Characteristics of Selected Somatic and Motor Abilities of Youth Soccer Players with Diabetes Type 1 Treated with Insulin Pump Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Protocol
2.2.1. Assessment of Anthropometric Parameters
2.2.2. Assessment of General Motor Abilities
2.3. Endurance (Ruffier Index)
2.4. Handgrip Strength
2.5. Running Speed (5 m dash)
2.6. Data Analysis
3. Results
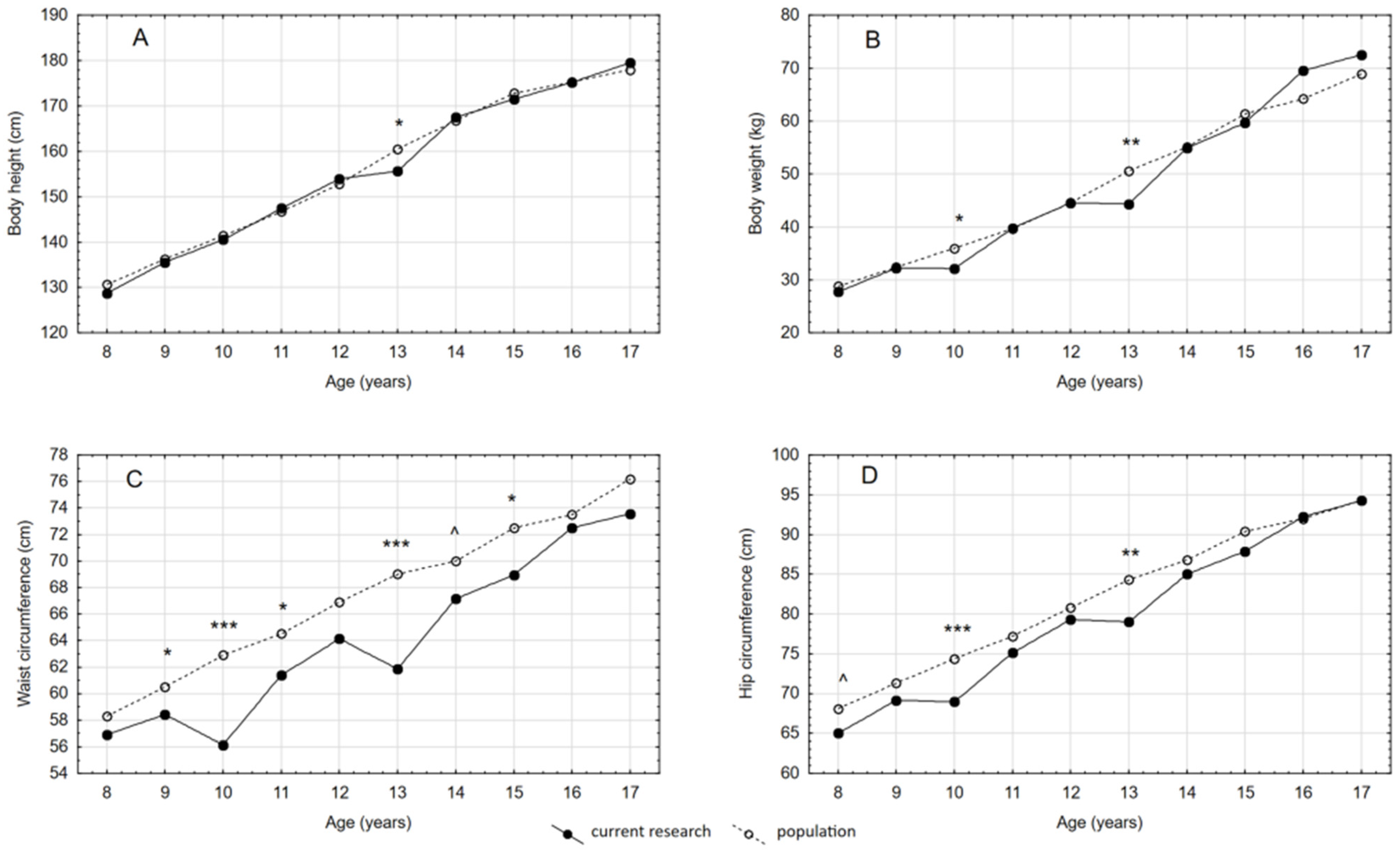
3.1. Anthropometric Parameters
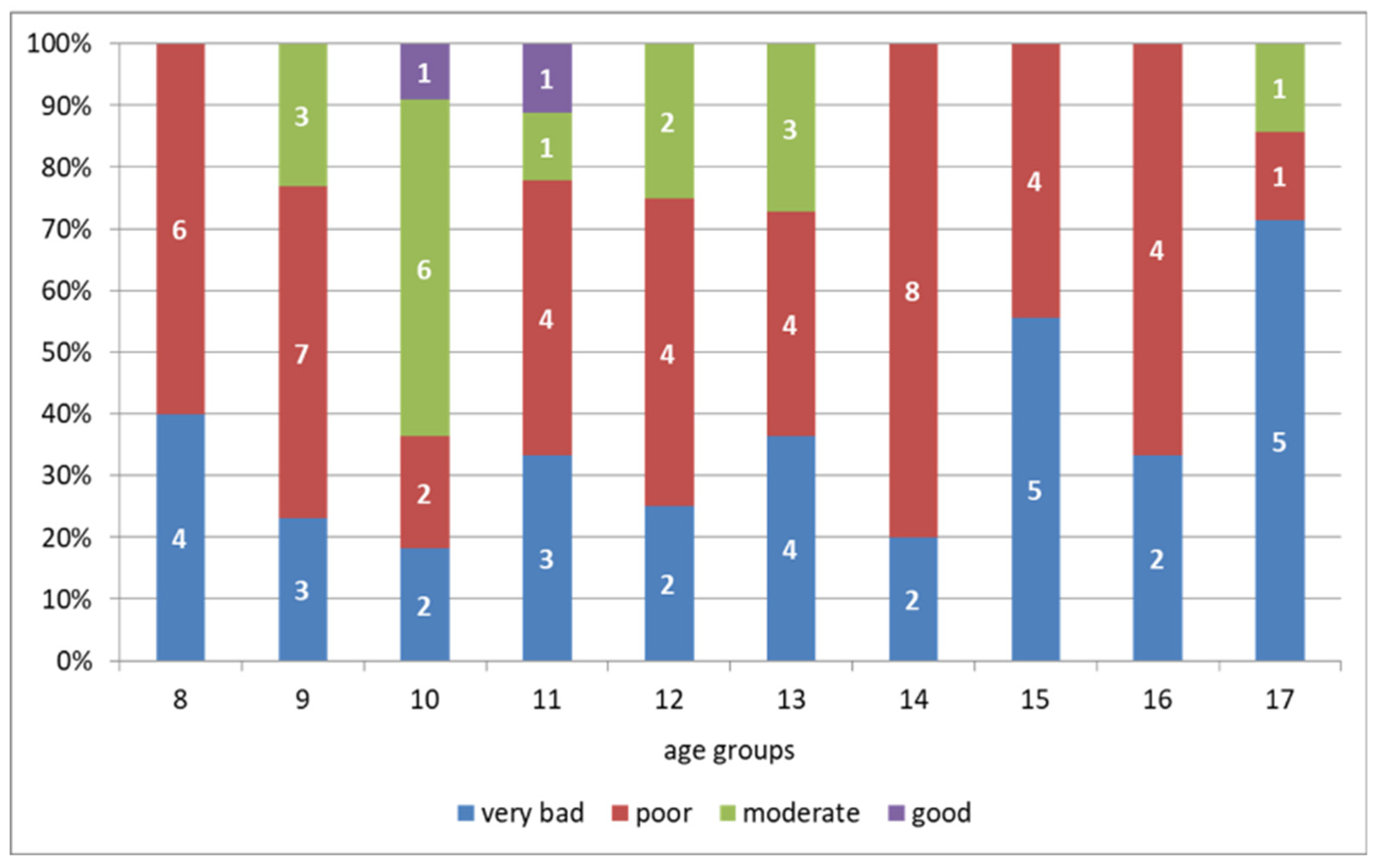
3.2. Motor Abilities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daneman, D. Type 1 diabetes. Lancet 2006, 367, 847–858. [Google Scholar] [CrossRef]
- Santi, E.; Tascini, G.; Toni, G.; Berioli, M.J.; Esposito, S. Linear Growth in Children and Adolescents with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2019, 16, 3677. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Quintos, J.B. Mauriac syndrome: Growth failure and type 1 diabetes mellitus. Pediatr. Endocrinol. Rev. 2008, 5, 989–993. [Google Scholar]
- Morris, M.; Johnson, S.B.; Gunnery, R.; Gayle, R.; Meek, P. Designing an education programme for type 1 diabetes: A focus group study. J. Diabetes Nurs. 2006, 10, 393–399. [Google Scholar]
- Thrower, S.L.; Bingley, P.J. What is type 1 diabetes? Medicine 2010, 38, 592–596. [Google Scholar] [CrossRef]
- Jabbour, G.; Henderson, M.; Mathieu, M.E. Barriers to Active Lifestyles in Children with Type 1 Diabetes. Can. J. Diabetes 2016, 40, 170–172. [Google Scholar] [CrossRef]
- Zaharieva, D.; Yavelberg, L.; Jamnik, V.; Cinar, A.; Turksoy, K.; Riddell, M.C. The Effects of Basal Insulin Suspension at the Start of Exercise on Blood Glucose Levels During Continuous Versus Circuit-Based Exercise in Individuals with Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Technol. Ther. 2017, 19, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr. Diabetes 2020, 21, 1375–1393. [Google Scholar] [PubMed]
- Gawrecki, A.; Zozulinska-Ziolkiewicz, D.; Matejko, B.; Hohendorff, J.; Malecki, M.T.; Klupa, T. Safe Completion of a Trail Running Ultramarathon by Four Men with Type 1 Diaetes. Diabetes Technol. Ther. 2018, 20, 147–152. [Google Scholar] [CrossRef]
- Bernardini, A.L.; Vanelli, M.; Chiari, G.; Lovene, B.; Gelmetti, C.; Vitale, R.; Errico, M.K. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed. Ateneo Parm. 2004, 75, 153–157. [Google Scholar]
