Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Procedure
2.3. Measures
2.3.1. WHO-5 Well-Being Test
2.3.2. Hospital Anxiety and Depression Scale
2.3.3. A Questionnaire on General Data, Behavioral Factors, and Self-Perceived Health
2.3.4. Objective Investigation
2.3.5. Weight, Height, and Waist Circumference Measurement
2.3.6. Rose’s Questionnaire
2.3.7. Laboratory Tests
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Psychoemotional State
3.3. Cardiovascular Risk Factors
3.4. Thyroid Biomarkers
3.5. Subject Groups According to Symptoms of Metabolic Syndrome
3.6. Thyroid Parameters Divided into Quartiles
3.7. HOMA-IR Cut-Offs for Diagnosing T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.; Aquilina, A.; Vassallo, J. Bisphenol A and Type 2 Diabetes Mellitus: A Review of Epidemiologic, Functional, and Early Life Factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef] [PubMed]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E.R. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2017, 15, 30–39. [Google Scholar] [CrossRef]
- Hettihewa, L.M.; Palangasinghe, S.; Jayasinghe, S.S.; Gunasekara, S.W.; Weerarathna, T.P. Comparison of Insulin Resistance by Indirect Methods—HOMA, QUICKI and McAuley—With Fasting Insulin in Patients with Type 2 Diabetes in Galle, Sri Lanka: A Pilot Study. Online J. Health Allied Sci. 2006, 5, 1–8. [Google Scholar]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Abdi, H.; Kazemian, E.; Gharibzadeh, S.; Amouzegar, A.; Mehran, L.; Tohidi, M.; Rashvandi, Z.; Azizi, F. Association between Thyroid Function and Body Mass Index: A 10-Year Follow-Up. Ann. Nutr. Metab. 2017, 70, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Jiang, X.; Zhang, X.; Guan, Q.; Yu, C.; Li, Y.; Gao, L.; Zhang, H.; Zhao, J. Association between thyroid hormones and body fat in euthyroid subjects. Clin. Endocrinol. 2014, 80, 585–590. [Google Scholar] [CrossRef]
- Salvatore, D.; Simonides, W.S.; Dentice, M.; Zavacki, A.M.; Larsen, P.R. Thyroid hormones and skeletal muscle--new insights and potential implications. Nature reviews. Endocrinology 2014, 10, 206–214. [Google Scholar] [CrossRef]
- Reinehr, T. Obesity and thyroid function. Mol. Cell. Endocrinol. 2010, 316, 165–171. [Google Scholar] [CrossRef]
- Fontenelle, L.C.; Feitosa, M.M.; Severo, J.S.; Freitas, T.E.; Morais, J.B.; Torres-Leal, F.L.; Henriques, G.S.; do Nascimento Marreiro, D. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm. Metab. Res. 2016, 48, 787–794. [Google Scholar] [CrossRef]
- Roef, G.; Lapauw, B.; Goemaere, S.; Zmierczak, H.G.; Toye, K.; Kaufman, J.M.; Taes, Y. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur. J. Endocrinol. 2012, 167, 719–726. [Google Scholar] [CrossRef]
