Heart Rate Variability Monitoring during Interferential Current Application in the Lower Back Area: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size
2.2. Ethics
2.3. Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jorge, S.; Parada, C.A.; Ferreira, S.H.; Tambeli, C.H. Interferential therapy produces antinociception during application in various models of inflammatory pain. Phys. Ther. 2006, 86, 800–808. [Google Scholar] [CrossRef]
- Fuentes, C.J.; Armijo-Olivo, S.; Magee, D.J.; Gross, D. Does amplitude-modulated frequency have a role in the hypoalgesic response of interferential current on pressure pain sensitivity in healthy subjects? A randomised crossover study. Physiotherapy 2010, 96, 22–29. [Google Scholar] [CrossRef]
- Zambito, A.; Bianchini, D.; Gatti, D.; Viapiana, O.; Rossini, M.; Adami, S. Interferential and horizontal therapies in chronic low back pain: A randomized, double blind, clinical study. Clin. Exp. Rheumatol. 2006, 24, 534–539. [Google Scholar] [PubMed]
- Adedoyin, R.A.; Olaogun, M.O.; Fagbeja, O.O. Effect of interferential current stimulation in management of osteo-arthritic knee pain. Physiotherapy 2002, 88, 493–499. [Google Scholar] [CrossRef]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; WB Saunders Co.: Philadelphia, PA, USA, 2012. [Google Scholar]
- Youn, J.I.; Lee, H.S.; Lee, S. Determination of effective treatment duration of interferential current therapy using electromyography. J. Phys. Ther. Sci. 2016, 28, 2400–2403. [Google Scholar] [CrossRef]
- García, P.; De La Cruz-Torres, B.; Naranjo, J.; Albornoz-Cabello, M. Autonomic Responses to Ultrasound-Guided Percutaneous Needle Electrolysis: Effect of Needle Puncture or Electrical Current? J. Altern. Complement. Med. 2018, 24, 69–75. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhao, X.; Li, Y.; Xi, Q.; Guo, Y. Adverse events following acupuncture: A systematic review of the Chinese literature for the years 1956–2010. J. Altern. Complement. Med. 2012, 18, 892–901. [Google Scholar] [CrossRef]
