Smoking Cessation during the Second Half of Pregnancy Prevents Low Birth Weight among Australian Born Babies in Regional New South Wales
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Sample
2.2. Study Setting
2.3. Study Outcome
2.4. Exposure Variables
2.5. Confounding Variables
2.6. Statistical Analyses
2.7. Ethical Consideration
3. Results
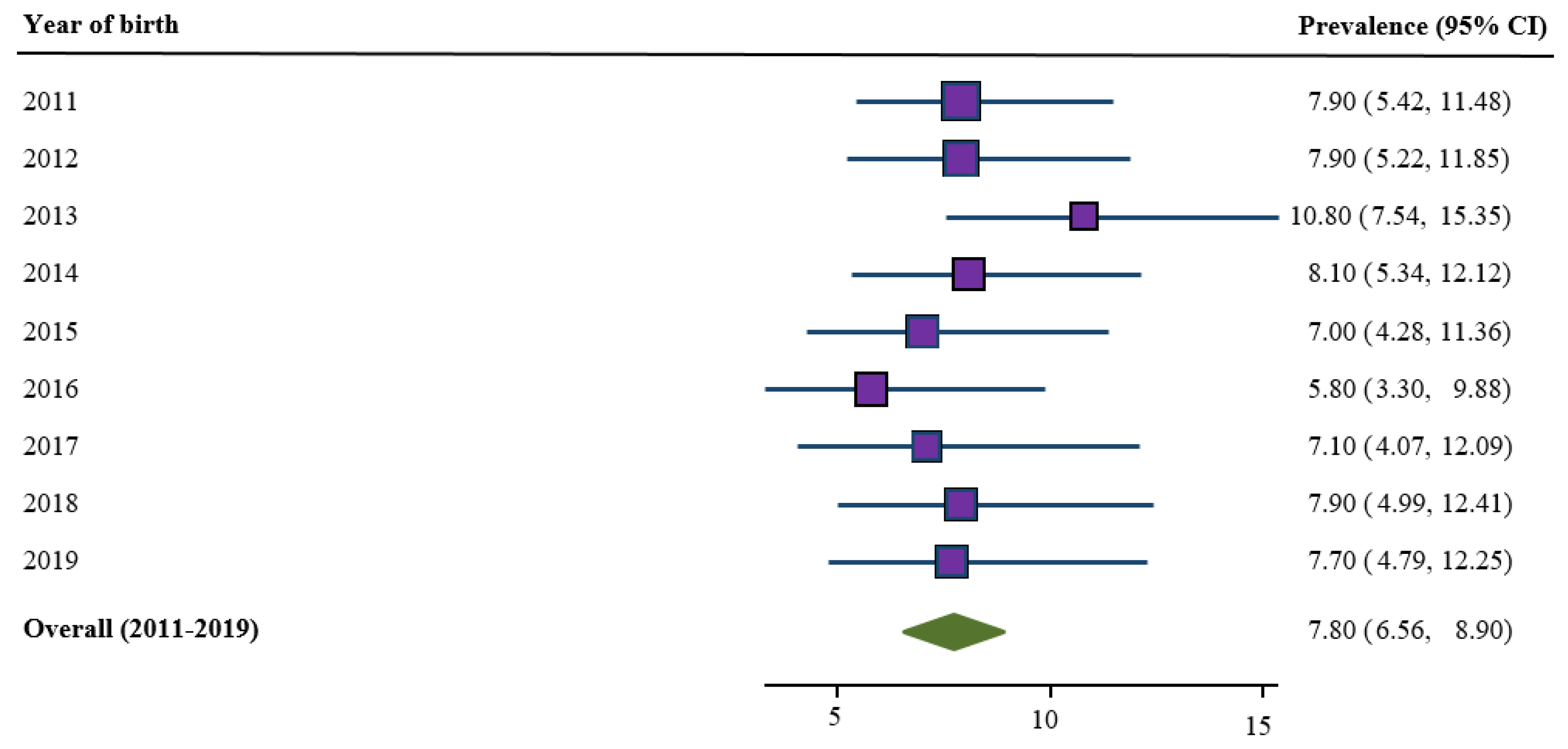
3.1. Trends in Prevalence of Low Birth Weight