- Kwon, H.; Cho, J.H.; Lee, D.Y.; Park, S.E.; Park, C.Y.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Rhee, E.J. Association between thyroid hormone levels, body composition and insulin resistance in euthyroid subjects with normal thyroid ultrasound: The Kangbuk Samsung Health Study. Clin. Endocrinol. 2018, 89, 649–655. [Google Scholar] [CrossRef]
- Schmitz, N.; Deschênes, S.S.; Burns, R.J.; Smith, K.J.; Lesage, A.; Strychar, I.; Rabasa-Lhoret, R.; Freitas, C.; Graham, E.; Awadalla, P.; et al. Depression and risk of type 2 diabetes: The potential role of metabolic factors. Mol. Psychiatry 2016, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Adriaanse, M.C.; Dekker, J.M.; Nijpels, G.; Heine, R.J.; Snoek, F.J.; Pouwer, F. Associations between depressive symptoms and insulin resistance: The Hoorn Study. Diabetologia 2006, 49, 2874–2877. [Google Scholar] [CrossRef]
- Khambaty, T.; Stewart, J.C.; Muldoon, M.F.; Kamarck, T.W. Depressive symptom clusters as predictors of 6-year increases in insulin resistance: Data from the Pittsburgh Healthy Heart Project. Psychosom. Med. 2014, 76, 363–369. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Moller, D.E.; Flier, J.S. Insulin resistance--mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. 2), S135–S148. [Google Scholar] [CrossRef]
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical Prevalence of Polycystic Ovary Syndrome as Determined by Region and Race/Ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- WHO. World Health Organization: Regional Office for Europe Well-Being measures in primary health care: The DepCare Project. In Proceedings of the Consensus Meeting, Stockholm, Sweden, 12–13 February 1998. [Google Scholar]
- Rose, G.A.; Blackburn, H.; Gillum, R.F.; Prineas, R.J. Cardiovascular Survey Methods; Monograph Series; World Health Organisation: Geneva, Switzerland, 1982; Volume 56, pp. 162–165. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit. Pathw. Cardiol. 2005, 4, 198–203. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Grabauskas, V.; Petkevičienė, J.; Šakalytė, E.; Kriaučionienė, V.; Veryga, A. Suaugusių Lietuvos žmonių gyvensenos tyrimas, 2011 = Health Behaviour among Lithuanian Adult Population, 2011; Kaunas LSMU: Kaunas, Lithuania, 2011. [Google Scholar]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef] [PubMed]
- Djukanovic, I.; Carlsson, J.; Årestedt, K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65–80 years old? A psychometric evaluation study. Health Qual. Life Outcomes 2017, 15, 193. [Google Scholar] [CrossRef]