- MacPherson, H.; Thomas, K.; Walters, S.; Fitter, M. A prospective survey of adverse events and treatment reactions following 34,000 consultations with professional acupuncturists. Acupunct. Med. 2001, 19, 93–102. [Google Scholar] [CrossRef]
- Jin, H.K.; Hwang, T.Y.; Cho, S.H. Effect of electrical stimulation on blood flow velocity and vessel size. Open Med. (Wars) 2017, 12, 5–11. [Google Scholar] [CrossRef]
- Task Force of ESC and NASPE: Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [CrossRef]
- Naranjo, J.; De la Cruz, B.; Sarabia, E.; De Hoyo, M.; Domínguez-Cobo, S. Heart rate variability: A follow-up in elite soccer players troughout the season. Int. J. Sports Med. 2015, 36, 881–886. [Google Scholar] [CrossRef]
- De la Cruz, B.; López, C.; Naranjo, J. Analysis of heart rate variability at rest and during aerobic exercise: A study in healthy people and cardiac patients. Br. J. Sports Med. 2008, 42, 715–720. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Kimura, K.; Yoshida, S. Spectral analysis of heart rate variability during trigger point acupuncture. Acupunct. Med. 2014, 32, 273–278. [Google Scholar] [CrossRef]
- Albornoz, M.; Rebollo, J.; García, R. Escala de Aprensión Psicológica Personal (EAPP) en Fisioterapia. Rev. Iberoam Fisioter Kinesol 2005, 2, 77–87. [Google Scholar] [CrossRef]
- Rajfur, J.; Pasternok, M.; Rajfur, K.; Walewicz, K.; Fras, B.; Bolach, B.; Dymarek, R.; Rosinczuk, J.; Halski, T.; Taradaj, J. Efficacy of Selected Electrical Therapies on Chronic Low Back Pain: A Comparative Clinical Pilot Study. Med. Sci. Monit. 2017, 23, 85–100. [Google Scholar] [CrossRef]
- Thiese, M.S.; Hughes, M.; Biggs, J. Electrical stimulation for chronic non-specific low back pain in a working-age population: A 12-week double blinded randomized controlled trial. BMC Musculoskelet. Disord 2013, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Paladini, J.H.; Korelo, R.G.; Liebano, R.E.; Brandt d Macedo, A.C. Effects of the Combination of Interferential Therapy Parameters on Chronic Low Back Pain: A Randomized Controlled Trial. Pain Pract. 2020, 20, 615–625. [Google Scholar] [CrossRef]