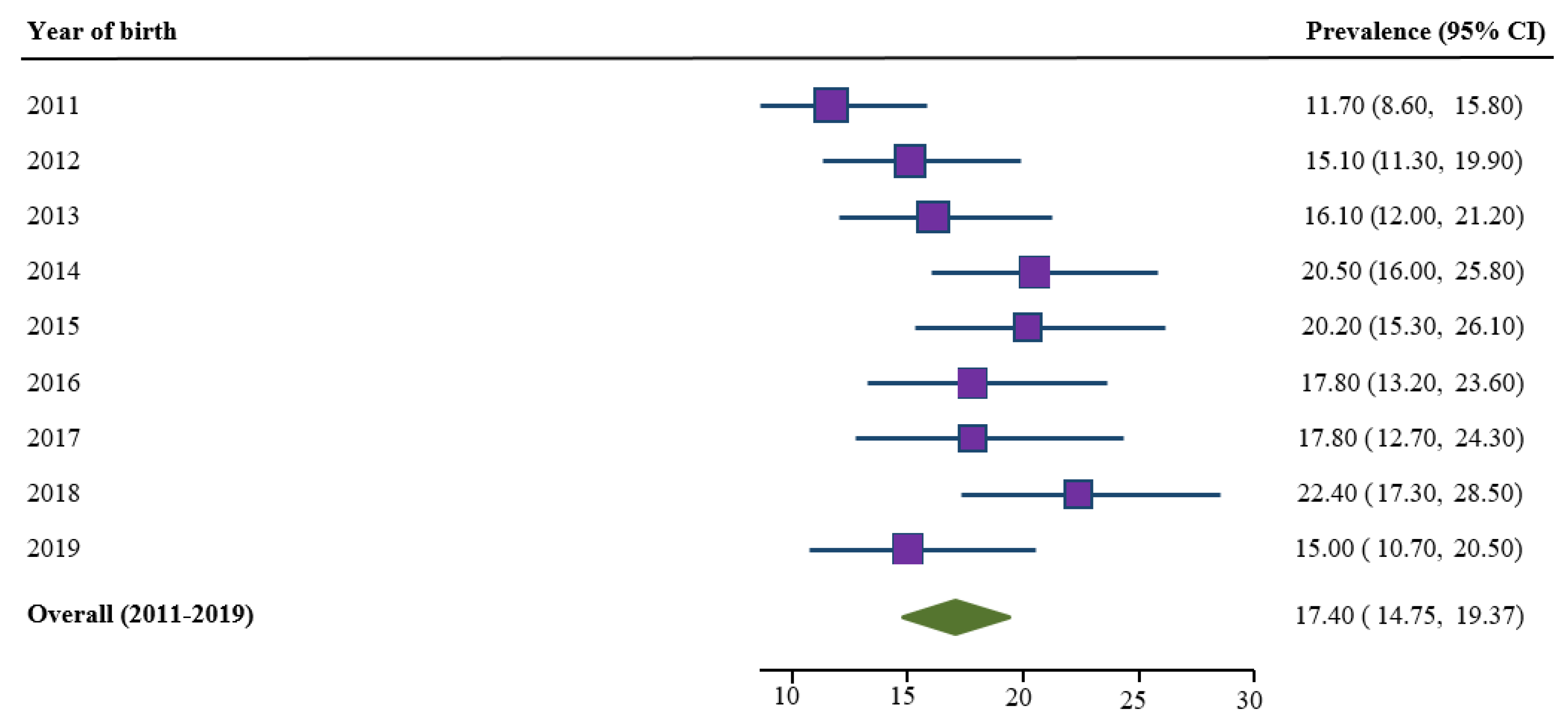
3.2. Trends in Prevalence of Smoking Cessation
3.3. Characteristics of Study Population
3.4. Impact of Smoking Cessation on Low Birth Weight
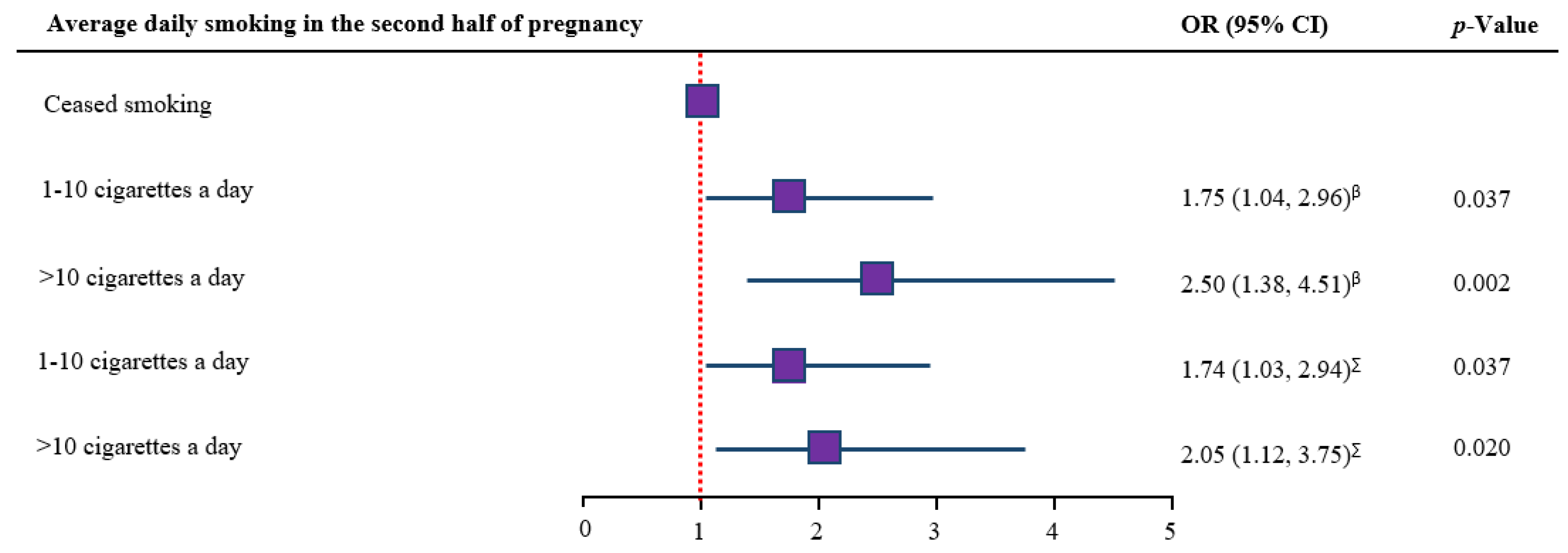
3.5. Dose-Response Analysis
3.6. Protective and Risk Factors for Low Birth Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems. Available online: https://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf (accessed on 2 September 2020).