- McPherson, A.; Martin, C.R. Is the Hospital Anxiety and Depression Scale (HADS) an appropriate screening tool for use in an alcohol-dependent population? J. Clin. Nurs. 2011, 20, 1507–1517. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Ben-Shlomo, Y.; Ebrahim, S.; Davey Smith, G.; Stansfeld, S.A.; Yarnell, J.W.; Gallacher, J.E. Insulin resistance and depressive symptoms in middle aged men: Findings from the Caerphilly prospective cohort study. BMJ (Clin. Res.) 2005, 330, 705–706. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Smith, G.D.; Ebrahim, S. Association of insulin resistance with depression: Cross sectional findings from the British Women’s Heart and Health Study. BMJ (Clin. Res.) 2003, 327, 1383–1384. [Google Scholar] [CrossRef]
- Paneni, F.; Costantino, S.; Cosentino, F. Insulin resistance, diabetes, and cardiovascular risk. Curr. Atheroscler. Rep. 2014, 16, 419. [Google Scholar] [CrossRef]
- Nóvoa, F.J.; Boronat, M.; Saavedra, P.; Díaz-Cremades, J.M.; Varillas, V.F.; La Roche, F.; Alberiche, M.P.; Carrillo, A. Differences in cardiovascular risk factors, insulin resistance, and insulin secretion in individuals with normal glucose tolerance and in subjects with impaired glucose regulation: The Telde Study. Diabetes Care 2005, 28, 2388–2393. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Farb, M.G.; Tiwari, S.; Karki, S.; Hamburg, N.M.; Vita, J.A.; Hess, D.T.; Lavalley, M.P.; Apovian, C.M.; Gokce, N. Insulin status and vascular responses to weight loss in obesity. J. Am. Coll. Cardiol. 2013, 62, 2297–2305. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Saito, T.; Ogihara, T.; Ishigaki, Y.; Yamada, T.; Imai, J.; Uno, K.; Gao, J.; Kaneko, K.; Shimosawa, T.; et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 2012, 125, 1122–1133. [Google Scholar] [CrossRef]
- Li, Q.; Park, K.; Li, C.; Rask-Madsen, C.; Mima, A.; Qi, W.; Mizutani, K.; Huang, P.; King, G.L. Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-β isoform in the endothelium. Circ. Res. 2013, 113, 418–427. [Google Scholar] [CrossRef]
- Saely, C.H.; Aczel, S.; Marte, T.; Langer, P.; Hoefle, G.; Drexel, H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J. Clin. Endocrinol. Metab. 2005, 90, 5698–5703. [Google Scholar] [CrossRef]
- Ambrosi, B.; Masserini, B.; Iorio, L.; Delnevo, A.; Malavazos, A.E.; Morricone, L.; Sburlati, L.F.; Orsi, E. Relationship of thyroid function with body mass index and insulin-resistance in euthyroid obese subjects. J. Endocrinol. Investig. 2010, 33, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Hua, S.C. High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker. J. Clin. Med. 2019, 8, 817. [Google Scholar] [CrossRef]
