Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Characterization and Sampling of Femoral Bone Samples and Determination the Elemental Metals
2.3. Radiographic Assessment
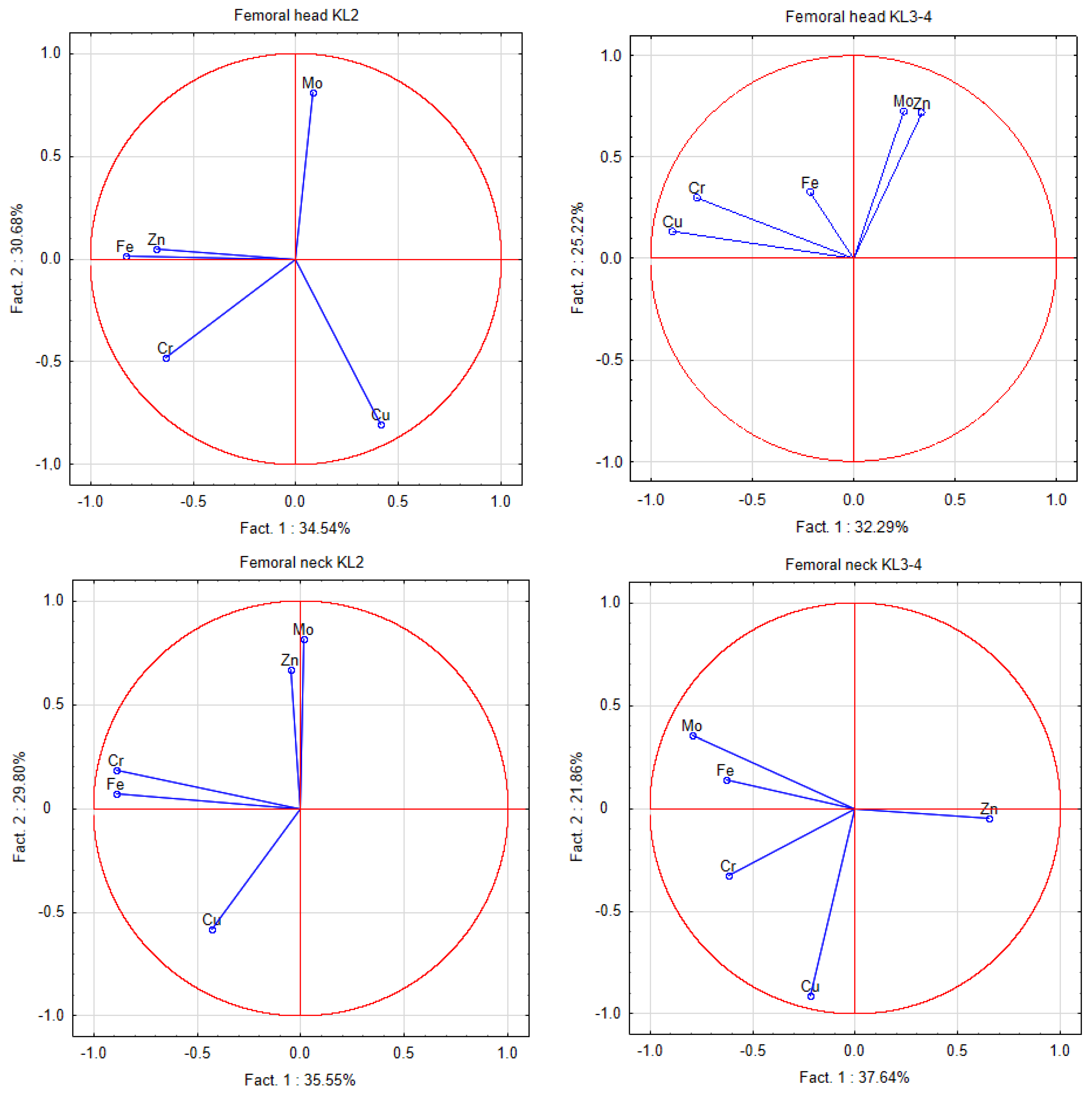
2.4. Statistical and Chemometrics Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Won, Y.; Shin, Y.; Chun, C.-H.; Cho, Y.; Ha, C.-W.; Kim, J.-H.; Chun, J.-S. Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis. Ann. Rheum. Dis. 2016, 75, 2045–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozler, K.; Aktas, E.; Atay, C.; Yilmaz, B.; Arikan, M.; Gungor, S. Serum and knee synovial fluid matrix metalloproteinase-13 and tumor necrosis factor-alpha levels in patients with late-stage osteoarthritis. Acta Orthop. Traumatol. Turc. 2016, 50, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, M.; Zioła-Frankowska, A.; Kubaszewski, Ł.; Rogala, P.; Frankowski, M. Urban and rural area differences in the interaction between oxidative process elements in human femoral bone. Environ. Sci. Pollut. Res. Int. 2018, 25, 30475–30487. [Google Scholar] [CrossRef] [PubMed]
- Zioła-Frankowska, A.; Kubaszewski, Ł.; Dąbrowski, M.; Kowalski, A.; Rogala, P.; Strzyżewski, W.; Łabędź, W.; Uklejewski, R.; Novotny, K.; Kanicky, V.; et al. The Content of the 14 Metals in Cancellous and Cortical Bone of the Hip Joint Affected by Osteoarthritis. BioMed Res. Int. 2015, 2015, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kosik-Bogacka, D.I.; Lanocha-Arendarczyk, N.; Kot, K.; Zietek, P.; Karaczun, M.; Prokopowicz, A.; Kupnicka, P.; Ciosek, Z. Calcium, magnesium, zinc and lead concentrations in the structures forming knee joint in patients with osteoarthritis. J. Trace Elem. Med. Biol. 2018, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Lanocha-Arendarczyk, N.; Kosik-Bogacka, D.I.; Prokopowicz, A.; Kalisinska, E.; Sokolowski, S.; Karaczun, M.; Zietek, P.; Podlasińska, J.; Pilarczyk, B.; Tomza-Marciniak, A.; et al. The Effect of Risk Factors on the Levels of Chemical Elements in the Tibial Plateau of Patients with Osteoarthritis following Knee Surgery. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowski, M.; Zioła-Frankowska, A.; Adamek, J.; Frankowski, M.; Kaczmarczyk, J.; Kubaszewski, Ł. Impact of the oxidative and enzymatic metals in degenerated intervertebral disc disease. Ann. Agric. Environ. Med. 2020. [Google Scholar] [CrossRef]
- Kubaszewski, L.; Ziola-Frankowska, A.; Gasik, Z.; Frankowski, M.; Dabrowski, M.; Molisak, B.; Kaczmarczyk, J.; Gasik, R. Chemometric evaluation of concentrations of trace elements in in-tervertebral disc tissue in patient with degenerative disc disease. Ann. Agric. Environ. Med. 2017, 24, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, K.A.; Papavassiliou, A.G. Redox and NF-kappaB signaling in osteoarthritis. Free Radic. Biol. Med. 2019, 132, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Zioła-Frankowska, A.; Dąbrowski, M.; Kubaszewski, Ł.; Rogala, P.; Frankowski, M. Factors affecting the aluminium content of human femoral head and neck. J. Inorg. Biochem. 