- Jornayvaz, F.R.; Vollenweider, P.; Bochud, M.; Mooser, V.; Waeber, G.; Marques-Vidal, P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 2016, 15, 73. [Google Scholar] [CrossRef]
- Arnold, L.; Hoy, W.; Wang, Z. Low birthweight increases risk for cardiovascular disease hospitalisations in a remote Indigenous Australian community–a prospective cohort study. Aust. N. Z. J. Public Health 2016, 40, S102–S106. [Google Scholar] [CrossRef]
- Mu, M.; Ye, S.; Bai, M.J.; Liu, G.L.; Tong, Y.; Wang, S.F.; Sheng, J. Birth weight and subsequent risk of asthma: A systematic review and meta-analysis. Heart. Lung Circ. 2014, 23, 511–519. [Google Scholar] [CrossRef]
- Matheson, M.C.; D’Olhaberriague, A.L.P.; Burgess, J.A.; Giles, G.G.; Hopper, J.L.; Johns, D.P.; Abramson, M.J.; Walters, E.H.; Dharmage, S.C. Preterm birth and low birth weight continue to increase the risk of asthma from age 7 to 43. J. Asthma 2017, 54, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.; Wang, Z.; Hoy, W. Low birth weight and reduced renal volume in Aboriginal children. Am. J. Kidney Dis. 2001, 37, 915–920. [Google Scholar] [CrossRef]
- Hack, M.; Klein, N.K.; Taylor, H.G. Long-term developmental outcomes of low birth weight infants. Future Child. 1995, 5, 176–196. [Google Scholar] [CrossRef]
- Bilgin, A.; Mendonca, M.; Wolke, D. Preterm birth/low birth weight and markers reflective of wealth in adulthood: A meta-analysis. Pediatrics 2018, 142, e20173625. [Google Scholar] [CrossRef] [PubMed]
- Lightwood, J.M.; Phibbs, C.S.; Glantz, S.A. Short-term health and economic benefits of smoking cessation: Low birth weight. Pediatrics 1999, 104, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.P.D.S.; Da Mata, F.A.; Figueiredo, A.C.G.; de Andrade, K.R.C.; Pereira, M.G. Maternal active smoking during pregnancy and low birth weight in the Americas: A systematic review and meta-analysis. Nicotine Tob. Res. 2017, 19, 497–505. [Google Scholar] [CrossRef]
- Mohsin, M.; Wong, F.; Bauman, A.; Bai, J.U.N. Maternal and neonatal factors influencing premature birth and low birth weight in Australia. J. Biosoc. Sci. 2003, 35, 161. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Sullivan, E.A. Teenage smoking in pregnancy and birthweight: A population study, 2001–2004. Med. J. Aust. 2008, 188, 392–396. [Google Scholar] [CrossRef]
- Ko, T.J.; Tsai, L.Y.; Chu, L.C.; Yeh, S.J.; Leung, C.; Chen, C.Y.; Chou, H.C.; Tsao, P.N.; Chen, P.C.; Hsieh, W.S. Parental smoking during pregnancy and its association with low birth weight, small for gestational age, and preterm birth offspring: A birth cohort study. Pediatr. Neonatol. 2014, 55, 20–27. [Google Scholar] [CrossRef]
- Chiolero, A.; Bovet, P.; Paccaud, F. Association between maternal smoking and low birth weight in Switzerland: The EDEN study. Swiss Med. Wkly. 2005, 135, 525–530. [Google Scholar] [PubMed]
- Mainous, A.G.; Hueston, W.J. The effect of smoking cessation during pregnancy on preterm delivery and low birthweight. J. Fam. Pract. 1994, 38, 262–267. [Google Scholar]
- Batech, M.; Tonstad, S.; Job, J.S.; Chinnock, R.; Oshiro, B.; Merritt, T.A.; Page, G.; Singh, P.N. Estimating the impact of smoking cessation during pregnancy: The San Bernardino County experience. J. Community Health 2013, 38, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Juárez, S.P.; Merlo, J. Revisiting the effect of maternal smoking during pregnancy on offspring birthweight: A quasi-experimental sibling analysis in Sweden. PLoS ONE 2013, 8, e61734. [Google Scholar] [CrossRef]
- Freund, M.A.; Campbell, E.M.; Paul, C.L.; Wiggers, J.H.; Knight, J.J.; Mitchell, E.N. Provision of smoking care in NSW hospitals: Opportunities for further enhancement. N. S. Wales Public Health Bull. 2008, 19, 50–55. [Google Scholar] [CrossRef] [PubMed]
- NSW Tobacco Strategy 2012–2021. Available online: https://www.health.nsw.gov.au/tobacco/Pages/tobacco-strategy-1221.aspx (accessed on 5 August 2020).