- Garduño-Garcia Jde, J.; Alvirde-Garcia, U.; López-Carrasco, G.; Padilla Mendoza, M.E.; Mehta, R.; Arellano-Campos, O.; Choza, R.; Sauque, L.; Garay-Sevilla, M.E.; Malacara, J.M.; et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur. J. Endocrinol. 2010, 163, 273–278. [Google Scholar] [CrossRef]
- Mousa, U.; Bozkuş, Y.; Kut, A.; Demir, C.C.; Tutuncu, N.B. Fat Distribution and Metabolic Profile in Subjects with Hashimoto’s Thyroiditis. Acta Endocrinol. (Buchar. Rom. 2005) 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Ruhla, S.; Weickert, M.O.; Arafat, A.M.; Osterhoff, M.; Isken, F.; Spranger, J.; Schöfl, C.; Pfeiffer, A.F.; Möhlig, M. A high normal TSH is associated with the metabolic syndrome. Clin. Endocrinol. 2010, 72, 696–701. [Google Scholar] [CrossRef]
- Jayanthi, R.; Srinivasan, A.R.; Hanifah, M.; Maran, A.L. Associations among Insulin Resistance, Triacylglycerol/High Density Lipoprotein (TAG/HDL ratio) and Thyroid hormone levels-A study on Type 2 diabetes mellitus in obese and overweight subjects. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S121–S126. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Y.; Fu, J.; Wang, G. Association BETWEEN Thyroid Hormones, Thyroid Antibodies, and Cardiometabolic Factors in Non-Obese Individuals with Normal Thyroid Function. Front. In Endocrinol. 2018, 9, 130. [Google Scholar] [CrossRef]
- Ferrannini, E.; Iervasi, G.; Cobb, J.; Ndreu, R.; Nannipieri, M. Insulin resistance and normal thyroid hormone levels: Prospective study and metabolomic analysis. American journal of physiology. Endocrinol. Metab. 2017, 312, E429–E436. [Google Scholar] [CrossRef]
- Raposo, L.; Martins, S.; Ferreira, D.; Guimarães, J.T.; Santos, A.C. Metabolic Syndrome, Thyroid Function and Autoimmunity—The PORMETS Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 75–83. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yu, T.Y.; Shih, S.R.; Huang, K.C.; Chang, T.C. Low total and free triiodothyronine levels are associated with insulin resistance in non-diabetic individuals. Sci. Rep. 2018, 8, 10685. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, J.; Jiang, F.; Wang, B.; Chen, Y.; Li, M.; Liu, H.; Li, C.; Xue, H.; Li, N.; et al. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr. J. 2011, 58, 23–30. [Google Scholar] [CrossRef]
- Nozarian, Z.; Abdollahi, A.; Mehrtash, V.; Nasiri Bonaki, H. Upper Normal Limit of Thyroid-Stimulating Hormone and Metabolic Syndrome in Iranian Patients with Obesity. Iran. J. Pathol. 2017, 12, 88–93. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Fiorillo, E.; Lakatta, E.G.; Schlessinger, D.; Cucca, F. Role of Adipokines in the Association between Thyroid Hormone and Components of the Metabolic Syndrome. J. Clin. Med. 2019, 8, 764. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, B.H. The Relationship between Thyroid Function and Different Obesity Phenotypes in Korean Euthyroid Adults. Diabetes Metab. J. 2019, 43, 867–878. [Google Scholar] [CrossRef]