2015, 152, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.M.A. Relationship between Serum Levels of Some Trace Elements, Disease Duration and Severity in Patients with Knee Osteoarthritis. Pharmacol. Pharm. 2015, 6, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Munjal, A.; Bapat, S.; Hubbard, D.; Hunter, M.; Kolhe, R.; Fulzele, S. Advances in Molecular biomarker for early diagnosis of Osteoarthritis. Biomol. Concepts 2019, 10, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Miller, R.J.; Malfait, A.M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, P.; Rojo, L.; Gonzalez-Gomez, A.; Roman, J.S. Self-assembling gradient copolymers of vinylimidazol and (acrylic)ibuprofen with anti-inflammatory and zinc chelating properties. Macromol. Biosci. 2013, 13, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial Fluid and Plasma Selenium, Copper, Zinc, and Iron Concentrations in Patients with Rheumatoid Arthritis and Osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef]
- Kraus, V.B. Osteoarthritis: The zinc link. Nature 2014, 507, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Abradelo, C.; Román, J.S.; Rojo, L. Bibliographic review on the state of the art of strontium and zinc based regenerative therapies. Recent developments and clinical applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; König, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Radakovich, L.; Marolf, A.; Santangelo, K. Systemic iron overload exacerbates osteoarthritis in the strain 13 guinea pig. Osteoarthr. Cartil. 2020, 28, 1265–1275. [Google Scholar] [CrossRef]
- Nganvongpanit, K.; Buddhachat, K.; Brown, J.L. Comparison of Bone Tissue Elements Between Normal and Osteoarthritic Pelvic Bones in Dogs. Biol. Trace Elem. Res. 2016, 171, 344–353. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Liu, L.; Shi, X.; Yin, L.; Zhang, Y.; Li, X.; Wang, Z.; Liu, G. Effects of Strontium on Collagen Content and Expression of Related Genes in Rat Chondrocytes Cultured In Vitro. Biol. Trace Elem. Res. 2013, 153, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Panahifar, A.; Chapman, L.D.; Weber, L.; Samadi, N.; Cooper, D.M.L. Biodistribution of strontium and barium in the develop-ing and mature skeleton of rats. J. Bone Miner. Metab. 2019, 37, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Coskun Benlidayi, I.; Gokcen, N.; Sarpel, T. Serum magnesium level is not associated with inflammation in patients with knee osteoarthritis. Turk. J. Phys. Med. Rehabil. 2017, 63, 249–352. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xu, J.; Zheng, N.; Wang, J.; Mok, S.; Lee, Y.; Shi, L.; Yue, J.; Yung, S.; Hu, P.; et al. Intra-articular injection of magnesium chloride attenuates osteoarthritis progression in rats. Osteoarthr. Cartil. 2019, 27, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Li, H.; Wei, J.; Yang, T.; Deng, Z.-H.; Yang, Y.; Zhang, Y.; Yang, T.-B.; Lei, G.-H. Association between Dietary Magnesium Intake and Radiographic Knee Osteoarthritis. PLoS ONE 2015, 10, e0127666. [Google Scholar] [CrossRef]
- Li, Y.; Yue, J.; Yang, C. Unraveling the role of Mg(++) in osteoarthritis. Life Sci. 2016, 147, 24–29. [Google Scholar] [CrossRef]
- Veronese, N.; La Tegola, L.; Caruso, M.G.; Maggi, S.; Guglielmi, G. The Association between Dietary Magnesium Intake and Magnetic Resonance Parameters for Knee Osteoarthritis. Nutrients 2019, 11, 1387. [Google Scholar] [CrossRef] [Green Version]
| AM ± SD/Min. and Max. | ||
|---|---|---|
| Age (years) | All patients | 70.6 ± 10.4/64–78 |
| KL2 | 72 ± 8.9/64–79 | |
| KL3–4 | 69.7 ± 11.2/64–77.5 | |
| BMI (kg·m−2) | All patients | 27.8 ± 5.1/24.3–30.5 |
| KL2 | 27.1 ± 4.3/25–29.9 | |
| KL3–4 | 28.3 ± 5.6/24.1–30.8 | |
| VAS | All patients | 7.6 ± 0.8/7–8 |
| KL2 | 7.2 ± 0.6/7–8 | |
| KL3–4 | 7.8 ± 0.8/7–8 | |
| Number of patients (%) | ||
| Sex (Female/Male) | All patients | 38 (65.5%)/20 (34.5%) |
| KL2 | 16 (72.7%)/6 (27.3%) | |
| KL3–4 | 22 (61.1%)/14 (38.9%) | |
| Place of residence | Village | 13 (22.4%) |
| City < 10,000 | 11 (19%) | |
| City > 10,000 | 34 (58.6%) | |
| Cigarette smoking | Nonsmoker | 48 (82.8%) |
| Irregular smoker | 4 (6.9%) | |
| Regular smoker | 6 (10.3%) | |
| Alcohol drinking | Nondrinker | 30 (51.8%) |
| Occasionally | 14 (24.1%) | |
| Often | 14 (24.1%) | |
| Comorbidities | Diabetes | 11 (19%) |
| Arterial hypertension | 38 (65.5%) | |
| Other heart diseases | 8 (13.8%) | |
| Other | 14 (24.1%) |
| Femoral Head | Femoral Neck | |||||
|---|---|---|---|---|---|---|
| Metal | KL2 (n = 22) | KL3–4 (n = 36) | p MW | KL2 (n = 22) | KL3–4 (n = 36) | p MW |
| AM ± SD | AM ± SD | AM ± SD | AM ± SD | |||
| Med. (IQR) | Med. (IQR) | Med. (IQR) | Med. (IQR) | |||
| Mo | 0.59 ± 0.56 | 0.54 ± 0.62 | NS | 0.8 ± 0.62 | 0.75 ± 0.82 | NS |
| 0.18 (0.18–1.15) | 0.18 (0.18–1.08) | 0.71 (0.18–1.38) | 0.18 (0.18–1.45) | |||
| Cr | 0.91 ± 0.98 | 1.44 ± 2.17 | NS | 1.56 ± 1.8 | 1.56 ± 1.82 | NS |
| 0.42 (0.12–1.68) | 0.49 (0.12–1.79) | 0.76 (0.24–2.05) | 0.85 (0.25–1.73) | |||
| Zn | 66.52 ± 16.65 | 75.12 ± 17.07 | 0.06 | 64.86 ± 13.3 | 68.17 ± 14.34 | NS |
| 63.21 (54.82–75.8) | 72.21 (63.89–90.47) | 62.7 (55.05–71.86) | 68.24 (57.87–75.44) | |||
| Cu | 0.62 ± 0.79 | 1.14 ± 0.96 | 0.03 * | 0.89 ± 0.9 | 1.12 ± 1.51 | NS |
| 0.04 (0.04–1.37) | 1.04 (0.42–1.61) | 0.68 (0.04–1.58) | 0.8 (0.04–1.31) | |||
| Fe | 112.18 ± 78.28 | 147.72 ± 131.91 | NS | 152.36 ± 187 | 129.08 ± 110.45 | NS |