- Maternal and Child Health Policies and Guidelines. Available online: https://www.health.nsw.gov.au/kidsfamilies/MCFhealth/child/pages/child-and-family-health-policies-and-guidelines.aspx (accessed on 7 August 2020).
- HealthStats NSW. Available online: http://www.healthstats.nsw.gov.au/Indicator/mab_smo_cat/mab_smo_cat (accessed on 7 August 2020).
- Mothers and Babies Reports. Available online: https://www.health.nsw.gov.au/hsnsw/Pages/mothers-and-babies-reports.aspx (accessed on 15 September 2020).
- Gedda, L.; Brenci, G.; Gatti, I. Low birth weight in twins versus singletons: Separate entities and different implications for child growth and survival. Twin Res. Acta Genet. Med. Gemellol. 1981, 30, 1–8. [Google Scholar] [CrossRef]
- Governance and Legislation for Local Health District and Speciality Network Boards. Available online: https://www.health.nsw.gov.au/lhd/boards/Pages/board_governance.aspx (accessed on 15 November 2020).
- Local Health Districts and Specialty Networks. Available online: https://www.health.nsw.gov.au/lhd/Pages/default.aspx (accessed on 15 April 2020).
- Population by Local Health District. Available online: http://www.healthstats.nsw.gov.au/Indicator/dem_pop_lhnmap/dem_pop_lhn_snap (accessed on 25 August 2020).
- Southern NSW Local Health District 2019–2020 year in Review. Available online: https://www.snswlhd.health.nsw.gov.au/getmedia/4ffcc029-7249-4408-ad77-d8683a15c72c/snswlhd_2019-20_year-in-review_single-page.pdf.aspx?ext=.pdf (accessed on 25 August 2020).
- Maternity Services. Available online: https://www.snswlhd.health.nsw.gov.au/our-services/maternity-services (accessed on 18 December 2020).
- Passmore, E.; McGuire, R.; Correll, P.; Bentley, J. Demographic factors associated with smoking cessation during pregnancy in New South Wales, Australia, 2000–2011. BMC Public Health 2015, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smoking and Pregnancy. Available online: https://www.aihw.gov.au/getmedia/f44090f1-1bed-42c1-b56f-dac0d3239d88/smoking-pregnancy.pdf.aspx?inline=true (accessed on 5 September 2020).
- Socio-Economic Indexes for Areas (SEIFA). Available online: https://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/8C5F5BB699A0921CCA258259000BA619?opendocument (accessed on 10 March 2020).
- Clinical Practice Guidelines: Pregnancy Care. Available online: https://www.health.gov.au/resources/pregnancy-care-guidelines (accessed on 2 August 2020).