- Mehran, L.; Amouzegar, A.; Rahimabad, P.K.; Tohidi, M.; Tahmasebinejad, Z.; Azizi, F. Thyroid Function and Metabolic Syndrome: A Population-Based Thyroid Study. Horm. Metab. Res. 2017, 49, 192–200. [Google Scholar] [CrossRef]
- Shinkov, A.; Borissova, A.M.; Kovatcheva, R.; Atanassova, I.; Vlahov, J.; Dakovska, L. The prevalence of the metabolic syndrome increases through the quartiles of thyroid stimulating hormone in a population-based sample of euthyroid subjects. Arq. Bras. Endocrinol. Metabol. 2014, 58, 926–932. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Fang, W.H.; Kao, T.W.; Wang, C.C.; Chang, Y.W.; Peng, T.C.; Wu, C.J.; Yang, H.F.; Chan, J.Y.; Chen, W.L. Exploring the association between thyroid- stimulating hormone and metabolic syndrome: A large population-based study. PLoS ONE 2018, 13, e0199209. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, K.J.; Cho, Y. Body Mass Index Is Associated with Hypercholesterolemia following Thyroid Hormone Withdrawal in Thyroidectomized Patients. Int. J. Endocrinol. 2014, 2014, 649016. [Google Scholar] [CrossRef] [PubMed]
- Tarcin, O.; Abanonu, G.B.; Yazici, D.; Tarcin, O. Association of metabolic syndrome parameters with TT3 and FT3/FT4 ratio in obese Turkish population. Metab. Syndr. Relat. Disord. 2012, 10, 137–142. [Google Scholar] [CrossRef]
- Amouzegar, A.; Kazemian, E.; Abdi, H.; Mansournia, M.A.; Bakhtiyari, M.; Hosseini, M.S.; Azizi, F. Association Between Thyroid Function and Development of Different Obesity Phenotypes in Euthyroid Adults: A Nine-Year Follow-Up. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 458–464. [Google Scholar] [CrossRef]
- Udenze, I.; Nnaji, I.; Oshodi, T. Thyroid function in adult Nigerians with metabolic syndrome. Pan Afr. Med. J. 2014, 18, 352. [Google Scholar] [CrossRef]
- Wolffenbuttel, B.H.R.; Wouters, H.; Slagter, S.N.; van Waateringe, R.P.; Muller Kobold, A.C.; van Vliet-Ostaptchouk, J.V.; Links, T.P.; van der Klauw, M.M. Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocr. Disord. 2017, 17, 65. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef]
