Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Design
2.2. Patients’ Characteristics
2.3. COVID-19 Period
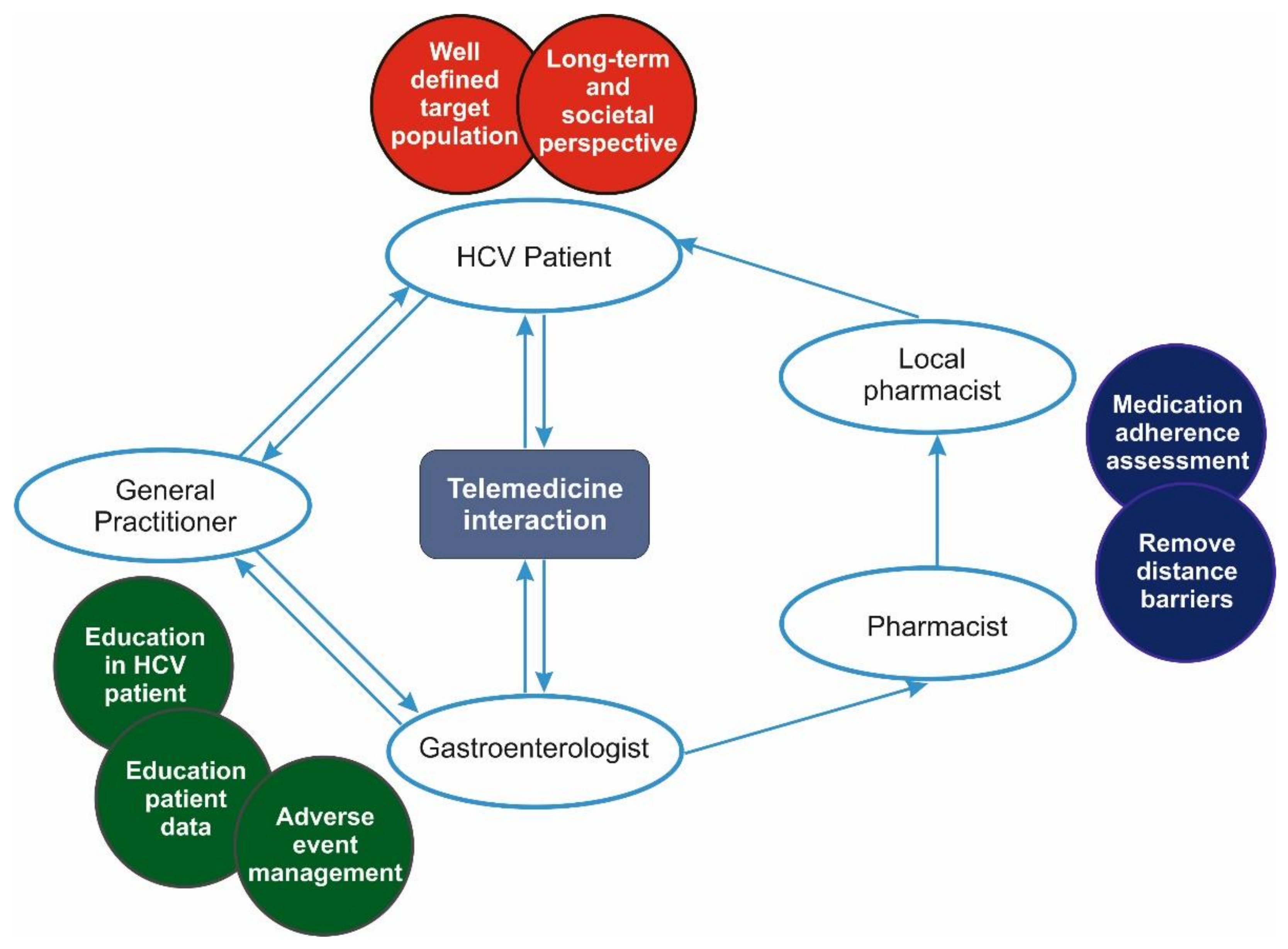
2.4. Telemedicine Protocol
2.5. Telemedicine Satisfaction Questionnaire
2.6. Medication Adherence
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stasi, C.; Silvestri, C.; Voller, F. Update on Hepatitis C Epidemiology: Unaware and Untreated Infected Population Could Be the Key to Elimination. SN Compr. Clin. Med. 2020, 2, 2808–2815. [Google Scholar] [CrossRef]
- Calvaruso, V.; Craxì, A. Hepatic benefits of HCV cure. J. Hepatol. 2020, 73, 1548–1556. [Google Scholar] [CrossRef]
- Polaris Observatory. The authoritative Resource for Epidemiological Data, Modeling Tools, Training, and Decision Analytics to Support Global Elimination of Hepatitis B and C by 2030. Available online: http://www.hepbunited.org/assets/Webinar-Slides/8b54a78215/POLARIS-Brief-181217.pdf (accessed on 30 December 2020).