- Agho, K.E.; Ezeh, O.K.; Ghimire, P.R.; Uchechukwu, O.L.; Stevens, G.J.; Tannous, W.K.; Fleming, C.; Ogbo, F.A.; Maternal, G. Exclusive Breastfeeding Rates and Associated Factors in 13 “Economic Community of West African States”(ECOWAS) Countries. Nutrients 2019, 11, 3007. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.R.; Goldstein, H.; Ross, E.M. Cigarette smoking in pregnancy: Its influence on birth weight and perinatal mortality. Br. Med. J. 1972, 2, 127–130. [Google Scholar] [CrossRef]
- Sabra, S.; Gratacós, E.; Roig, M.D.G. Smoking-induced changes in the maternal immune, endocrine, and metabolic pathways and their impact on fetal growth: A Topical Review. Fetal. Diagn. Ther 2017, 41, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Secker-Walker, R.H.; Vacek, P.M.; Flynn, B.S.; Mead, P.B. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet. Gynecol. 1997, 89, 648–653. [Google Scholar] [CrossRef]
- Milnerowicz-Nabzdyk, E.; Bizoń, A. Effect of cigarette smoking on vascular flows in pregnancies complicated by intrauterine growth restriction. Reprod. Toxicol. 2014, 50, 27–35. [Google Scholar] [CrossRef]
- Pattenden, S.; Antova, T.; Neuberger, M.; Nikiforov, B.; De Sario, M.; Grize, L.; Heinrich, J.; Hruba, F.; Janssen, N.; Luttmann-Gibson, H.; et al. Parental smoking and children’s respiratory health: Independent effects of prenatal and postnatal exposure. Tob. Control 2006, 15, 294–301. [Google Scholar] [CrossRef]
- Tager, I.B.; Ngo, L.; Hanrahan, J.P. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am. J. Respir. Crit. Care Med. 1995, 152, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Bjerg, A.; Hedman, L.; Perzanowski, M.; Lundbäck, B.; Rönmark, E. A strong synergism of low birth weight and prenatal smoking on asthma in schoolchildren. Pediatrics 2011, 127, e905–e912. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.S.; Munn, J.; Avuri, S.; Hoff, S.; Cadet-James, Y.; McEwen, A.; Clough, A.R. “Nobody smokes in the house if there’s a new baby in it”: Aboriginal perspectives on tobacco smoking in pregnancy and in the household in regional NSW Australia. Women Birth 2013, 26, 246–253. [Google Scholar] [CrossRef]
- Wood, L.; France, K.; Hunt, K.; Eades, S.; Slack-Smith, L. Indigenous women and smoking during pregnancy: Knowledge, cultural contexts and barriers to cessation. Soc. Sci. Med. 2008, 66, 2378–2389. [Google Scholar] [CrossRef] [PubMed]
- Mahumud, R.A.; Sultana, M.; Sarker, A.R. Distribution and determinants of low birth weight in developing countries. J. Prev. Med. Public Health 2017, 50, 18. [Google Scholar] [CrossRef]
- Goisis, A.; Remes, H.; Barclay, K.; Martikainen, P.; Myrskylä, M. Advanced maternal age and the risk of low birth weight and preterm delivery: A within-family analysis using Finnish population registers. Am. J. Epidemiol. 2017, 186, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum. Reprod. 2007, 22, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Tough, S.C.; Newburn-Cook, C.; Johnston, D.W.; Svenson, L.W.; Rose, S.; Belik, J. Delayed childbearing and its impact on population rate changes in lower birth weight, multiple birth, and preterm delivery. Pediatrics 2002, 109, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Raatikainen, K.; Heiskanen, N.; Heinonen, S. Under-attending free antenatal care is associated with adverse pregnancy outcomes. BMC Public Health 2007, 7, 268. [Google Scholar] [CrossRef]
- Trinh, L.T.T.; Rubin, G. Late entry to antenatal care in New South Wales, Australia. Reprod. Health 2006, 3, 8. [Google Scholar] [CrossRef][Green Version]