- Martín, J.M.; Cabello, M.A.; Maldonado, G.D. Estudio piloto del dolor lumbar tratado con corrientes interferenciales [Pilot study on low back pain treated with interferential currents]. Fisioterapia 2011, 33, 243–247. [Google Scholar] [CrossRef]
- Fuentes, J.; Armijo-Olivo, S.; Funabashi, M. Enhanced therapeutic alliance modulates pain intensity and muscle pain sensitivity in patients with chronic low back pain: An experimental controlled study. Phys. Ther. 2014, 94, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Bogdány, T.; Boros, S.; Szemerszky, R.; Köteles, F. Validation of the Firstbeat TeamBelt and BodyGuard2 systems. Magy. Sporttudományi Szle. 2016, 17, 5–12. [Google Scholar]
- Brennan, M.; Palaniswami, M.; Kamen, P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans. Biomed. Eng. 2001, 48, 134–137. [Google Scholar] [CrossRef]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Rouillon, J.D.; Regnard, J. Quantitative Poincaré plot analysis of heart rate variability: Effect of endurance training. Eur. J. Appl. Physiol. 2004, 91, 79–87. [Google Scholar] [CrossRef]
- Hoshi, R.A.; Pastre, C.M.; Vanderlei, L.C.; Godoy, M.F. Poincaré plot indexes of heart rate variability: Relationships with other nonlinear variables. Auton. Neurosci. 2013, 177, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.; De la Cruz, B.; Sarabia, E.; De Hoyo, M.; Domínguez-Cobo, S. Two new indexes for the assessment of autonomic balance in elite soccer players. Int. J. Sports Physiol. Perform. 2015, 10, 452–457. [Google Scholar]
- Kelley, K.; Preacher, K.J. On effect size. Psych. Methods 2012, 17, 137–152. [Google Scholar] [CrossRef]
