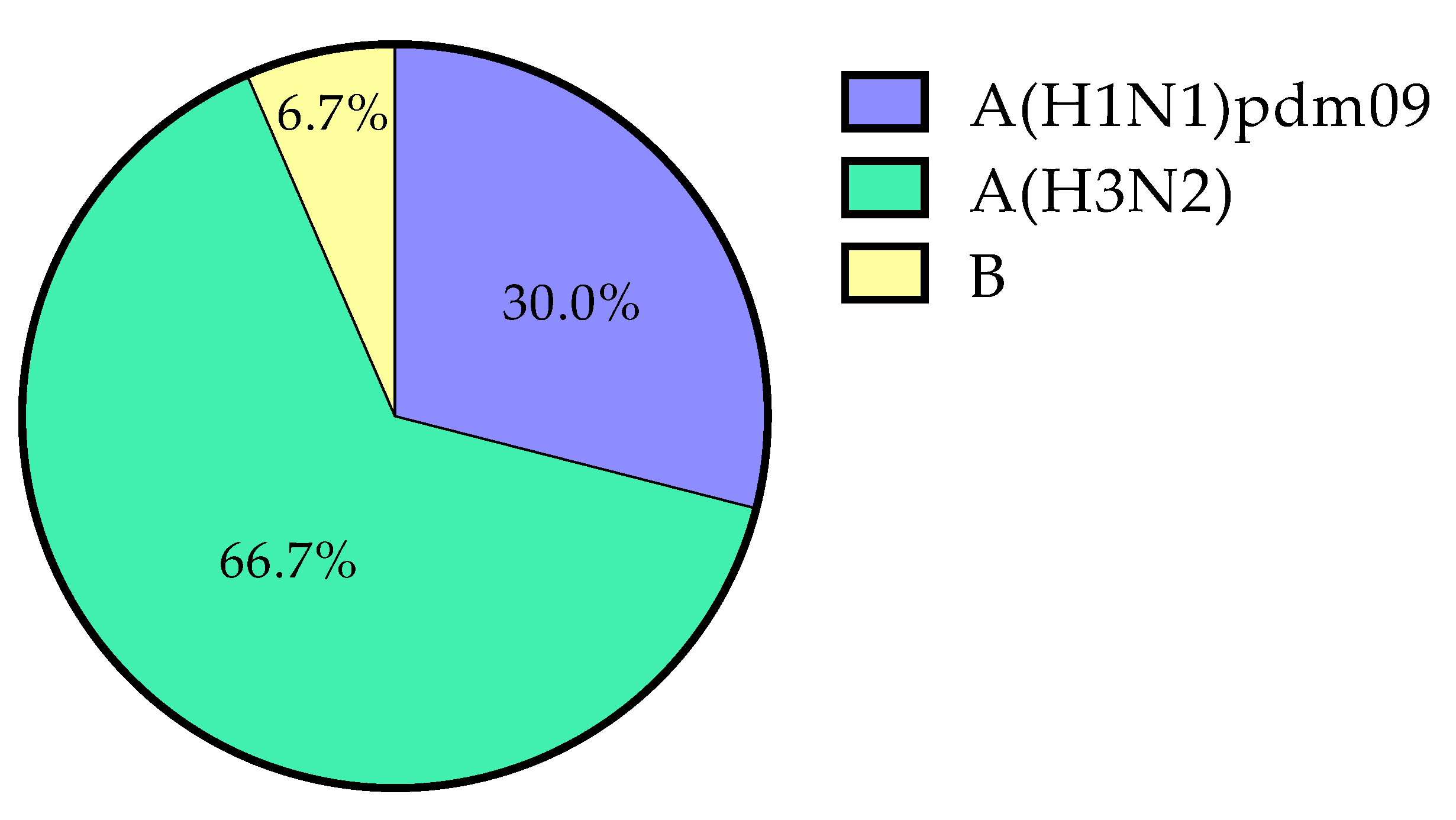
| Season | A(H1N1)pdm09 | A(H3N2) | B | Total | % (95% CI) |
|---|---|---|---|---|---|
| 2015/2016 | 1 | 0 | 0 | 1 | 2.4% (0.0–13.7) |
| 2016/2017 | 0 | 5 | 0 | 5 | 26.3% (11.4–49.1) |
| 2017/2018 | 1 * | 1 * | 2 | 3 | 17.6% (9.0–47.8) |
| 2018/2019 | 2 | 6 | 0 | 8 | 21.1% (10.8–36.6) |
| 2019/2020 | 5 | 8 | 0 | 13 | 19.4% (11.6–30.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manini, I.; Camarri, A.; Marchi, S.; Trombetta, C.M.; Vicenti, I.; Dragoni, F.; Lazzeri, G.; Bova, G.; Montomoli, E.; Capecchi, P.L. Surveillance for Severe Acute Respiratory Infections among Hospitalized Subjects from 2015/2016 to 2019/2020 Seasons in Tuscany, Italy. Int. J. Environ. Res. Public Health 2021, 18, 3875. https://doi.org/10.3390/ijerph18083875
Manini I, Camarri A, Marchi S, Trombetta CM, Vicenti I, Dragoni F, Lazzeri G, Bova G, Montomoli E, Capecchi PL. Surveillance for Severe Acute Respiratory Infections among Hospitalized Subjects from 2015/2016 to 2019/2020 Seasons in Tuscany, Italy. International Journal of Environmental Research and Public Health. 2021; 18(8):3875. https://doi.org/10.3390/ijerph18083875
Chicago/Turabian StyleManini, Ilaria, Andrea Camarri, Serena Marchi, Claudia Maria Trombetta, Ilaria Vicenti, Filippo Dragoni, Giacomo Lazzeri, Giovanni Bova, Emanuele Montomoli, and Pier Leopoldo Capecchi. 2021. "Surveillance for Severe Acute Respiratory Infections among Hospitalized Subjects from 2015/2016 to 2019/2020 Seasons in Tuscany, Italy" International Journal of Environmental Research and Public Health 18, no. 8: 3875. https://doi.org/10.3390/ijerph18083875
APA StyleManini, I., Camarri, A., Marchi, S., Trombetta, C. M., Vicenti, I., Dragoni, F., Lazzeri, G., Bova, G., Montomoli, E., & Capecchi, P. L. (2021). Surveillance for Severe Acute Respiratory Infections among Hospitalized Subjects from 2015/2016 to 2019/2020 Seasons in Tuscany, Italy. International Journal of Environmental Research and Public Health, 18(8), 3875. https://doi.org/10.3390/ijerph18083875