- McDonald, S.D.; Han, Z.; Mulla, S.; Beyene, J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: Systematic review and meta-analyses. BMJ 2010, 341. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.; Da Mata, F.A.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal anemia and low birth weight: A systematic review and meta-analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy is associated with risk of low birth weight: A systematic review and dose-response meta-analysis. BMC Med. 2014, 12, 174. [Google Scholar] [CrossRef]
- Ferdos, J.; Rahman, M.M. Maternal experience of intimate partner violence and low birth weight of children: A hospital-based study in Bangladesh. PLoS ONE 2017, 12, e0187138. [Google Scholar]
- Berhanie, E.; Gebregziabher, D.; Berihu, H.; Gerezgiher, A.; Kidane, G. Intimate partner violence during pregnancy and adverse birth outcomes: A case-control study. Reprod. Health 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Low Birth Weight | ||
|---|---|---|---|
| Confounding variables | N (%) | n | % (95% CI) |
| Maternal SES category (N = 2096) | |||
| High | 208 (9.9) | 5 | 2.4 (1.0, 5.6) |
| Middle | 1220 (58.1) | 98 | 8.0 (6.6, 9.7) |
| Low | 668 (31.8) | 63 | 9.4 (7.4, 11.9) |
| Maternal Aboriginal status (N = 2096) | |||
| Non-aboriginal | 1745 (83.1) | 129 | 7.4 (6.3, 8.7) |
| Aboriginal | 351 (16.7) | 37 | 10.5 (7.7, 14.2) |
| Hospital of birth | |||
| Cooma Health Service | 217 (10.3) | 14 | 6.5 (3.9, 10.6) |
| Goulburn Base Hospital | 524 (25.0) | 42 | 8.0 (6.0, 10.7) |
| Moruya District Hospital | 586 (27.9) | 58 | 9.9 (7.7, 12.6) |
| Queanbeyan Health Service | 403 (19.2) | 19 | 4.7 (3.0, 7.3) |
| South East Regional Hospital | 369 (17.6) | 33 | 8.9 (6.4, 12.3) |
| Maternal age at birth | |||
| <20 years | 207 (9.9) | 15 | 7.2 (4.4, 11.7) |
| 20–34 years | 1647 (78.5) | 122 | 7.4 (6.2, 8.8) |
| 35–49 years | 245 (11.7) | 29 | 11.8 (8.3, 16.5) |
| History of previous pregnancy (N = 2093) | |||
| 2 or more previous pregnancies | 856 (40.8) | 71 | 8.3 (6.6, 10.3) |
| 1 previous pregnancy | 576 (27.4) | 41 | 7.1 (5.3, 9.5) |
| no previous pregnancy | 661 (31.5) | 54 | 8.2 (6.3, 10.5) |
| Sex of baby | |||
| Male | 1069 (50.9) | 78 | 7.3 (5.9, 9.0) |
| Female | 1030 (49.1) | 88 | 8.5 (6.9, 10.3) |
| Type of delivery | |||
| Normal vaginal | 1436 (68.4) | 111 | 7.7 (6.5, 9.2) |
| Caesarean section | 490 (23.3) | 42 | 8.6 (6.4, 11.4) |
| Assisted vaginal | 173 (8.3) | 13 | 7.5 (4.4, 12.5) |
| Diagnosed with gestational diabetes (N = 2094) | |||
| No | 1990 (94.8) | 158 | 7.9 (6.8, 9.2) |
| Yes | 104 (4.9) | 8 | 7.7 (3.9, 14.6) |
| Diagnosed with gestational hypertension | |||
| No | 2060 (98.1) | 161 | 7.8 (6.7, 9.1) |
| Yes | 39 (1.9) | 5 | 12.8 (5.4, 27.3) |
| Number of antenatal care visits (N = 2071) | |||
| <7 visits | 357 (17.0) | 53 | 14.8 (11.5, 18.9) |
| 7+ visits | 1703 (81.1) | 102 | 6.0 (4.9, 7.2) |
| Duration of pregnancy at first antenatal visit (N = 2082) | |||
| <20 weeks | 1365 (65.0) | 106 | 7.8 (6.5, 9.3) |
| 20+ weeks | 717 (34.2) | 53 | 7.4 (5.7, 9.6) |
| Exposure variables | |||
| Smoking cessation during the second half of pregnancy | |||
| Continued smoking | 1740 (82.9) | 149 | 8.6 (7.3, 10.0) |
| Ceased smoking | 359 (17.1) | 17 | 4.7 (3.0, 7.5) |
| Average daily smoking in the second half of pregnancy (N = 2073) | |||
| Ceased smoking | 359 (17.1) | 17 | 4.7 (3.0, 7.5) |
| 1–10 cigarettes a day | 1361 (64.8) | 109 | 8.0 (6.7, 9.6) |
| >10 cigarettes a day | 353 (16.8) | 39 | 11.0 (8.2, 14.7) |
| Characteristics | Unadjusted Model | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confounding Variables | OR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value |