- Anderson, B.; Nielsen, A.; McKee, D.; Jeffres, A.; Kligler, B. Acupuncture and heart rate variability:a systems level approach to understanding mechanism. Explore 2012, 8, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Telles, S.; Sharma, S.K.; Gupta, R.K.; Bhardwaj, A.K.; Balkrishna, A. Heart rate variability in chronic low back pain patients randomized to yoga or standard care. BMC Complement Altern. Med. 2016, 16, 279. [Google Scholar] [CrossRef] [PubMed]
- Naumann, J.; Grebe, J.; Kaifel, S.; Weinert, T.; Sadaghiani, C.; Huber, R. Effects of hyperthermic baths on depression, sleep and heart rate variability in patients with depressive disorder: A randomized clinical pilot trial. BMC Complement. Altern. Med. 2017, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; De la Cruz, B.; Medina, M.; Garrido, M.A.; Naranjo, J. Heart rate variability after three badminton matches. Are there gender differences? Arch. Med. Deporte 2011, XXVIII, 257–264. [Google Scholar]
- Chuang, C.-Y.; Tsai, C.-N.; Kao, M.-T.; Huang, S.H. Effects of massage therapy intervention on autonomic nervous system promotion in integrated circuit design company employees. IFMBE Proc. 2014, 43, 562–564. [Google Scholar]
- Tbuttagat, V.; Eungpinichpong, W.; Chatchawan, U.; Kharmwan, S. The immediate effects of traditional Thai massage on heart rate variability and stress-related parameters in patients with back pain associated with myofascial trigger points. J. Bodyw Mov. Ther. 2011, 15, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Girsberger, W.; Bänziger, U.; Lingg, G.; Lothaller, H.; Endler, P.C. Heart rate variability and the influence of craniosacral therapy on autonomous nervous system regulation in persons with subjective disconforts: A pilot study. J. Integr. Med. 2014, 12, 156–161. [Google Scholar] [CrossRef]
- Huang, H.; Zhong, Z.; Chen, J.; Huang, Y.; Luo, J.; Wu, J.; Liao, H.; Zhen, E.; Lin, R.; Fasmer, O.B.; et al. Effect of acupuncture at HT7 on heart rate variability: An exploratory study. Acupunct. Med. 2015, 33, 30–35. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, B.; Albornoz, M.; García, P.; Naranjo, J. Autonomic responses to ultrasound-guided percutaneous needle electrolysis of the patellar tendon in healthy male footballers. Acupunct. Med. 2016, 34, 275–279. [Google Scholar] [CrossRef]