- Jiménez-Pavón, D.; Ortega, F.B.; Valtueña, J.; Castro-Piñero, J.; Gómez-Martínez, S.; Zaccaria, M.; Gottrand, F.; Molnár, D.; Sjöström, M.; González-Gross, M.; et al. Muscular strength and markers of insulin resistance in European adolescents: The HELENA Study. Eur. J. Appl. Physiol. 2012, 112, 2455–2465. [Google Scholar]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar]
- Gawrecki, A.; Araszkiewicz, A.; Szadkowska, A.; Biegański, G.; Konarski, J.; Domaszewska, K.; Michalak, A.; Skowrońska, B.; Adamska, A.; Naskręt, D.; et al. Assessment of Safety and Glycemic Control During Football Tournament in Children and Adolescents With Type 1 Diabetes-Results of GoalDiab Study . Pediatr. Exerc. Sci. 2019, 31, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Toutain, C.; Delamarche, P.; Berthon, P.; Briard, D.; Youssef, H.; DeKerdanet, M.; Gratas-Delamarche, A. Exercise training and cardiovascular risk factors in type 1 diabetic adolescent girls. Pediatr. Exerc. Sci. 2007, 19, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Laan, R.; Dassau, E.; Kerr, D. Physical activity and type 1 diabetes: Time for a rewire? J. Diabetes Sci. Technol. 2015, 9, 609–618. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194–197. [Google Scholar]
- Cole, T.J.; Lobstein, T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012, 7, 284–294. [Google Scholar]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Świąder-Leśniak, A.; Litwin, M. Siatki centylowe dla oceny wzrastania i stanu odżywienia polskich dzieci i młodzieży od urodzenia do 18 roku życia. Stand. Med. Pediatr. 2015, 12, 119–135. [Google Scholar]
- Świąderek-Leśniak, A.; Kułaga, Z.; Grajda, A. Wartości referencyjne obwodu talii i bioder polskich dzieci i młodzieży w wieku 3–18 lat. Stand. Med. Pediatr. 2015, 12, 137–150. [Google Scholar]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, J.H. International Standards for Anthropometric Assessment; International Society for the Advanced of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Malina, R.M.; Bouchard, C.; Bar-Or, O. Growth, Maturation, and Physical Activity, 2rd ed.; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Li, C.; Ford, E.S.; Mokdad, A.H.; Cook, S. Recent trends in waist circumference and waist-height ratio among US children and adolescent. Pediatrics 2006, 118, 1390–1398. [Google Scholar] [CrossRef]
- Demerath, E.W.; Guo, S.S.; Chumlea, W.; Towne, B.; Roche, A.F.; Siervogel, R.M. Comparison of percent body fat estimates using air displacement plethysmography and hydrodensitometry in adults and children. Int. J. Obes. 2002, 26, 389–397. [Google Scholar] [CrossRef]
- Heyward, V.H.; Wagner, D.R. Applied Body Composition Assessment; Human Kinetics: Champaign, IL, USA, 2004. [Google Scholar]