- Chan, S.L.; Kudo, M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer 2020, 9, 491–502. [Google Scholar] [CrossRef]
- Decree Signed by the PRESIDENT of Romania, Mr. Klaus Iohannis, Regarding the Establishment of the Lockdown on the Romanian Territory. Available online: https://www.presidency.ro/ro/media/decret-semnat-de-presedintele-romaniei-domnul-klaus-iohannis-privind-instituirea-starii-de-urgenta-pe-teritoriul-romaniei (accessed on 30 December 2020).
- Portnoy, J.; Waller, M.; Elliott, T. Telemedicine in the Era of COVID-19. J. Allergy Clin. Immunol. Pr. 2020, 8, 1489–1491. [Google Scholar] [CrossRef]
- Jiménez-Rodríguez, D.; García, A.S.; Robles, J.M.; Salvador, M.D.M.R.; Ronda, F.J.M.; Arrogante, O. Increase in Video Consultations During the COVID-19 Pandemic: Healthcare Professionals’ Perceptions about Their Implementation and Adequate Management. Int. J. Environ. Res. Public Health 2020, 17, 5112. [Google Scholar] [CrossRef]
- Coombes, C.E.; Gregory, M.E. The Current and Future Use of Telemedicine in Infectious Diseases Practice. Curr. Infect. Dis. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Romanian Health Insurance House. Available online: http://www.cnas.ro/category/lista-medicamentelor.html (accessed on 30 December 2020).
- American Telemedicine Association. About Telemedicine. Available online: https://www.americantelemed.org (accessed on 30 December 2020).
- Stotts, M.J.; Grischkan, J.A.; Khungar, V. Improving cirrhosis care: The potential for telemedicine and mobile health technologies. World J. Gastroenterol. 2019, 25, 3849–3856. [Google Scholar] [CrossRef]
- Efe, C.; Simşek, C.; Batıbay, E.; Calışkan, A.R.; Wahlin, S. Feasibility of telehealth in the management of autoimmune hepatitis before and during the COVID-19 pandemic. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1215–1219. [Google Scholar] [CrossRef]
- Morey, S.; Hamoodi, A.; Jones, D.; Young, T.; Thompson, C.; Dhuny, J.; Buchanan, E.; Miller, C.; Hewett, M.; Valappil, M.; et al. Increased diagnosis and treatment of hepatitis C in prison by universal offer of testing and use of telemedicine. J. Viral Hepat. 2019, 26, 101–108. [Google Scholar] [CrossRef]
- Galán, G.J.; Alia, C.A.; González, M.V.; González, F.F.; Rodríguez, C.M.F.; Fernández, M.G.; García, M.L.G.; Losa, J.E.; Velasco, M.; Moreno, L.; et al. The contribution of telemedicine to hepatitis C elimination in a correctional facility. Rev. Española Enferm. Dig. 2019, 111, 550–555. [Google Scholar] [CrossRef]
- Popa, D.E.; Manea, N.C.; Gheonea, D.I.; Pirlog, M.C. Development of a Software for Treat-To-Target Strategy Implemen-tation and Increasing Quality of Life in Patients with Inflammatory Bowel Disease. Curr. Health Sci. J. 2020, 46, 103–110. [Google Scholar]
- Emergency Ordinance Regarding Telemedicine to Amend and Complete the Romanian Law no. 95/2006 on Health Care Reform. Available online: http://legislatie.just.ro/Public/DetaliiDocument/233458 (accessed on 14 January 2021).
- Decision, no. 252/2020 on the Establishment of Measures in the Field of Health during the Establishment of the State of Emergency on Romania’s Territory. Available online: http://www.cnas.ro/cjastm/post/type/local/hg-252-2020-privind-stabilirea-unor-masuri-in-domeniul-sanatatii-pe-perioada-instituirii-starii-de-u.html (accessed on 14 January 2021).