| Year of birth | ||||||||||||
| 2011 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| 2012 | 1.18 (0.61, 2.26) | 0.619 | 1.21 (0.62, 2.34) | 0.578 | 1.23 (0.63, 2.40) | 0.537 | 1.23 (0.63, 2.40) | 0.539 | 1.31 (0.66, 2.59) | 0.433 | 1.35 (0.68, 2.66) | 0.389 |
| 2013 | 1.74 (0.94, 3.21) | 0.078 | 1.70 (0.91, 3.17) | 0.096 | 1.76 (0.93, 3.31) | 0.081 | 1.74 (0.93, 3.28) | 0.085 | 1.97 (1.03, 3.74) | 0.039 | 2.02 (1.06, 3.84) | 0.033 |
| 2014 | 1.20 (0.62, 2.30) | 0.584 | 1.16 (0.60, 2.23) | 0.665 | 1.16 (0.60, 2.25) | 0.654 | 1.16 (0.60, 2.24) | 0.652 | 1.31 (0.67, 2.57) | 0.425 | 1.40 (0.72, 2.75) | 0.323 |
| 2015 | 1.09 (0.54, 2.20) | 0.812 | 1.05 (0.51, 2.16) | 0.887 | 1.06 (0.52, 2.18) | 0.873 | 1.06 (0.51, 2.17) | 0.882 | 1.27 (0.61, 2.64) | 0.516 | 1.34 (0.64, 2.78) | 0.437 |
| 2016 | 0.80 (0.37, 1.73) | 0.574 | 0.75 (0.35, 1.62) | 0.466 | 0.78 (0.36, 1.68) | 0.52 | 0.78 (0.36, 1.69) | 0.53 | 0.9 (0.42, 1.95) | 0.789 | 0.93 (0.43, 2.01) | 0.852 |
| 2017 | 1.10 (0.52, 2.34) | 0.797 | 1.08 (0.51, 2.31) | 0.844 | 1.05 (0.49, 2.24) | 0.895 | 1.05 (0.49, 2.24) | 0.904 | 1.09 (0.51, 2.33) | 0.827 | 1.12 (0.52, 2.40) | 0.779 |
| 2018 | 1.18 (0.59, 2.35) | 0.639 | 1.14 (0.57, 2.30) | 0.713 | 1.11 (0.55, 2.26) | 0.766 | 1.12 (0.55, 2.28) | 0.749 | 1.24 (0.6, 2.55) | 0.566 | 1.31 (0.63, 2.70) | 0.469 |
| 2019 | 1.13 (0.56, 2.28) | 0.734 | 1.08 (0.53, 2.20) | 0.821 | 1.05 (0.52, 2.14) | 0.885 | 1.06 (0.52, 2.18) | 0.867 | 1.19 (0.58, 2.45) | 0.627 | 1.22 (0.59, 2.50) | 0.595 |
| Maternal SES category | ||||||||||||
| High | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Middle | 4.12 (1.50, 11.36) | 0.006 | 2.96 (0.99, 8.82) | 0.051 | 2.92 (0.98, 8.66) | 0.054 | 2.92 (0.98, 8.66) | 0.054 | 2.97 (1.01, 8.78) | 0.049 | 2.94 (1.00, 8.62) | 0.050 |
| Low | 5.05 (1.81, 14.09) | 0.002 | 3.92 (1.25, 12.26) | 0.019 | 4.01 (1.29, 12.5) | 0.017 | 3.99 (1.28, 12.42) | 0.017 | 4.15 (1.34, 12.87) | 0.014 | 4.04 (1.31, 12.44) | 0.015 |
| Maternal Aboriginal status | ||||||||||||
| Non-Aboriginal | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Aboriginal | 1.48 (0.99, 2.20) | 0.057 | 1.38 (0.92, 2.07) | 0.117 | 1.50 (0.99, 2.27) | 0.058 | 1.49 (0.98, 2.26) | 0.062 | 1.39 (0.91, 2.13) | 0.131 | 1.36 (0.89, 2.10) | 0.156 |
| Hospital of birth | ||||||||||||
| Cooma Health Service | 1.00 | |||||||||||
| Goulburn Base Hospital | 1.18 (0.63, 2.23) | 0.605 | 0.84 (0.41, 1.71) | 0.622 | 0.84 (0.41, 1.72) | 0.629 | 0.83 (0.41, 1.72) | 0.622 | 0.73 (0.35, 1.51) | 0.394 | 0.75 (0.37, 1.55) | 0.443 |
| Moruya District Hospital | 1.50 (0.82, 2.76) | 0.192 | 1.13 (0.60, 2.13) | 0.707 | 1.12 (0.59, 2.14) | 0.724 | 1.12 (0.59, 2.13) | 0.737 | 0.96 (0.5, 1.86) | 0.912 | 0.99 (0.52, 1.91) | 0.986 |
| Queanbeyan Health Service | 0.57 (0.27, 1.22) | 0.147 | 0.65 (0.30, 1.43) | 0.286 | 0.66 (0.30, 1.45) | 0.299 | 0.66 (0.30, 1.44) | 0.296 | 0.61 (0.27, 1.36) | 0.226 | 0.61 (0.27, 1.35) | 0.220 |
| South East Regional Hospital | 1.39 (0.72, 2.66) | 0.326 | 1.12 (0.58, 2.15) | 0.744 | 1.09 (0.56, 2.11) | 0.799 | 1.08 (0.56, 2.09) | 0.814 | 0.95 (0.48, 1.85) | 0.873 | 0.96 (0.49, 1.87) | 0.902 |
| Maternal age at birth | ||||||||||||
| 20–34 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| <20 years | 0.96 (0.54, 1.70) | 0.88 | 0.78 (0.42, 1.47) | 0.446 | 0.79 (0.42, 1.48) | 0.462 | 0.72 (0.38, 1.37) | 0.322 | 0.69 (0.36, 1.30) | 0.250 | ||
| 35–49 years | 1.69 (1.08, 2.63) | 0.021 | 1.73 (1.08, 2.78) | 0.023 | 1.73 (1.07, 2.80) | 0.027 | 1.88 (1.15, 3.07) | 0.012 | 1.87 (1.14, 3.05) | 0.013 | ||
| History of previous pregnancy | ||||||||||||