- Faik, A.; Vanderhulst, E.; Rossem, I.; Devroey, D. Influence of physical activity and interest for food and sciences versus weight disorders in children aged 8 to 18 years. J. Prev. Med. Hyg. 2017, 58, E105–E113. [Google Scholar]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardized approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Wachowski, E.; Strzelczyk, R.; Osiński, W. Pomiar cech sprawności motorycznej osobników uprawiających sport. In Wyniki Badań; Measurement of the Features of Motor Fitness of Individuals Practicing Sport. Main Results; AWF Poznań: Królowej Jadwigi, Poland, 1987; Volume 238. (In Polish) [Google Scholar]
- Konarski, J.M.; Strzelczyk, R.; Karpowicz, K.; Janowski, J. Teoria sportu. Wybrane metody diagnostyczne (Theory of sport. Chosen diagnostic methods). In Hokej na Trawie. Historia-Teoria-Metodyka-Praktyka; Strzelczyk, R., Konarski, J.M., Podgórski, T., Pawlak, M., Eds.; AWF Poznań: Królowej Jadwigi, Poland, 2015; Volume 439, pp. 252–276. [Google Scholar]
- Zając, A.; Waśkiewicz, Z. Diagnoza Przygotowania Kondycyjnego Piłkarzy Nożnych (Diagnose of Motor Preparation of Soccer Players); AWF: Katowice, Poland, 1998. (In Polish) [Google Scholar]
- Dobosz, J. Tabele Punktacyjne Testów Eurofit, Międzynarodowego i COOPERA dla Uczniów i Uczennic Gimnazjów Oraz Szkół Ponadgimnazjalnych (Eurofit, International and Cooper Test Scoring Tables for Junior High School and High School Students); AWF: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Bonfig, W.; Kapellen, T.; Dost, A.; Fritsch, M.; Rohrer, T.; Wolf, J.; Holl, R.W. Diabetes Patienten Verlaufsdokumentationssystem Initiative of the German Working Group for Pediatric Diabetology and the German Bundesministerium für Bildung und Forschung Competence Net for Diabetes Mellitus. Growth in children and adolescents with type 1 diabetes. J. Pediatr. 2012, 160, 900–903. [Google Scholar]
- Rohrer, J.E.; Arif, A.; Denison, A.; Young, R.; Adamson, S. Overall self-rated health as an outcome indicator in primary care. J. Eval. Clin. Pract. 2007, 13, 882–888. [Google Scholar]
- Pozo, J.; Argente, J. Ascertainment and treatment of delayed puberty. Horm. Res. Paediatr. 2003, 60, 35–48. [Google Scholar]
- Lebl, J.; Schober, E.; Zidek, T.; Baldis, S.; Rami, B.; Pruhova, S.; Kolouskova, S.; Snajderova, M.; Frisch, H. Growth data in large series of 587 children and adolescents with type 1 diabetes mellitus. Endocr. Regul. 2003, 37, 153–161. [Google Scholar]