| 89.54 (66.04–118.3) | 113.96 (54.82–144.13) | 82.07 (47.13–155.29) | 107.05 (55.71–164.66) | |||
| Sr | 33.74 ± 15.2 | 49.94 ± 29.65 | 0.01 * | 39.44 ± 17.45 | 46.45 ± 20.22 | NS |
| 29.59 (23.38–37.97) | 38.71 (29.76–59.66) | 34.75 (27.18–46.61) | 42.81 (29.83–58.01) | |||
| Ca | 122.07 ± 36.18 | 145.24 ± 33.98 | 0.01 * | 149.36 ± 42.98 | 153.37 ± 41.95 | NS |
| 111.96 (97.2–130.33) | 143.95 (115.68–167.91) | 135.94 (121.05–171.4) | 151.21 (125.96–177.87) | |||
| Mg | 1.29 ± 0.44 | 1.54 ± 0.31 | 0.00 * | 1.49 ± 0.35 | 1.57 ± 0.34 | NS |
| 1.23 (0.95–1.4) | 1.57 (1.31–1.8) | 1.44 (1.26–1.75) | 1.61 (1.37–1.81) | |||
| P | 55.49 ± 16.78 | 66.48 ± 15.69 | 0.01 * | 67.02 ± 18.41 | 68.85 ± 19.36 | NS |
| 52.1 (42.99–58.39) | 66.29 (52.6–78.89) | 62.75 (53.6–81) | 68.03 (56.96–77.81) |
| Femoral Head | Mo | Cr | Zn | Cu | Fe | Sr |
|---|---|---|---|---|---|---|
| KL3–4 | ||||||
| Mo | x | −0.02 | 0.27 | −0.16 | −0.08 | 0 |
| Cr | −0.18 | x | −0.12 | 0.74 * | 0.26 | −0.14 |
| Zn | −0.17 | 0.26 | x | −0.07 | 0.11 | 0.57 * |
| Cu | −0.46 * | 0.11 | −0.27 | x | 0.45 * | −0.16 |
| Fe | 0.05 | 0.51 * | 0.17 | −0.47 * | x | −0.02 |
| Sr | −0.45 * | 0.30 | 0.63 * | −0.06 | 0.26 | x |
| KL2 | ||||||
| Femoral Neck | Mo | Cr | Zn | Cu | Fe | Sr |
|---|---|---|---|---|---|---|
| KL3–4 | ||||||
| Mo | x | 0.30 | −0.46 * | −0.02 | 0.20 | −0.33 * |
| Cr | 0.11 | x | 0.03 | 0.21 | 0.25 | 0.00 |
| Zn | 0.34 | −0.09 | x | −0.05 | −0.20 | 0.48 * |
| Cu | −0.25 | 0.33 | −0.06 | x | 0.39 | −0.35 * |
| Fe | −0.09 | 0.64 * | −0.27 | 0.30 | x | −0.23 |
| Sr | 0.31 | −0.08 | 0.43 * | −0.02 | −0.03 | x |
| KL2 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, M.; Zioła-Frankowska, A.; Frankowski, M.; Kaczmarczyk, J.; Kubaszewski, Ł. Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis. Int. J. Environ. Res. Public Health 2021, 18, 3260. https://doi.org/10.3390/ijerph18063260
Dąbrowski M, Zioła-Frankowska A, Frankowski M, Kaczmarczyk J, Kubaszewski Ł. Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis. International Journal of Environmental Research and Public Health. 2021; 18(6):3260. https://doi.org/10.3390/ijerph18063260
Chicago/Turabian StyleDąbrowski, Mikołaj, Anetta Zioła-Frankowska, Marcin Frankowski, Jacek Kaczmarczyk, and Łukasz Kubaszewski. 2021. "Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis" International Journal of Environmental Research and Public Health 18, no. 6: 3260. https://doi.org/10.3390/ijerph18063260
APA StyleDąbrowski, M., Zioła-Frankowska, A., Frankowski, M., Kaczmarczyk, J., & Kubaszewski, Ł. (2021). Comparison of Bone Tissue Trace Element Content in the Different Radiological Stages of Hip Osteoarthritis. International Journal of Environmental Research and Public Health, 18(6), 3260. https://doi.org/10.3390/ijerph18063260