- Horáková, D.; Štěpánek, L.; Janout, V.; Janoutová, J.; Pastucha, D.; Kollárová, H.; Petráková, A.; Štěpánek, L.; Husár, R.; Martiník, K. Optimal Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) Cut-Offs: A Cross-Sectional Study in the Czech Population. Medicina 2019, 55, 158. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shih, A.Z.; Woo, Y.C.; Fong, C.H.; Leung, O.Y.; Janus, E.; Cheung, B.M.; Lam, K.S. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A 15-Year Prospective Study in Chinese. PLoS ONE 2016, 11, e0163424. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | Total (n = 835) | Without-IR Group | With-IR Group | p-Value |
|---|---|---|---|---|
| HOMA-IR ≤ 2.7, (n = 557) | HOMA-IR > 2.7, (n = 278) | |||
| Age, years; mean ± SD | 63.6 ± 10.3 | 63.0 ± 10.5 | 64.8 ± 9.7 | 0.015 |
| Gender; n (%) | 0.003 | |||
| Male | 300 (35.9) | 181 (32.5) | 119 (42.8) | |
| Female | 535 (64.1) | 376 (67.5) | 159 (57.2) | |
| Marital status; n (%) | 0.533 | |||
| Married | 557 (66.7) | 376 (67.5) | 181 (65.1) | |
| Alone | 278 (33.3) | 181 (32.5) | 97 (34.9) | |
| Education; n (%) | 0.001 | |||
| Lower than higher | 565 (67.7) | 351 (63.0) | 214 (77.0) | |
| Higher | 270 (32.3) | 206 (37.0) | 64 (23.0) | |
| Employment; n (%) | 0.142 | |||
| Employed | 400 (47.9) | 277 (49.7) | 123 (44.2) | |
| No employed | 435 (52.1) | 280 (50.3) | 155 (55.8) |
| Psychoemotional State Characteristics | Total (n = 835) | Without-IR Group | With-IR Group | p-Value |
|---|---|---|---|---|
| HOMA-IR ≤ 2.7, (n = 557) | HOMA-IR > 2.7, (n = 278) | |||
| WHO-5 scores; mean ± SD | 55.9 ± 18.2 | 55.8 ± 18.3 | 55.9 ± 18.2 | 0.942 |
| WHO-5 with bad possible quality of life; n (%) | 276 (33.1) | 186 (33.4) | 90 (32.4) | 0.815 |
| HADS-A scores; median (IQR) | 6.0 (3.0–9.0) | 6.0 (3.0–9.0) | 6.0 (3.0–9.0) | 0.810 |
| HADS-D scores, median (IQR) | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) | 4.5 (2.0–7.0) | 0.291 |
| HADS-A ≥ 8; n (%) | 300 (36.1) | 202 (36.4) | 98 (35.4) | 0.818 |
| HADS-D ≥ 8; n (%) | 170 (20.4) | 104 (18.7) | 66 (23.8) | 0.100 |
| HADS with anxiety and depression ≥ 8; n (%) | 124 (14.9) | 81 (14.5) | 43 (15.5) | 0.757 |
| Cardiovascular Risk Factors Characteristics | Total (n = 835) | Without-IR Group | With-IR GROUP | p-Value |
|---|---|---|---|---|
| HOMA-IR ≤ 2.7, (n = 557) | HOMA-IR > 2.7, (n = 278) | |||
| Systolic blood pressure, mm Hg; mean ± SD | 129.5 ± 12.9 | 128.3 ± 12.9 | 132.2 ± 12.5 | <0.001 |
| Diastolic blood pressure, mm Hg; mean ± SD | 74.6 ± 7.3 | 74.0 ± 7.2 | 75.7 ± 7.4 | 0.002 |
| Presence of Disease; n (%) | 0.001 | |||
| Without AH and CAD | 159 (19.0) | 122 (21.9) | 37 (13.3) | |
| AH | 398 (47.7) | 256 (46.0) | 142 (51.1) | |
| CAD | 43 (5.1) | 35 (6.3) | 8 (2.9) | |
| With AH and CAD | 235 (28.1) | 144 (25.9) | 91 (32.9) | |
| Rose questionnaire: angina pectoris; n (%) | 88 (10.5) | 51 (9.2) | 37 (13.3) | 0.065 |
| BMI, kg/m2; mean ± SD | 28.1 ± 4.8 | 26.6 ± 4.1 | 31.0 ± 4.7 | <0.001 |