- García, P.; De la Cruz, B.; Naranjo, J.; Albornoz, M. Autonomic activity in women during percutaneous needle electrolysis. Eur. J. Integr. Med. 2017, 11, 53–58. [Google Scholar] [CrossRef]
- Wälchli, C.; Saltzwedel, G.; Krüerke, D.; Kaufmann, C.; Schnorr, B.; Rist, L.; Eberhard, J.; Decker, M.; Simões-Wüst, A.P. Physiologic effects of rhythmical massage: A prospective exploratory cohort study. J. Altern. Complement. Med. 2014, 20, 507–515. [Google Scholar] [CrossRef]
- Hideaki, W.; Tatsuya, H.; Shogo, M.; Naruto, Y.; Hideaki, T.; Yoichi, M.; Yoshihiro, O.; Kazuo, U.; Hidenori, T. Effect of 100 Hz electroacupuncture on salivary immunoglobulin A and the autonomic nervous system. Acupunct. Med. 2015, 33, 451–456. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
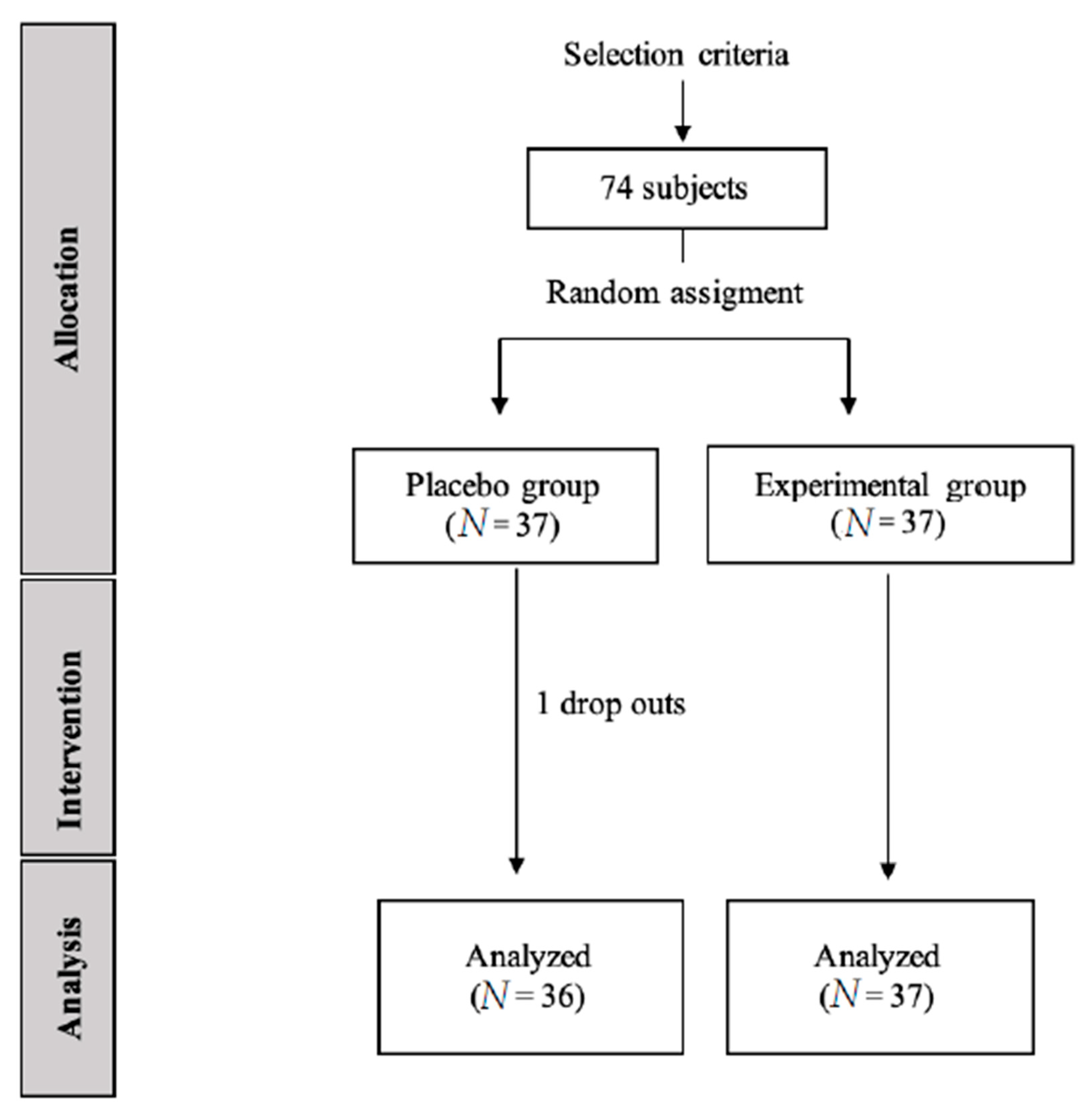

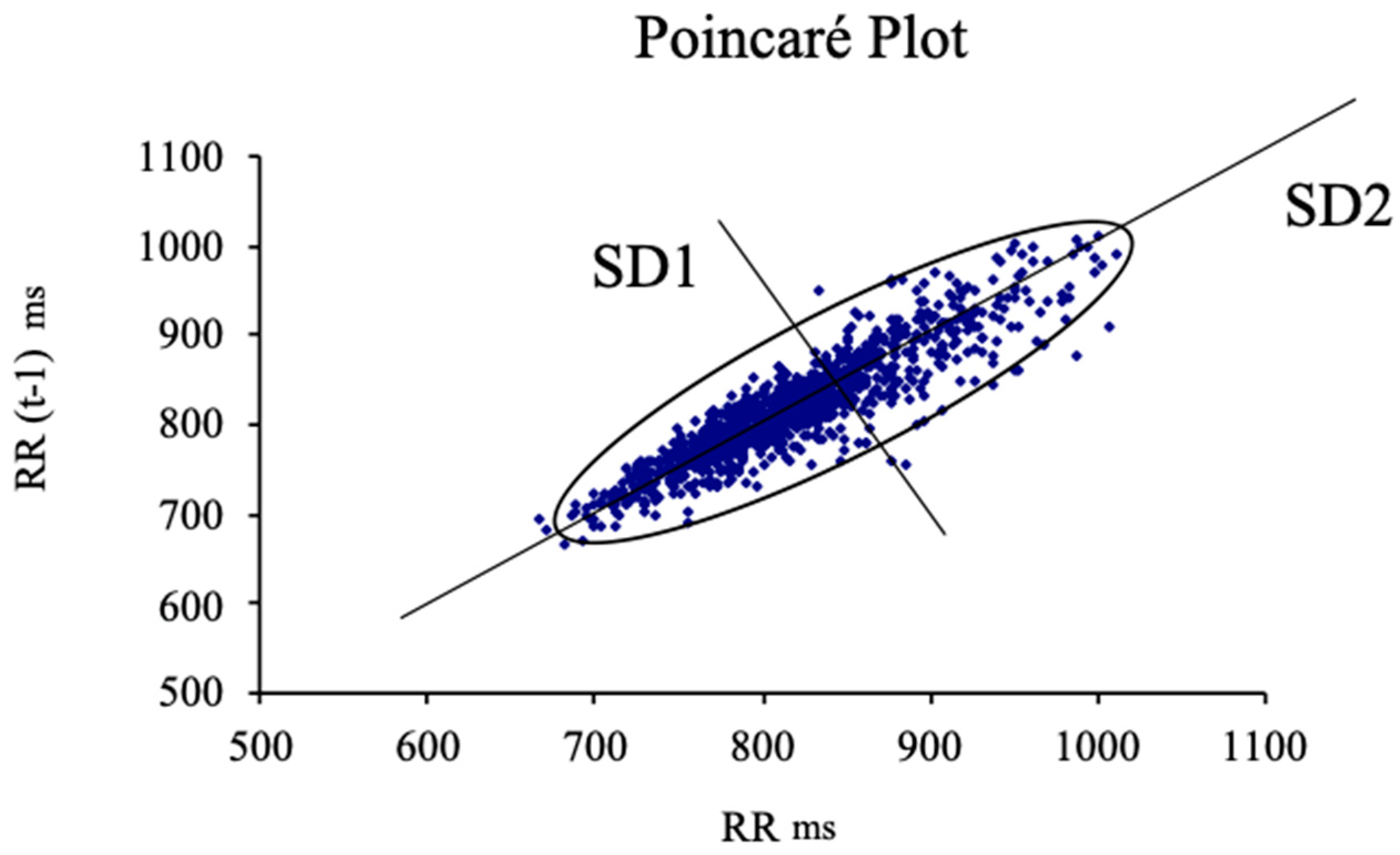
| Total Sample (N = 73) | IFC Group (n = 37) | Control Group (n = 36) | p-Value * | |
|---|---|---|---|---|
| Mean age (years) | 21 (5.12) | 22 (3.57) | 20 (2.83) | 0.16 |
| Height (cm) | 178.22 (6.66) | 178.97 (7.69) | 177.44 (5.4) | 0.33 |
| Body Mass (Kg) | 73.36 (9.84) | 75.76 (11.11) | 70.89 (7.73) | 0.07 |
| Body Mass Index (Kg/m2) | 23.06 (2.68) | 23.64 (3.22) | 22.47 (1.83) | 0.16 |
| Gender (F/M) | 73 (51/22) | 37 (25/12) | 36 (26/10) | n/a |
| PPAS | 24.75 (4.80) | 23.51 (4.15) | 23.97 (5.17) | 0.14 |
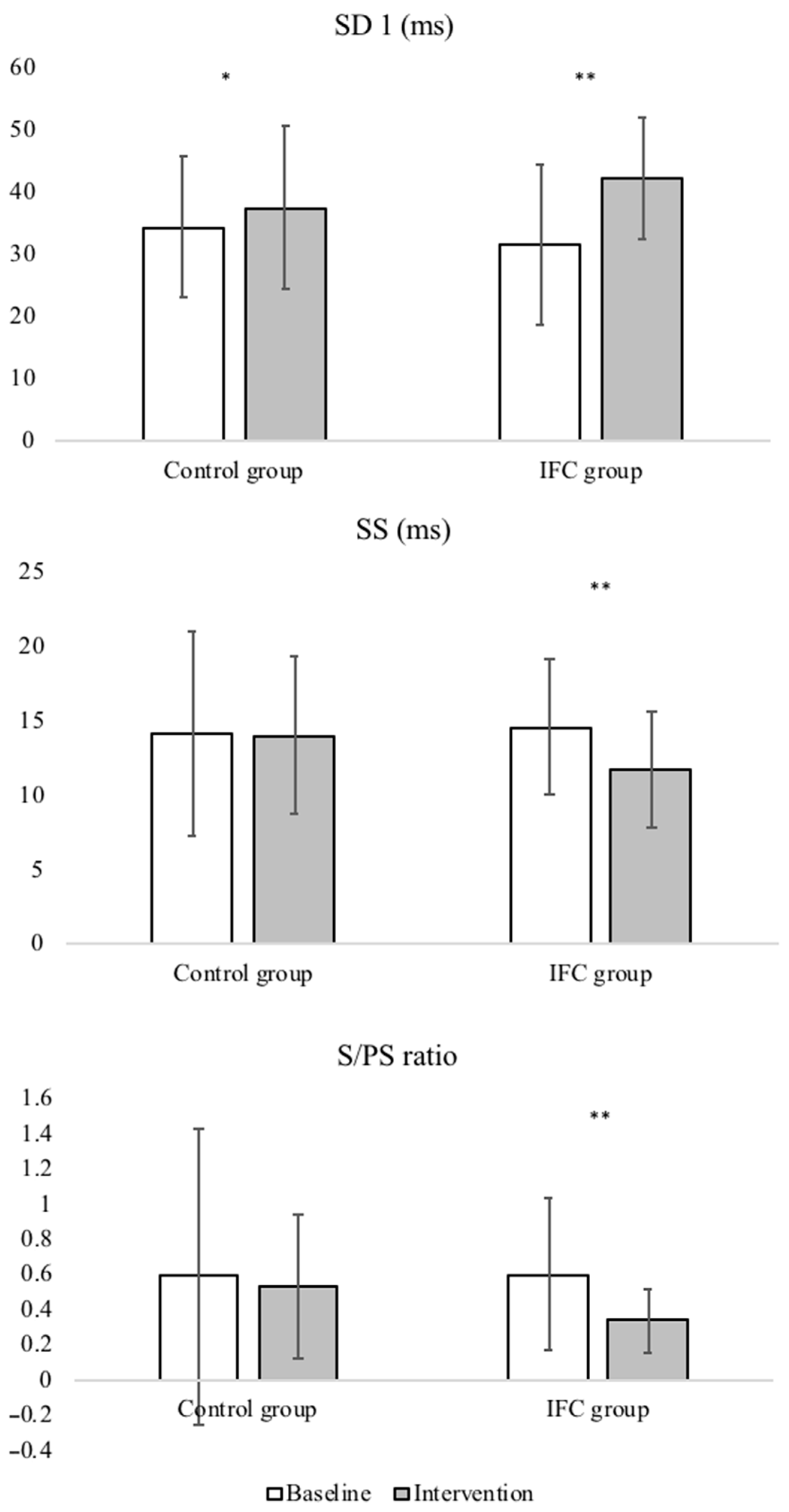
| Variable | Group | Baseline | Intervention | Within-Group Mean Changes | Between-Groups Mean Changes |
|---|---|---|---|---|---|
| SD1 (ms) | Control group | 34.36 (30.49/38.22) | 37.49 (30.49/38.22) | 3.13 (5.97/0.29) * | 4.63 (0.75/10.02) |
| IFC group | 31.57 (27.32/35.81) | 42.13 (33.05/41.93) | 10.56 (13.24/7.88) ** | ||
| SD2 (ms) | Control group | 82.96 (72.71/93.21) | 89.41 (83.41/95.41) | 6.45 (0.35/13.26) | 14.26 (2.83/25.7) † |