- Holl, R.W.; Grabert, M.; Heinze, E.; Sorgo, W.; Debatin, K.M. Age at onset and long-term metabolic control affect height in type-1 diabetes mellitus. Eur. J. Pediatr. 1998, 157, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Meira Sde, O.; Morcillo, A.M.; Lemos-Marini, S.H.; Paulino, M.F.; Minicucci, W.J.; Guerra-Júnior, G. Crescimento puberal e altura final em 40 pacientes com diabetes mellitus tipo 1 [Pubertal growth and final height in 40 patients with type 1 diabetes mellitus]. Arq. Bras. Endocrinol. Metabol. 2005, 49, 396–402. [Google Scholar]
- Elamin, A.; Hussein, O.; Tuvemo, T. Growth, puberty, and final height in children with Type 1 diabetes. J. Diabetes Complicat. 2006, 20, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.E.; Kolb, E.L.; Sauder, S.E. Effect of glycemic control on growth velocity in children with IDDM. Diabetes Care 1992, 15, 826–830. [Google Scholar]
- Brown, M.; Ahmed, M.L.; Clayton, K.L.; Dunger, D.B. Growth during childhood and final height in type 1 diabetes. Diabet. Med. 1994, 11, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Demir, K.; Altıncık, A.; Abacı, A.; Büyükgebiz, A.; Böber, E. Growth of Children with Type 1 Diabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Du Caju, M.V.; Rooman, R.P.; op de Beeck, L. Longitudinal data on growth and final height in diabetic children. Pediatr. Res. 1995, 38, 607–611. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sunil, E.; Suresh, V.; Sachan, A.; Rajitha, D.; Sarala, S.; Arun, M.; Sailaja, A.; Srinivasrao, C.; Shalini, P.; Sangeetha, S. Growth and skeletal maturation in children with type 1 diabetes mellitus. J. Clin. Sci. Res. 2016, 5, 20–27. [Google Scholar]
- Hartz, A.J.; Rupley, D.C.; Kalkhoff, R.; Rimm, A.A. Relationship of obesity to diabetes: Influence of obesity level and body fat distribution. Prev. Med. 1983, 12, 351–357. [Google Scholar] [CrossRef]
- Koster, A.; Leitzmann, M.F.; Schatzkin, A.; Mouw, T.; Adams, K.F.; van Eijk, J.T.; Hollenbeck, A.R.; Harris, T.B. Waist circumference and mortality. Am. J. Epidemiol. 2008, 167, 1465–1475. [Google Scholar]
- Wei-li, Y.; He, B.; Yao, H.; Dai, J.; Cui, J.; Ge, D.; Zheng, Y.; Li, L.; Guo, Y.; Xiao, K.; et al. Waist-to-height ratio is an accurate and easier index for evaluating obesity in children and adolescents. Obesity 2007, 15, 748–752. [Google Scholar]
- McCarthy, H.D.; Jarrett, K.V.; Emmett, P.M.; Rogers, I. Trends in waist circumferences in young British children: A comparative study. Int. J. Obes. 2005, 29, 157–162. [Google Scholar] [CrossRef]