| BMI ≥30; n (%) | 261 (31.3) | 99 (17.8) | 162 (58.3) | <0.001 |
| Waist circumference men’s, cm; mean ± SD | 98.8 ± 11.2 | 93.9 ± 9.8 | 104.8 ± 9.8 | <0.001 |
| Waist circumference women’s, cm; mean ± SD | 88.7 ± 12.7 | 84.6 ± 10.6 | 98.5 ± 11.7 | <0.001 |
| Low physical activity; n (%) | 239 (29.7) | 145 (26.9) | 94 (35.2) | 0.015 |
| Total cholesterol, mmol/L; mean ± SD | 6.0 ± 1.2 | 6.0 ± 1.1 | 6.0 ± 1.2 | 0.778 |
| LDL, mmol/L; mean ± SD | 3.8 ± 1.1 | 3.8 ± 1.0 | 3.9 ± 1.1 | 0.356 |
| HDL men’s, mmol/L; mean ± SD | 1.5 ± 0.43 | 1.6 ± 0.43 | 1.3 ± 0.37 | <0.001 |
| HDL women’s, mmol/L; mean ± SD | 1.78 ± 0.48 | 1.9 ± 0.46 | 1.6 ± 0.47 | <0.001 |
| Triglyceride, mmol/L; mean ± SD | 1.4 ± 0.70 | 1.2 ± 0.58 | 1.7 ± 0.83 | <0.001 |
| With metabolic syndrome; n (%) | 204 (24.5) | 61 (11.0) | 143 (51.4) | <0.001 |
| Smoking regular; n (%) | 123 (14.7) | 85 (15,3) | 38 (13.7) | 0.541 |
| Type 2 diabetes mellitus; n (%) | 62 (7.4) | 17 (3.1) | 45 (16.2) | <0.001 |
| Fasting glucose, mmol/L; mean ± SD | 5.4 ± 1.1 | 5.1 ± 0.47 | 6.1 ± 1.7 | <0.001 |
| Fasting glucose ≥ 6.1; n (%) | 95 (11.4) | 8 (1.4) | 87 (31.3) | <0.001 |
| Fasting insulin, mU/L; mean ± SD | 5.4 ± 1.1 | 7.4 ± 2.4 | 16.9 ± 6.8 | <0.001 |
| Thyroid Biomarkers Characteristics | Total (n = 835) | Without-IR Group | With-IR Group | p-Value |
|---|---|---|---|---|
| HOMA-IR ≤ 2.7, (n = 557) | HOMA-IR > 2.7, (n = 278) | |||
| TSH, mIU/L; median (IQR) | 2.0 (1.4–3.1) | 2.1 (1.4–3.2) | 2.0 (1.3–3.0) | 0.338 |
| TSH; n (%) | 0.494 | |||
| <0.55 | 25 (3.0) | 14 (2.5) | 11 (4.0) | |
| 0.55–4.78 | 740 (88.6) | 495 (88.9) | 245 (88.1) | |
| >4.78 | 70 (8.4) | 48 (8.6) | 22 (7.9) | |
| Anti-TPO, U/mL; median (IQR) | 51.8 (44.2–62.7) | 50.9 (43.6–63.7) | 53.5 (45.5–62.3) | 0.316 |
| Anti-TPO; n (%) | 0.676 | |||
| 0–60 | 596 (71.4) | 395 (70.9) | 201 (72.3) | |
| >60 | 239 (28.6) | 162 (29.1) | 77 (27.7) | |
| FT3, pmol/L; (mean ± SD) | 5.2 ± 0.66 | 5.2 ± 0.62 | 5.2 ± 0.74 | 0.431 |
| FT3; n (%) | 0.870 | |||
| ≤6.5 | 815 (97.6) | 544 (97.7) | 271 (97.5) | |
| >6.5 | 20 (2.5) | 13 (2.3) | 7 (2.5) | |
| FT4, pmol/L; (mean ± SD) | 15.5 ± 2.1 | 15.4 ± 2.1 | 15.6 ± 2.0 | 0.348 |
| FT4, n (%) | 0.870 | |||
| 11.5–22.7 | 835 (100) | 557 (100) | 278 (100) |
| Parameter | All | Without MetS, n = 631 | With MetS, n = 204 | p |
|---|---|---|---|---|
| TSH, mIU/L; median (IQR) | 2.0 (1.4–3.1) | 2.0 (1.3–3.0) | 2.3 (1.5–3.3) | 0.049 |
| TSH; n (%) | 0.339 | |||
| <0.55 | 25 (3.0) | 21 (3.3) | 4 (2.0) | |
| 0.55–4.78 | 740 (88.6) | 561 (88.9) | 179 (87.7) | |
| >4.78 | 70 (8.4) | 49 (7.8) | 21 (10.3) | |
| Anti-TPO, U/mL; median (IQR) | 51.8 (44.2–62.7) | 51.3 (43.8–62.2) | 53.1 (45.4–63.9) | 0.235 |