| 2 or more previous pregnancy | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| 1 previous pregnancy | 0.81 (0.53, 1.22) | 0.308 | 0.98 (0.63, 1.52) | 0.92 | 0.97 (0.63, 1.50) | 0.898 | 1 (0.64, 1.58) | 0.983 | 1.01 (0.64, 1.58) | 0.975 | ||
| no previous pregnancy | 0.91 (0.62, 1.34) | 0.645 | 1.22 (0.77, 1.94) | 0.396 | 1.20 (0.76, 1.91) | 0.437 | 1.39 (0.86, 2.27) | 0.179 | 1.46 (0.90, 2.36) | 0.126 | ||
| Diagnosed with chronic hypertension | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Yes | 2.73 (0.58, 12.76) | 0.202 | 2.21 (0.44, 11.18) | 0.338 | 2.26 (0.45, 11.49) | 0.324 | 2.98 (0.56, 15.9) | 0.2 | 3.32 (0.57, 19.41) | 0.182 | ||
| Sex of baby | ||||||||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Female | 1.22 (0.88, 1.69) | 0.239 | 1.23 (0.88, 1.71) | 0.224 | 1.22 (0.88, 1.71) | 0.23 | 1.27 (0.91, 1.77) | 0.155 | 1.27 (0.91, 1.77) | 0.161 | ||
| Type of delivery | ||||||||||||
| Normal vaginal | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Caesarean section | 1.17 (0.80, 1.71) | 0.414 | 1.14 (0.76, 1.69) | 0.533 | 1.13 (0.76, 1.69) | 0.538 | 1.13 (0.75, 1.7) | 0.556 | 1.15 (0.76, 1.74) | 0.497 | ||
| Assisted vaginal | 0.87 (0.46, 1.66) | 0.678 | 0.85 (0.43, 1.67) | 0.628 | 0.85 (0.43, 1.68) | 0.642 | 0.82 (0.41, 1.62) | 0.563 | 0.87 (0.44, 1.73) | 0.688 | ||
| Diagnosed with gestational diabetes | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 1.02 (0.48, 2.13) | 0.967 | 0.89 (0.40, 1.96) | 0.764 | 1 (0.45, 2.2) | 0.996 | 0.99 (0.45, 2.16) | 0.975 | ||||
| Diagnosed with gestational hypertension | ||||||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 1.82 (0.70, 4.72) | 0.22 | 1.40 (0.54, 3.67) | 0.492 | 1.48 (0.54, 4.08) | 0.445 | 1.49 (0.53, 4.16) | 0.445 | ||||
| Number of antenatal care visits | ||||||||||||
| <7 visits | 1.00 | 1.00 | 1.00 | |||||||||
| 7+ visits | 0.37 (0.26, 0.52) | <0.001 | 0.33 (0.22, 0.49) | <0.001 | 0.34 (0.23, 0.50) | <0.001 | ||||||
| Duration of pregnancy at first antenatal visit | ||||||||||||
| <20 weeks | 1.00 | 1.00 | 1.00 | |||||||||
| 20 + weeks | 1.06 (0.75, 1.50) | 0.743 | 1.26 (0.86, 1.86) | 0.235 | 1.28 (0.86, 1.88) | 0.221 | ||||||
| Exposure variables | ||||||||||||
| Smoking cessation during the second half of pregnancy | ||||||||||||
| Continued smoking | 1.00 | 1.00 | ||||||||||
| Ceased smoking | 0.57 (0.34, 0.95) | 0.031 | 0.56 (0.34, 0.94) | 0.028 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, P.R.; Mooney, J.; Fox, L.; Dubois, L. Smoking Cessation during the Second Half of Pregnancy Prevents Low Birth Weight among Australian Born Babies in Regional New South Wales. Int. J. Environ. Res. Public Health 2021, 18, 3417. https://doi.org/10.3390/ijerph18073417
Ghimire PR, Mooney J, Fox L, Dubois L. Smoking Cessation during the Second Half of Pregnancy Prevents Low Birth Weight among Australian Born Babies in Regional New South Wales. International Journal of Environmental Research and Public Health. 2021; 18(7):3417. https://doi.org/10.3390/ijerph18073417
Chicago/Turabian StyleGhimire, Pramesh Raj, Julie Mooney, Louise Fox, and Lorraine Dubois. 2021. "Smoking Cessation during the Second Half of Pregnancy Prevents Low Birth Weight among Australian Born Babies in Regional New South Wales" International Journal of Environmental Research and Public Health 18, no. 7: 3417. https://doi.org/10.3390/ijerph18073417
APA StyleGhimire, P. R., Mooney, J., Fox, L., & Dubois, L. (2021). Smoking Cessation during the Second Half of Pregnancy Prevents Low Birth Weight among Australian Born Babies in Regional New South Wales. International Journal of Environmental Research and Public Health, 18(7), 3417. https://doi.org/10.3390/ijerph18073417