- Malina, R.M.; Bouchard, C.; Beunen, G. Human Growth: Selected aspects of current research on well-nourished children. Annu. Rev. Anthropol. 1988, 17, 187–219. [Google Scholar] [CrossRef]
- Malina, R.M. Growth and maturation: Normal variation and the effects of training. In Perspectives in Exercise Science and Sports Medicine; Gisolfi, C.V., Lamb, D.R., Eds.; Benchmark Press: Indianapolis, Indiana, 1989; pp. 223–265. [Google Scholar]
- Tuvemo, T.; Kobbah, M.; Proos, L.A. Growth and subcutaneous fat during the first five years of insulin-dependent diabetes in children. Acta Paediatr. Suppl. 1997, 418, 1–5. [Google Scholar]
- Bartz, J.; Sulzbach, U.; Heinze, E.; Teller, W.M.; Holl, R.W. Body composition in type 1 diabetes mellitus. Bio-impedance measurements in 274 diabetic children, adolescents and young adults. Dtsch. Med. Wochenschr. 1997, 122, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.B.; Rizzoli, R.R.; Marchand, L.M.; Ferrari, S.; Beghetti, M.; Farpour-Lambert, N.J. Physical activity increases bone mineral density in children with type 1 diabetes. Med. Sci. Sports Exerc. 2012, 44, 1206–1211. [Google Scholar] [CrossRef]
- Adolfsson, P.; Nilsson, S.; Albertsson-Wikland, K.; Lindblad, B. Hormonal response during physical exercise of different intensities in adolescents with type 1 diabetes and healthy controls. Pediatr. Diabetes 2012, 13, 587–596. [Google Scholar] [CrossRef]
- Komatsu, W.R.; Gabbay, M.A.; Castro, M.L.; Saraiva, G.L.; Chacra, A.R.; de Barros Neto, T.L.; Dib, S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes 2005, 6, 145–149. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Regensteiner, J.G.; Bauer, T.A.; Brown, M.S.; Dorosz, J.L.; Hull, A.; Zeitler, P.; Draznin, B.; Reusch, J.E. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J. Clin. Endocrinol. Metab. 2010, 95, 513–521. [Google Scholar] [CrossRef]
- Hagag, A.A.; Seyam, M.K.; Zaky, N.A.; Salem, E.Y. Evaluation of physical fitness in children with type 1 diabetes. Int. J. Physiother. Res. 2017, 5, 2332–2336. [Google Scholar] [CrossRef][Green Version]
- Gajewska, E.; Kalinska, K.; Bogdanski, P.; Sobieska, M. Cardiorespiratory endurance in relation to body mass in Polish rural children: Preliminary report. HOMO 2015, 66, 278–285. [Google Scholar] [CrossRef]
- Reilly, T. The Science of Training—Soccer: A Scientific Approach to Developing Strength, Speed and Endurance, 1st ed.; Routledge: London, UK, 2007. [Google Scholar]
- World Health Organization. Global Recommendations on Physical Activity for Health. 2010. Available online: https://www.who.int/publications/i/item/9789241599979 (accessed on 22 January 2021).