| Anti-TPO; n (%) | 0.423 | |||
| 0–60 | 596 (71.4) | 455 (72.1) | 141 (69.1) | |
| >60 | 239 (28.6) | 176 (27.9) | 63 (30.9) | |
| FT3, pmol/L; (mean ± SD) | 5.2 ± 0.66 | 5.2 ± 0.65 | 5.1 ± 0.70 | 0.025 |
| FT3; n (%) | 0.870 | |||
| ≤6.5 | 815 (97.6) | 616 (97.6) | 199 (97.5) | |
| >6.5 | 20 (2.5) | 15 (2.4) | 5 (2.5) | |
| FT4, pmol/L; (mean ± SD) | 15.5 ± 2.1 | 15.5 ± 2.1 | 15.6 ± 2.0 | 0.574 |
| FT4, n (%) | 0.870 | |||
| 11.5–22.7 | 835 (100) | 631 (100) | 204 (100) | |
| FT3/FT4 | 0.34 ± 0.05 | 0.34 ± 0.05 | 0.33 ± 0.05 | 0.013 |
| HOMA_IR | 2.1 (1.5–3.1) | 1.9 (1.3–2.6) | 3.7 (2.5–5.1) | <0.001 |
| HOMA-IR > 2.7 | 278 (33.3) | 135 (21.4) | 143 (70.1) | <0.001 |
| Parameter | Q1 | Q2 | Q3 | Q4, | p | Q1:Q2 | Q1:Q3 | Q1:Q4 |
|---|---|---|---|---|---|---|---|---|
| TSH | n = 210 | n = 208 | n = 208 | n = 208 | ||||
| MetS, n (%) | 42 (20.0) | 51 (24.5) | 55 (26.4) | 56 (26.9) | 0.336 | ns | ns | ns |
| FT3 | n = 193 | n = 187 | n = 211 | n = 243 | ||||
| MetS, n (%) | 63 (32.6) | 43 (23.0) | 45 (21.3) | 53 (21.8) | 0.026 | 0.037 | 0.011 | 0.011 |
| FT4 | n = 198 | n = 205 | n = 221 | n = 210 | ||||
| MetS, n (%) | 46 (23.2) | 52 (25.4) | 51 (23.1) | 55 (26.2) | 0.844 | ns | ns | ns |
| FT3/FT4 | n = 194 | n = 214 | n = 208 | n = 218 | ||||
| MetS, n (%) | 61 (31.4) | 56 (26.2) | 37 (17.8) | 50 (22.9) | 0.013 | ns | 0.002 | 0.053 |
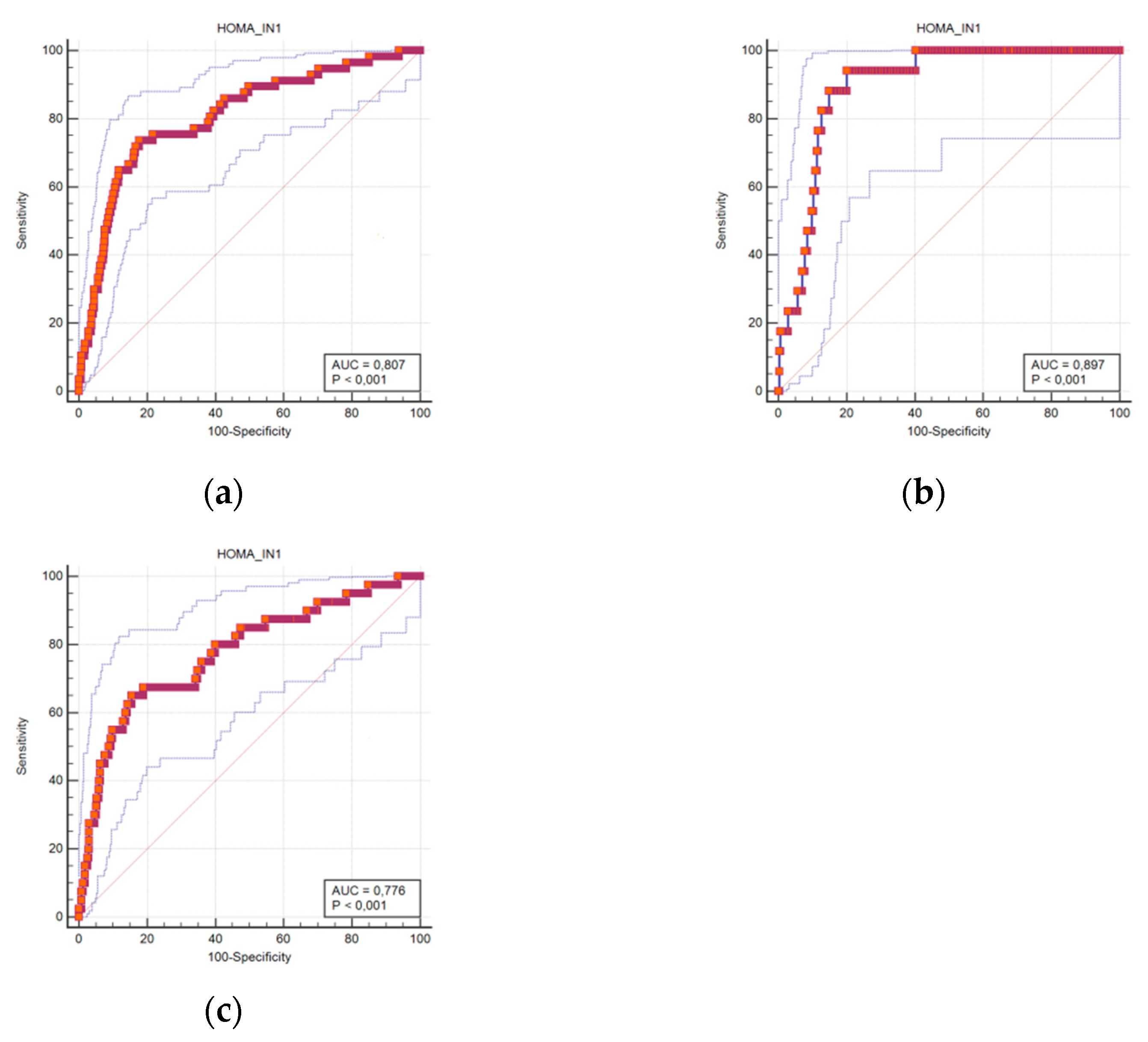
| Cut-Off | Sensitivity | Specificity | Accuracy (95%CI) | |
|---|---|---|---|---|
| HOMA-IR men + women | 3.45 | 73.7 | 82.3 | 0.807 (0.779–0.834) |
| HOMA-IR men | 3.52 | 94.1 | 79.9 | 0.897 (0.857–0.929) |
| HOMA-IR women | 3.35 | 65.0 | 84.4 | 0.776 (0.738–0.810) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazukauskiene, N.; Podlipskyte, A.; Varoneckas, G.; Mickuviene, N. Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 3388. https://doi.org/10.3390/ijerph18073388
Kazukauskiene N, Podlipskyte A, Varoneckas G, Mickuviene N. Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors. International Journal of Environmental Research and Public Health. 2021; 18(7):3388. https://doi.org/10.3390/ijerph18073388
Chicago/Turabian StyleKazukauskiene, Nijole, Aurelija Podlipskyte, Giedrius Varoneckas, and Narseta Mickuviene. 2021. "Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors" International Journal of Environmental Research and Public Health 18, no. 7: 3388. https://doi.org/10.3390/ijerph18073388
APA StyleKazukauskiene, N., Podlipskyte, A., Varoneckas, G., & Mickuviene, N. (2021). Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors. International Journal of Environmental Research and Public Health, 18(7), 3388. https://doi.org/10.3390/ijerph18073388