| IFC group | 76.82 (68.88/84.76) | 103.68 (100.48/106.88) | 26.86 (16.9/36.82) ** | ||
| SS (ms) | Control group | 14.13 (11.79/16.48) | 14.46 (12.67/16.26) | 0.33 (1.98/1.32) | 2.72 (0.54/4.90) † |
| IFC group | 14.62 (13.10/16.14) | 11.74 (10.42/13.06) | 2.87 (1.36/4.38) ** | ||
| Ratio S/PS | Control group | 0.59 (0.31/0.88) | 0.47 (0.32/0.62) | 0.06 (−0.12/0.25) | 0.19 (0.04/0.34) † |
| IFC group | 0.60 (0.46/0.75) | 0.90 (0.67/01.14) | 0.26 (0.13/0.39) ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De-la-Cruz-Torres, B.; Martínez-Jiménez, E.; Navarro-Flores, E.; Palomo-López, P.; Abuín-Porras, V.; Díaz-Meco-Conde, R.; López-López, D.; Romero-Morales, C. Heart Rate Variability Monitoring during Interferential Current Application in the Lower Back Area: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3394. https://doi.org/10.3390/ijerph18073394
De-la-Cruz-Torres B, Martínez-Jiménez E, Navarro-Flores E, Palomo-López P, Abuín-Porras V, Díaz-Meco-Conde R, López-López D, Romero-Morales C. Heart Rate Variability Monitoring during Interferential Current Application in the Lower Back Area: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2021; 18(7):3394. https://doi.org/10.3390/ijerph18073394
Chicago/Turabian StyleDe-la-Cruz-Torres, Blanca, Eva Martínez-Jiménez, Emmanuel Navarro-Flores, Patricia Palomo-López, Vanesa Abuín-Porras, Raquel Díaz-Meco-Conde, Daniel López-López, and Carlos Romero-Morales. 2021. "Heart Rate Variability Monitoring during Interferential Current Application in the Lower Back Area: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 18, no. 7: 3394. https://doi.org/10.3390/ijerph18073394
APA StyleDe-la-Cruz-Torres, B., Martínez-Jiménez, E., Navarro-Flores, E., Palomo-López, P., Abuín-Porras, V., Díaz-Meco-Conde, R., López-López, D., & Romero-Morales, C. (2021). Heart Rate Variability Monitoring during Interferential Current Application in the Lower Back Area: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 18(7), 3394. https://doi.org/10.3390/ijerph18073394