- Perrone, G.; Zerbo, S.; Bilotta, C.; Malta, G.; Argo, A. Telemedicine during COVID-19 pandemic: Advantage or critical issue? Medico-Legal J. 2020, 88, 76–77. [Google Scholar] [CrossRef]
- Turcu-Stiolica, A.; Gheonea, D.I.; Ungureanu, B.S.; Doica, I.P.; Subtirelu, M.S.; Rogoveanu, I. Translation and Cultural Adaptation of the Telemedicine Satisfaction Questionnaire for Use in Romania. Value Health 2021, in press. [Google Scholar]
- Yip, M.P.; Chang, A.M.; Chan, J.; MacKenzie, A.E. Development of the Telemedicine Satisfaction Questionnaire to evaluate patient satisfaction with telemedicine: A preliminary study. J. Telemed. Telecare 2003, 9, 46–50. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Ágh, T.; Malmenäs, M.; Raval, A.D.; Bennett, B.M.; Borah, B.J.; Hutchins, D.S.; Manias, E.; Williams, A.F.; Hiligsmann, M.; et al. Methods for Measuring Multiple Medication Adherence: A Systematic Review–Report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health 2019, 22, 139–156. [Google Scholar] [CrossRef]
- Hayes, A.F.; Coutts, J.J. Use Omega Rather than Cronbach’s Alpha for Estimating Reliability. But…. Commun. Methods Meas. 2020, 14, 1–24. [Google Scholar] [CrossRef]
- McDonald, R.P. Test Theory: A Unified Treatment; Lawrence Erlbaum: Mahwah, NJ, USA, 1999. [Google Scholar]
- Kelley, K. Methods for the Behavioral, Educational, and Social Sciences: An R package. Behav. Res. Methods 2007, 39, 979–984. [Google Scholar] [CrossRef]
- Implementing Telemedicine Services during COVID-19: Guiding Principles and Considerations for a Stepwise Approach. Available online: https://iris.wpro.who.int/bitstream/handle/10665.1/14651/WPR-DSE-2020-032-eng.pdf (accessed on 14 January 2021).
- Ungureanu, B.S.; Vladut, C.; Bende, F.; Sandru, V.; Tocia, C.; Turcu-Stiolica, R.-A.; Groza, A.; Balan, G.G.; Turcu-Stiolica, A. Impact of the COVID-19 Pandemic on Health-Related Quality of Life, Anxiety, and Training Among Young Gastroenterologists in Romania. Front. Psychol. 2020, 11, 579177. [Google Scholar] [CrossRef]
- Boettler, T.; Marjot, T.; Newsome, P.N.; Mondelli, M.U.; Maticic, M.; Cordero, E.; Jalan, R.; Moreau, R.; Cornberg, M.; Berg, T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020, 2, 100169. [Google Scholar] [CrossRef]
- Moon, A.M.; Webb, G.J.; Aloman, C.; Armstrong, M.J.; Cargill, T.; Dhanasekaran, R.; Genescà, J.; Gill, U.S.; James, T.W.; Jones, P.D.; et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J. Hepatol. 2020, 73, 705–708. [Google Scholar] [CrossRef]
- Case, L.; Wright, J.; Ryan, Y. Comparison of hepatitis C treatment outcomes between telehepatology and specialty care clinics in the era of direct-acting antivirals. J. Telemed. Telecare 2019, 1357633X19885750. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, S.; Ocak, S.; Saygılı, S.; Hopurcuoğlu, D.; Haşlak, F.; Kıykım, E.; Zeybek, Ç.A.; Celkan, T.; Demirgan, E.B.; Kasapçopur, Ö.; et al. Telemedicine Applications in a Tertiary Pediatric Hospital in Turkey During COVID-19 Pandemic. Telemed. e-Health 2020. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Zingone, F.; Camera, S.; Siniscalchi, M.; Ciacci, C. Telemedicine in the COVID-19 era for Liver Trans-plant Recipients: An Italian lockdown area experience. Clin. Res. Hepatol. Gastroenterol. 2020. [Google Scholar] [CrossRef]
- Butaru, A.E.; Doica, I.P.; Gheonea, D.I.; Rogoveanu, I.; Diculescu, M.; Oancea, C.N. Preliminary Results of the Micro-Elimination Project of Hepatitis C in a Disadvantaged Town in South-West of Romania-Orşova. Curr. Health Sci. J. 2020, 46, 217–221. [Google Scholar]
- Ajlan, A.A.; Al-Gain, R.; Ahmed, M.; Abu-Riash, T.; Alquaiz, M.; Alkhail, F.A.; Alashgar, H.; Alkhairallah, T.; Alkortas, D.; Al-Jedai, A. Developing a multidisciplinary HCV direct-acting antivirals utilization management and assessment program. J. Am. Pharm. Assoc. 2021, 61, e159–e170. [Google Scholar] [CrossRef]
- Gauld, N.; Perry, J.; Jackson, C.; Gane, E. Feasibility and outcomes of a hepatitis C screening programme in community pharmacies. N. Z. Med. J. 2020, 133, 74–83. [Google Scholar]
- Jiménez-Rodríguez, D.; Ruiz-Salvador, D.; Salvador, M.D.M.R.; Pérez-Heredia, M.; Ronda, F.J.M.; Arrogante, O. Consensus on Criteria for Good Practices in Video Consultation: A Delphi Study. Int. J. Environ. Res. Public Heal. 2020, 17, 5396. [Google Scholar] [CrossRef]
- Greenhalgh, T.; IRIHS Research Group. Video Consultations: A Guide to Practice. Available online: https://bjgplife.com/wp-content/uploads/2020/03/Video-consultations-a-guide-for-practice.pdf (accessed on 14 January 2021).