- Name, M.V.; Hilliard, M.; Boyle, C.; Miller, K.; DeSalvo, D.; Anderson, B.; Laffel, L.; Woerner, S.; DiMeglio, L.; Tamborlane, W. Nighttime is the worst time: Parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr. Diabetes 2018, 19, 114––120. [Google Scholar]
- Brazeau, A.S.; Mircescu, H.; Desjardins, K.; Dubé, M.C.; Weisnagel, S.J.; Lavoie, C.; Rabasa-Lhoret, R. The Barriers to Physical Activity in Type 1 Diabetes (BAPAD-1) scale: Predictive validity and reliability. Diabetes Metab. 2012, 38, 164–170. [Google Scholar] [CrossRef]
- Maly, T.; Zahalka, F.; Hráský, P.; Malá, L.; Ižovská, J.; Bujnovský, D.; Dragijský, M.; Mihal, J. Age-Related Differences in Linear SprInt. and Power Characteristics in Youth Elite Soccer Players. J. Phys. Educ. Sport 2015, 15. [Google Scholar]
- Jürimäe, T.; Hurbo, T.; Jürimäe, J. Relationship of handgrip strength with anthropometric and body composition variables in prepubertal children. HOMO 2009, 60, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Lafortuna, C.L.; Pogliaghi, S.; Trecate, L. The impact of gender, body dimension and body composition on handgrip strength in healthy children. J. Endocrinol. Investig. 2002, 25, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.C.; Torode, M.E.; Singh, M.A. Muscular strength and cardiorespiratory fitness are associated with higher insulin sensitivity in children and adolescent. Int. J. Pediatr. Obes. 2006, 1, 222–231. [Google Scholar] [CrossRef] [PubMed]
| Variable | Boys’ Age Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| (n = 10) | (n = 13) | (n = 11) | (n = 9) | (n = 8) | (n = 11) | (n = 10) | (n = 9) | (n = 6) | (n = 7) | |
| Diabetes duration (years) | 2.8 (1.53) | 4.54 (2.44) | 5.18 (2.52) | 5.00 (3.20) | 4.46 (3.74) | 4.91 (3.11) | 4.13 (2.69) | 7.67 (4.09) | 7.50 (5.05) | 10.42 (5.41) |
| HbA1c (%) | 7.15 (0.78) | 7.3 (0.58) | 7.3 (1.10) | 7.3 (1.03) | 7.5 (1.08) | 7.6 (072) | 8.3 (1.99) | 7.6 (0.66) | 8.2 (1.28) | 8.0 (1.21) |
| Body height (cm) | 128.7 (4.72) | 135.5 (5.85) | 140.5 (9.95) | 147.4 (7.87) | 153.9 (5.79) | 155.6 (6.59) | 167.5 (4.82) | 171.5 (6.46) | 175.2 (10.60) | 179.5 (5.22) |
| Body weight (kg) | 27.76 (4.79) | 32.23 (5.29) | 32.15 (5.06) | 39.75 (6.78) | 44.57 (7.31) | 44.35 (5.79) | 54.95 (6.28) | 59.7 (9.42) | 69.56 (12.67) | 72.52 (7.51) |
| Waist circumference (cm) | 56.9 (4.22) | 58.42 (3.04) | 56.13 (3.26) | 61.38 (3.22) | 64.12 (5.39) | 61.86 (2.41) | 67.15 (4.28) | 68.94 (4.19) | 72.5 (4.59) | 73.57 (4.71) |
| Hip circumference (cm) | 65 (4.73) | 69.15 (4.93) | 68.95 (3.82) | 75.11 (5.39) | 79.31 (5.89) | 79 (4.51) | 85 (4.85) | 87.88 (5.91) | 92.25 (6.24) | 94.25 (5.48) |
| WHR (cm) | 0.875 (0.03) | 0.846 (0.03) | 0.814 (0.02) | 0.819 (0.04) | 0.81 (0.02) | 0.78 (0.04) | 0.79 (0.03) | 0.79 (0.02) | 0.79 (0.03) | 0.78 (0.04) |
| WHTR (%) | 44.18 (2.20) | 43.10 (1.72) | 40.04 (2.46) | 41.67 (1.62) | 41.62 (2.78) | 39.79 (1.85) | 40.10 (2.62) | 40.21 (2.30) | 41.42 (2.09) | 40.99 (2.82) |
| FAT (%) | 21.62 (3.88) | 21.25 (2.67) | 19.60 (2.91) | 20.40 (3.23) | 21.63 (3.89) | 18.66 (2.41) | 16.29 (3.12) | 15.94 (3.15) | 17.08 (2.02) | 16.57 (3.08) |
| FFM (%) | 78.37 (3.88) | 78.74 (2.67) | 80.39 (2.91) | 79.59 (3.23) | 78.36 (3.89) | 81.33 (2.41) | 83.70 (3.12) | 84.05 (3.15) | 82.91 (2.02) | 83.42 (3.08) |
| TBW (%) | 57.42 (2.86) | 57.69 (1.95) | 58.88 (2.13) | 58.25 (2.39) | 57.34 (2.80) | 59.57 (1.79) | 61.25 (2.29) | 61.56 (2.29) | 60.71 (1.47) | 61.03 (2.26) |
| BMI (kg/m2) | 16.67 (1.70) | 17.43 (1.69) | 16.24 (1.67) | 18.17 (1.58) | 18.84 (2.29) | 18.15 (1.52) | 19.55 (1.80) | 20.21 (2.30) | 22.49 (2.14) | 22.48 (1.95) |
| General Fitness | ||||||||||
| The Ruffier test (points) | 14.16 (2.67) | 13.27 (2.74) | 10.64 (4.97) | 13.68 (5.23) | 12.26 (4.63) | 14.63 (4.98) | 14.08 (3.54) | 14.51 (3.09) | 13.63 (2.31) | 15.41 (3.36) |
| 5 m dash (s) | 1.440 (0.08) | 1.354 (0.13) | 1.35 (0.09) | 1.32 (0.09) | 1.27 (0.13) | 1.22 (0.05) | 1.22 (0.07) | 1.218 (0.12) | 1.176 (0.11) | 1.14 (0.07) |
| Grip Strength, RH (kG) | 11.1 (1.73) | 14.46 (3.15) | 14.45 (3.58) | 22.22 (9.91) | 20.87 (2.03) | 24.63 (7.62) | 29.3 (3.53) | 32.66 (6.78) | 38.83 (9.54) | 38.14 (13.15) |
| BMI (Cole’s Cutoffs) | Boys’ Age Groups | Total | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |||||||||||||
| (n = 10) | (n = 13) | (n = 11) | (n = 9) | (n = 8) | (n = 11) | (n = 10) | (n = 9) | (n = 6) | (n = 7) | (n = 94) | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Thin | 1 | 1.06 | 1 | 1.06 | 1 | 1.06 | 1 | 1.06 | 4 | 4.26 | ||||||||||||
| Normal weight | 8 | 8.51 | 11 | 11.70 | 9 | 9.52 | 9 | 9.57 | 7 | 7.45 | 10 | 10.64 | 9 | 9.57 | 7 | 7.45 | 4 | 4.26 | 6 | 6.38 | 80 | 85.11 |
| Overweight | 2 | 2.13 | 2 | 2.13 | 1 | 1.00 | 1 | 1.06 | 1 | 1.06 | 2 | 2.13 | 1 | 1.06 | 10 | 10.64 | ||||||
| Obese | ||||||||||||||||||||||
| Total (%) | 10.64 | 13.83 | 11.70 | 9.57 | 8.51 | 11.70 | 10.64 | 9.57 | 6.38 | 7.45 | 100.0 | |||||||||||
| Thinness | ||||||||||||||||||||||
| Mild thinness | 1 | 25.0 | 1 | 25.0 | ||||||||||||||||||
| Moderate thinness | 1 | 25.0 | 1 | 25.0 | 1 | 25.0 | 3 | 75.0 | ||||||||||||||
| Severe thinness | ||||||||||||||||||||||
| Age Groups | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5m dash (s) | |||||||||||