- Hernandez, J.L.P.; Mendoza, R.L.; Martinez, J.L.; Roldan, J.F.T.; Rosales, P.A.C.; Arredondo, H.A.M.; Gonzalez, V.R.; Silva, L.D.L.C.; Santana-Vargas, D.; Tijera, M.D.F.H.D.L.; et al. Chronic viral hepatitis c micro-elimination program using telemedicine. The mexican experience. Rev. Española Enferm. Dig. 2020. [Google Scholar] [CrossRef]
| Characteristics | Pre-COVID Phase March–May 2019 (n = 223) | Lockdown Phase March–May 2020 (n = 41) | p-Value |
|---|---|---|---|
| Age (years) | 0.9819 | ||
| Mean ± SD | 61.6 ± 11.4 | 62.1 ± 11.1 | |
| Range | 19–82 | 34–86 | |
| Gender (n, %) | 0.6904 | ||
| Men | 52, 23% | 11, 27% | |
| Women | 171, 77% | 30, 73% | |
| Health insurance type (n, %) | 0.0232 *,a | ||
| Employee | 34, 15% | 9, 22% | |
| Pensioner | 146, 66% | 19, 46% | |
| Co-insured | 23, 10% | 5, 12% | |
| Direct payment | 6, 3% | 3, 7% | |
| Social aid | 5, 2% | 3, 7% | |
| Other | 9, 4% | 2, 5% | |
| Medication adherence (Mean ± SD) | 94.7 ± 15.4 | 100 ± 0 | 0.0328 * |
| New patients/month (n, %) | 0.037 *,b | ||
| March | 87, 39% | 22, 54% | |
| April | 77, 35% | 9, 22% | |
| May | 59, 26% | 10, 27% |
| Outcomes | Lockdown Phase–Telemedicine Patients (n = 41) |
|---|---|
| Treatment (n, %) | |
| ombitasvir/paritaprevir/ritonavir + dasabuvir | 7, 17% |
| Ledipasvir + sofosbuvir | 34, 83% |
| Sustained virological response (n, %) | 41, 100% |
| Telemedicine satisfaction (mean ± SD) | |
| General satisfaction | 4.92 ± 0.11 |
| Quality of care provided | 4.84 ± 0.17 |
| Similarity to in-person face-to-face interaction | 4.94 ± 0.10 |
| Perception of the interaction | 4.98 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doica, I.P.; Florescu, D.N.; Oancea, C.N.; Turcu-Stiolica, A.; Subtirelu, M.-S.; Dumitra, G.; Rogoveanu, I.; Gheonea, D.I.; Ungureanu, B.S. Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3694. https://doi.org/10.3390/ijerph18073694
Doica IP, Florescu DN, Oancea CN, Turcu-Stiolica A, Subtirelu M-S, Dumitra G, Rogoveanu I, Gheonea DI, Ungureanu BS. Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(7):3694. https://doi.org/10.3390/ijerph18073694
Chicago/Turabian StyleDoica, Irina Paula, Dan Nicolae Florescu, Carmen Nicoleta Oancea, Adina Turcu-Stiolica, Mihaela-Simona Subtirelu, Gindrovel Dumitra, Ion Rogoveanu, Dan Ionut Gheonea, and Bogdan Silviu Ungureanu. 2021. "Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 7: 3694. https://doi.org/10.3390/ijerph18073694
APA StyleDoica, I. P., Florescu, D. N., Oancea, C. N., Turcu-Stiolica, A., Subtirelu, M.-S., Dumitra, G., Rogoveanu, I., Gheonea, D. I., & Ungureanu, B. S. (2021). Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A Pilot Study. International Journal of Environmental Research and Public Health, 18(7), 3694. https://doi.org/10.3390/ijerph18073694