| Type 1 Diabetes boys | 1.44 (0.08) | 1.35 (0.13) | 1.35 (0.10) | 1.32 (0.10) | 1.27 (0.13) | 1.22 (0.05) | 1.22 (0.07) | 1.21 (0.12) | 1.18 (0.11) | 1.14 (0.07) | |
| norm † | ♦ | 1.35–1.40 | 1.20–1.25 | 1.08–1.12 | 1.04–1.08 | 1.00–1.04 | |||||
| ♦♦ | 1.30–1.34 | 1.15–1.19 | 1.05–1.07 | 1.00–1.03 | 0.95–0.99 | ||||||
| ♦♦♦ | <1.25 | <1.14 | <1.05 | ≤1.00 | ≤0.95 | ||||||
| Above any norm (%) | 46 | 18 | 78 | 50 | 100 | 100 | 89 | 83 | 100 | ||
| Handgrip strength (kg) | |||||||||||
| T1D groups | 11.1 (1.72) | 14.6 (3.15) | 14.5 (3.58) | 22.2 (9.91) | 20.9 (2.03) | 24.6 (7.61) | 29.3 (3.53) | 32.7 (6.78) | 38.8 (9.54) | 38.1 (13.56) | |
| Population †† | 10–15 | 12–18 | 14–20 | 17–23 | 20–27 | 23–34 | 28–40 | 33–46 | 39–51 | 42–55 | |
| Below population range (%) | 10 | 15 | 36 | 22 | 0 | 45 | 20 | 44 | 33 | 29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykała, M.; Domaszewska, K.; Woźniewicz-Dobrzyńska, M.; Kryściak, J.; Konarska, A.; Araszkiewicz, A.; Zozulińska-Ziółkiewicz, D.; Gawrecki, A.; Biegański, G.; Konarski, J.M. Characteristics of Selected Somatic and Motor Abilities of Youth Soccer Players with Diabetes Type 1 Treated with Insulin Pump Therapy. Int. J. Environ. Res. Public Health 2021, 18, 3493. https://doi.org/10.3390/ijerph18073493
Krzykała M, Domaszewska K, Woźniewicz-Dobrzyńska M, Kryściak J, Konarska A, Araszkiewicz A, Zozulińska-Ziółkiewicz D, Gawrecki A, Biegański G, Konarski JM. Characteristics of Selected Somatic and Motor Abilities of Youth Soccer Players with Diabetes Type 1 Treated with Insulin Pump Therapy. International Journal of Environmental Research and Public Health. 2021; 18(7):3493. https://doi.org/10.3390/ijerph18073493
Chicago/Turabian StyleKrzykała, Magdalena, Katarzyna Domaszewska, Małgorzata Woźniewicz-Dobrzyńska, Jakub Kryściak, Agata Konarska, Aleksandra Araszkiewicz, Dorota Zozulińska-Ziółkiewicz, Andrzej Gawrecki, Grzegorz Biegański, and Jan M. Konarski. 2021. "Characteristics of Selected Somatic and Motor Abilities of Youth Soccer Players with Diabetes Type 1 Treated with Insulin Pump Therapy" International Journal of Environmental Research and Public Health 18, no. 7: 3493. https://doi.org/10.3390/ijerph18073493
APA StyleKrzykała, M., Domaszewska, K., Woźniewicz-Dobrzyńska, M., Kryściak, J., Konarska, A., Araszkiewicz, A., Zozulińska-Ziółkiewicz, D., Gawrecki, A., Biegański, G., & Konarski, J. M. (2021). Characteristics of Selected Somatic and Motor Abilities of Youth Soccer Players with Diabetes Type 1 Treated with Insulin Pump Therapy. International Journal of Environmental Research and Public Health, 18(7), 3493. https://doi.org/10.3390/ijerph18073493






